Abstract
Herpes simplex virus amplicon vectors expressing RANTES (HSVrantes) and the T-cell costimulatory ligand B7.1 (HSVB7.1) were studied for their ability to elicit a tumor-specific T-cell response in a murine lymphoma model. HSVB7.1- and HSVrantes-transduced EL4 cells expressed high levels of B7.1 and RANTES as analyzed by flow cytometry and enzyme-linked immunosorbent assay, respectively. Inoculation of ex vivo HSVB7.1 transduced cells in syngeneic mice resulted in regression of both transduced cells and nontransduced cells inoculated contralaterally. Direct intratumoral injection of HSVB7.1 and/or HSVrantes alone or in combination into established EL4 tumors led to complete tumor regression in injected tumors as well as in nontransduced contralaterally implanted tumor, whereas control tumors or tumors injected with HSVlac expressing β-galactosidase did not regress. Maximal protection was achieved with combined injection of HSVB7.1 and HSVrantes; mice showing tumor regression were resistant to rechallenge with parental EL4 cells, and tumor cell-specific cytolytic T-cell activity was observed in mice demonstrating regression. HSV amplicon-mediated delivery of immune effector molecules may represent a useful strategy for immunotherapy in the setting of pre-existing tumor.
CONVENTIONAL THERAPIES for hematological malignancies frequently fail to eradicate minimal residual disease. Because malignancies of B- or T-cell origin possess antigen presentation capabilities, the application of immunotherapy represents an attractive strategy for elimination of minimal residual disease. Peptide fragments derived from abnormal fusion proteins due to chromosomal translocations, mutated oncogenes, and viral and tumor-suppressor gene products may all serve as suitable immunologic targets to T cells. Although such antigens may be presented by major histocompatibility complex class I or II proteins (MHC-I/MHC-II), hematologic tumors generally are not rejected. Failure of immune rejection of these tumors may be partially attributed to a lack of T-cell costimulation and consequent establishment of tumor-specific tolerance by T cells. To generate protective immunity, neoplastic cells need to deliver at least two signals to T cells reactive to tumor; first, an antigen-specific signal is delivered by antigenic peptide bound by MHC class I or II proteins to the antigen-specific T-cell receptor (TCR); second, a costimulus is communicated by members of the B7 family (CD80 or B7.1, CD86 or B7.2, B7.x) via the T-cell counterreceptor CD28 and must be delivered within a short interval after the presentation of the first signal.1,2 Presentation of the first without the second signal may lead to the induction of T-cell anergy3: such T cells lack the ability to mount a tumor-specific immune response and become tolerized.4 A number of studies in humans and animals indicate that once established such tolerance cannot be reversed by costimulation with CD80 alone.5 However, combined CD80 stimulation and treatment with cytokines can restore the ability of tolerized T cells to lyse tumor cells.5-7 These data suggest that immunotherapy of hematologic malignancies may require several interventions including provision of a costimulatory signal, reversal of T-cell tolerance with suitable cytokines,8 or the recruitment of nontolerized T cells.
Chemokines are low molecular weight proteins that can attract T cells, monocytes, dendritic cells, and other immune effector cells. RANTES, a member of the C-C family of chemokines, is a potent chemoattractant of monocytes as well as unstimulated CD4+, CD8+, CD45RO+ memory T cells.9 RANTES also has the ability to induce activation and proliferation of T cells when present in high concentrations and may augment the effect of CD80 activation.10 RANTES expression has directly been shown to generate a tumor-specific immune response in a mouse sarcoma model.11
Tumor immunotherapy approaches involving gene transfer are based primarily on the requirement of rapid high level expression of the desired genes required to render a tumor immunogenic. A variety of viral vectors, including retroviruses, adenovirus, vaccinia virus, and others, have been used to enhance immunogenicity through delivery and expression of cytokines, CD80, and other immune effector molecules.7,12-15 Replication defective herpes simplex virus (HSV) amplicon vectors offer several potential advantages in this setting, including high transduction efficiency and expression levels and the ability to package many copies of the transcriptional unit of interest per virion.16-18 We have recently demonstrated the successful use of HSV amplicon vectors expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-2) to prevent hepatic metastases in a rat hepatoma model.19
In this report, we evaluated the effectiveness of HSV amplicon vectors to transduce CD80 and/or RANTES and elicit a protective immune response to preestablished lymphoma. We demonstrate that in virtually all animals transduction of CD80 and RANTES resulted in the eradication of preestablished tumors, the generation of tumor-specific cytotoxic T-cell (CTL) immunity, and immunologic memory.
MATERIALS AND METHODS
Cell Lines
EL4 cells were maintained in Iscove’s Dulbecco modified Eagles Medium (DMEM) with 10% fetal bovine serum (ID-10). EL4-B7.1 is an EL4 cell line transduced with a Moloney murine leukemia virus-derived retroviral vector encoding human CD80 cDNA.20 EL4-B7.1 cells were maintained in ID-10 containing G418 at 500 μg/mL (GIBCO-BRL, Grand Island, NY). RR1 cells used for packaging the HSVamplicon constructs are a BHK-derived cell line engineered to stably express the HSV-IE3 gene.21 NIH 3T3 cells (ATCC, Rockville, MD) as well as the RR1 cell line were maintained in DMEM with 10% (vol/vol) fetal bovine serum (FBS), penicillin (100 μg/mL), streptomycin (100 μg/mL), and geneticin (G418; 400 μg/mL; GIBCO-BRL). Murine splenocytes or enriched murine T cells were maintained in RPMI-1640 medium with 10% FBS, penicillin, and streptomycin, along with 5 × 10−5 mol/L β-mercaptoethanol (RP-10). YAC-1, a natural killer cell target cell line, was maintained in RP-10 medium.
Construction of HSV Amplicon Plasmids
Construction of the HSV amplicon for human CD80.
The coding sequences for human CD80 or human RANTES were cloned into the polylinker region of the pHSVPrPUC plasmid. Specifically, pBJ.huB7.1 plasmid (kindly provided by Dr Lewis Lanier, DNAX, Palo Alto, CA) was digested with HindIII and filled in to generate a blunt end and digested with Xba I. AHindIII/Xba I fragment encoding the human CD80 (B7.1) cDNA was gel-purified and used as insert in the ligation with the vector. The HSV amplicon vector pHSVPrPUC was digested withEcoRI and filled in with Klenow, followed by Xba I digestion. The EcoRI/Xba I vector fragment was gel-purified and ligated with the insert. Orientation of the coding sequences of huB7.1 with respect to the HSV-1 IE4/5 promoter was verified and the amplicon used in the generation of the HSVB7.1 amplicon virus.
Construction of the HSV amplicon for human RANTES.
The SK+pBS-RANTES plasmid (kindly provided by Dr Tom Schall, ChemoCentryx, Mountain View, CA) was partially digested withKpn I followed by digestion with Xba I. A KpnI/Xba I fragment encoding the human RANTES cDNA was gel-purified and ligated to the HSV amplicon vector pHSVPrPUC plasmid digested with Kpn I and Xba I. Orientation of the coding sequences for huRANTES with respect to the HSV-1 IE4/5 promoter was verified and the amplicon used in the generation of the HSVrantes amplicon virus.
Virus Production and Titration
Amplicon DNA was packaged into HSV-1 particles by transfecting 5 μg of plasmid DNA into RR1 cells with lipofectamine as recommended by the manufacturer (GIBCO-BRL). After incubation for 24 hours, the transfected monolayer was superinfected with the HSV strain 17, IE3 deletion mutant virus D30EBA22 at a multiplicity of infection (MOI) of 0.2. Once cytopathic changes were observed in the infected monolayer, the cells were harvested, freeze-thawed, and sonicated using a cup sonicator (Misonix, Inc, Farmingdale, NY). Viral supernatants were clarified by centrifugation at 5,000g for 10 minutes before repeat passage on RR1 cells. This second viral passage was harvested as described above and concentrated overnight by ultracentrifugation in a 25% sucrose gradient as previously described.23 Viral pellets were resuspended in phosphate-buffered saline (PBS; Ca2+ and Mg2+ free) and stored at −80°C for future use. Stocks were titered for helper virus by standard plaque assay methods. Amplicon titers were determined as follows: NIH 3T3 cells were plated in a 24-well plate at a density of 1 × 105 cells/well and infected with the virus. Twenty-four hours after viral infection, the monolayers were washed twice in PBS and either fixed with 4% paraformaldehyde and stained by X-gal histochemistry (5 mmol/L Potassium Ferricyanide; 5 mmol/L Potassium Ferrocyanide; 0.02% NP-40; 0.01% sodium deoxycholic acid; 2 mmol/L MgCl2; and 1 mg/mL Xgal dissolved in PBS) or harvested for total DNA using lysis buffer (100 mmol/L NaCl, 10 mmol/L Tris, pH 8.0, 25 mmol/L EDTA, 0.5% sodium dodecyl sulfate [SDS]) followed by phenol/chloroform extraction and ethanol precipitation. Polymerase chain reaction (PCR) was performed on duplicate samples using primers corresponding to the β-lactamase gene present in the amplicon plasmid under the following conditions: 94°C for 2 minutes; and then 20, 23, or 26 cycles of 94°C for 30 seconds and 58°C for 30 seconds, followed by 72°C for 7 minutes. PCR products from early and late cycles were run on a 1% ethidium bromide gel, and the 450-bp band intensities were assessed using the Fotodyne Foto/Eclipse system (Fotodyne, Inc, Hartland, WI) and Collage Image Analysis Software. HSVB7.1 and HSVrantes titers were estimated by comparison with HSVlac virus as standards. Plaque forming unit (pfu/mL) and amplicon (bfu/mL) titers obtained from these measurements were used to calculate amplicon titer and thus standardize experimental viral delivery. Amplicon titer in the different virus preparations ranged from 1 to 10 × 107 bfu/mL and the helper titers were in the range of 5 to 15 × 107 pfu/mL.
Flow Analysis for CD80 Expression
EL4 cells were infected in vitro either with HSVB7.1 or HSVlac amplicon virus at an MOI of 1 pfu per cell. Specifically, 106 EL4 cells were adsorbed with the amplicon virus in a volume of 0.5 mL at 37°C, 5% CO2 for 4 hours. At the end of 4 hours, 0.5 mL of fresh ID-10 medium was added and incubation was continued for another 12 hours. The infected cells were harvested after 16 hours and 106 cells in 0.1 mL of chilled PBS were stained with 1:10 diluted phycoerythrin-conjugated anti-B7.1 antibody (anti-CD80 PE; Becton Dickinson, San Jose, CA) for 30 minutes at 4°C. Uninfected EL4 cells (negative control) or EL4 stably expressing B7.1 (EL4-B7.1 as positive control) were also stained simultaneously with the anti-CD80 PE antibody. The stained cells were analyzed by flow cytometry using an EPICS flow cytometry instrument.
In Vitro T-Cell Proliferation
T cells were enriched using a murine T-cell enrichment column (R&D Systems, Minneapolis, MN). T cells (105) were incubated in the presence of 5 × 104 γ-irradiated stimulator cells. EL4 or CHO cells infected with HSV amplicon were used as stimulator cells. Retrovirally transduced EL4-B7.1 (derived in the laboratory) or CHO-B7.1 (kindly provided by Dr Peter Linsley, Bristol Myers Squibb Pharmaceutical Research Institute, Seattle, WA) were used as positive controls for B7.1 expression and parental EL4 and CHO cells served as negative controls. Stimulator cells were irradiated with 7,500 rad using a137Cesium-gamma source. Either anti-CD3 antibody (2C11) used as 1:50 dilution of hybridoma cell supernatant or phorbol myristate (10 ng/mL) with ionophore (0.1 ng/mL) was added and the cells were cocultured for 3 days at 37°C. To assay for proliferative responses, triplicate cultures were labeled for 16 hours with 1 μCi3H-thymidine (NEN, Boston, MA; 2 Ci/mmol, 1 μCi/0.2 mL). Cells were harvested on glass fiber filters using a cell harvester (Packard Instruments, Downers Grove, IL) and incorporated 3H-radioactivity measured using a β-counter (Packard Instruments). Results are expressed as the mean (of triplicate cultures) ± standard deviation. T-cell proliferation index (normalized cpm) was determined as the ratio of3H-thymidine incorporated in stimulated versus unstimulated control cultures.
Enzyme-Linked Immunosorbent Assay (ELISA) for Analysis of RANTES Production
EL4 cells were infected with HSVrantes or HSVlac amplicon at an MOI of 1. EL4 cells at 1 × 106 were adsorbed with the amplicon virus in a volume of 0.5 mL at 37°C, 5% CO2for 4 hours, and then 0.5 mL of fresh medium was added and incubation was continued for another 20 hours. Cell culture supernatants were harvested at the end of 24 hours and supernatants were tested for RANTES in a sandwich ELISA using anti-RANTES antibody (R&D Systems) for RANTES capture and biotinylated anti-RANTES (R&D Systems) for detection followed by alkaline phosphatase-conjugated avidin.Para-nitrophenyl phosphate was used as a substrate and absorbance at 405 nm was read in a Bio-Rad (Hercules, CA) microplate ELISA reader. Serial twofold dilutions of standard recombinant human-RANTES (R&D Systems) were run in parallel to quantitate the amount of RANTES in the culture supernatant of infected cells.
T-Cell Migration Assay
Primary murine T cells were purified using T-cell enrichment columns (R & D Systems) from splenocytes of a normal C57BL6 adult mouse. T cells (105) in X-Vivo 10 serum free medium (BioWhitaker Inc, Walkersville, MD) were placed in the upper well of a transwell chamber with a 3-μm pore size membrane (Costar Inc, Cambridge, MA). Samples in lower wells contained either recombinant human RANTES (R&D Systems) or HSVrantes-infected EL4 culture supernatant with known titer of RANTES. HSVlac-infected EL4 culture supernatant was used as a negative control. All the samples were diluted in triplicate in X-Vivo 10 medium and an estimated concentration of 10, 1, or 0.1 ng/mL of soluble RANTES was added to the lower wells. Control wells contained the medium only and the background migration was monitored. The plates were incubated at 37°C for 4 hours, and cells that migrated to the lower wells were counted using a hemocytometer. The results are represented as the mean ± SD of the number of migrated cells in the lower wells assayed in triplicate.
Tumor Growth in Mice
Adult C57BL/6 (H-2b) female mice (8 weeks old) were obtained from Charles River Laboratories (Wilmington, MA) and maintained at the Animal Facility, University of Rochester Medical Center (Rochester, NY). The mice were handled under an approved laboratory animal handling and care protocol. Mice (6 per group) were shaved on the dorsal side of the hind limb and inoculated subcutaneously (SC) with 1 × 106 viable EL4 cells ex vivo infected at an estimated MOI of 1 with HSVB7.1, HSVrantes, or HSVlac amplicon virus, or with uninfected EL4 cells. In some experiments, 106 uninfected EL4 cells were inoculated contralaterally at the same time on the other hind limb. Tumor growth was measured every 2 to 3 days using a caliper and size reported in millimeters diameter. Animals were killed when the tumor size exceeded 22 mm.
Intratumoral Delivery of HSV Amplicon
For intratumoral inoculation of the HSV amplicons, 106viable EL4 cells were inoculated SC on the dorsal side of a shaved hind limb and the tumor was allowed to grow to a size of 5 to 6 mm (6 to 7 days). At this point, the mice were grouped and either HSVB7.1, HSVrantes, HSVB7.1 + HSVrantes, or HSVlac amplicon virus diluted in PBS to a concentration of 2 × 106 amplicon containing virus particles in 50 μL was inoculated intratumorally (10 to 12 mice/group). Control animals with pre-established EL4 tumor received only the diluent PBS. Flow cytometry analysis for B7.1 expression performed 48 hours after intratumoral delivery of HSVB7.1 amplicon into pre-established EL4 tumor on day 7 showed approximately 20% B7.1-positive cells in several samples. The excised tumor cells were stained immediately after the removal and dispersal of tumors (data not shown). A second inoculation of the HSV amplicons was administered on day 14, and the tumor growth was measured every 2 to 3 days. Tumors were allowed to grow to a maximal size of 22 to 23 mm, at which point the animals were killed. Mice from each group that showed no signs of tumor growth at 1 month were selected for rechallenge with parental EL4 cells. The secondary tumor growth after rechallenge was monitored every 2 to 3 days.
In another experiment, 106 EL4 cells were inoculated contralaterally at the same time on the both hind limbs. HSVB7.1 or HSVrantes diluted in PBS to a concentration of 2 × 106 amplicon containing virus particles plus 2 × 106 HSVlac amplicon, 2 × 106 HSVB7.1 plus 2 × 106 HSVrantes, or 4 × 106HSVlac amplicon virus diluted in PBS in 50 μL were injected into tumor on the right hind limb only on days 7 and 14 (10 mice/group). Tumor growth on both sides was measured every 2 to 3 days and size was reported in millimeters diameter. Animals were killed when the tumor size reached 22 to 23 mm.
CTL Assay
Spleens were harvested from C57BL/6 mice that had been inoculated with EL4 cells and injected intratumorally with either HSVB7.1 or HSVrantes or a combination of HSVB7.1 and HSVrantes. Control splenocytes were obtained from mice that were inoculated intratumorally with HSVlac virus or with PBS diluent alone. Splenocytes were prepared according to standard procedures and red blood cells were lysed using AKC lysis buffer. To obtain cytolytic T cells, splenocyte cell suspensions (2 × 106/mL in RP-10) were cultured together with γ-irradiated (7,500 rad) EL4 cells (0.5 × 106cells/mL) in a 25-cm2 flask at 5% CO2, 37°C for 6 days. These in vitro cocultured splenocytes were then used as effector cells in the CTL assays. On the day of assay, EL4 target cells were washed with PBS and resuspended in RP10 medium (0.1 mL) at a concentration of 1.5 to 2 × 106 cells/mL and Na51CrO4 (NEN; 100 μCi; stock concentration, 1 mCi/mL) added for 90 minutes at 37°C. These cells were washed 3 times with PBS and resuspended in 1 mL RP-10, and the viable cell count was measured with a hemocytometer. 51Cr-labeled target cells (l04 cells/0.1 mL) were added to the wells of a V-shaped 96-well plate, and threefold serial dilutions of effector cells were made in triplicate, resulting in final effector-target cell ratios (E:T ratios) of 100:1, 33:1, 11:1, 3:1, and 1:1. Spontaneous release of radioactivity from labeled target cells was measured by culturing the target cells with medium alone in six wells. Total release of radioactivity was determined by lysing the target cells with 2% Triton-X 100 detergent. Plates containing effector and target cells were spun at 1,000 rpm for 2 minutes and incubated for 4 hours at 37°C, 5% CO2. The plates were then centrifuged at 2,000 rpm for 4 minutes and half of the culture supernatant (100 μL) was counted for 51Cr release in a γ counter (Packard Instruments). Mean values are calculated for the replicate wells and the results are expressed as the percentage of specific lysis according to the formula: experimental counts − spontaneous counts/total counts − spontaneous counts × 100.
The mean spontaneous release for virus-infected and uninfected controls averaged between 10% and 20% of the total counts.
Antibody Blocking
Monoclonal antibodies to murine CD4 (GK1.5), CD8 (3.155), or Thy-1 (30H-12) were used in the CTL assay to selectively block either CD4+, CD8+, or Thy-1+ cells. The effector cells for the antibody blocking assay were generated from splenocytes of HSVB7.1 and HSVrantes amplicon inoculated mice with known lytic activity as described above. These antibodies were used in the 51Cr release assay as hybridoma culture supernatants and were diluted 1:2 in a CTL assay. To test for the presence of natural killer (NK) cell activity, the NK cell sensitive target Yac-1 cells (a lymphoma cell line) were used in the assay. Results of triplicate cultures were expressed as the percentage of specific lysis as described above. Average spontaneous release values ranged from 10% to 20% of the total 51Cr incorporated.
RESULTS
Expression and Bioactivity of B7.1 in HSVB7.1 Amplicon-Infected Cells
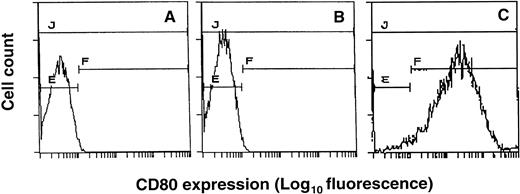
EL4 T-lymphoma cells were transduced with the HSVB7.1 or HSVlac amplicon virus (Fig 1). Infected cells were harvested 24 hours later, immunostained for the expression of B7.1 using PE-conjugated anti-B7.1 antibody (anti-CD80 PE), and analyzed by flow cytometry. Control uninfected EL4 cells or EL4 cells infected with HSVlac were negative for B7.1 expression (Fig 2A and B). In contrast, approximately 95% of EL4 cells infected at an estimated MOI of 1 stained positively for B7.1 (Fig 2C). HSVB7.1 amplicon virus-infected EL4 cells showed significantly higher levels of B7.1 expression than those seen with retrovirally transduced EL4-B7.1 cells (data not shown). Expression of B7.1 in HSVB7.1 infected cells was maintained for up to 60 hours postinfection (data not shown).
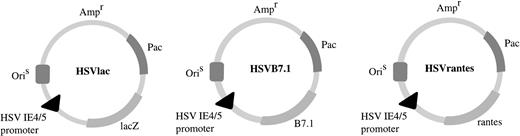
HSV amplicon vectors. Coding sequences for human B7.1 or human RANTES were cloned downstream of the HSV IE4/5 promoter in pHSVPrPUC amplicon plasmid as indicated. HSVlac, which places theEscherichia coli β-galactosidase gene (lacZ) under the transcriptional control of the IE 4/5 promoter, has been described previously.19 Ampr denotes location of the ampicillin resistance gene. OriS represents HSV-1 replication origin and Pac is the HSV-1 cleavage and packaging sequence.
HSV amplicon vectors. Coding sequences for human B7.1 or human RANTES were cloned downstream of the HSV IE4/5 promoter in pHSVPrPUC amplicon plasmid as indicated. HSVlac, which places theEscherichia coli β-galactosidase gene (lacZ) under the transcriptional control of the IE 4/5 promoter, has been described previously.19 Ampr denotes location of the ampicillin resistance gene. OriS represents HSV-1 replication origin and Pac is the HSV-1 cleavage and packaging sequence.
Expression of CD80 (B7.1) in HSVB7.1 amplicon-infected EL4 cells. EL4 cells (A), EL4 cells transduced with HSVlac (B), or HSVB7.1 (C) at an estimated MOI of 1 pfu/cell were stained with PE-conjugated anti-CD80 antibody and analyzed by flow cytometry as described in Materials and Methods.
Expression of CD80 (B7.1) in HSVB7.1 amplicon-infected EL4 cells. EL4 cells (A), EL4 cells transduced with HSVlac (B), or HSVB7.1 (C) at an estimated MOI of 1 pfu/cell were stained with PE-conjugated anti-CD80 antibody and analyzed by flow cytometry as described in Materials and Methods.
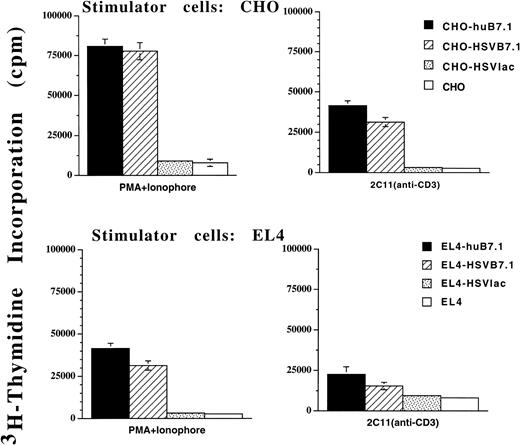
The bioactivity of HSV vector-expressed B7.1 was studied in an in vitro proliferation assay (Fig 3). Murine T cells isolated from C57BL/6 mice were cocultured together with either γ-irradiated EL4 or CHO cells that had been infected with either HSVB7.1 or HSVlac as stimulator cells. Retrovirally transduced EL4-B7.1 or CHO-B7.1 cell lines were used as positive controls, whereas untransduced EL4 or CHO cells served as negative controls. When stimulated with anti-CD3 antibody (2C11) or a mixture of phorbol myristate acetate (PMA) and ionophore to provide signal one, a significant proliferative response was observed for T cells cocultured with HSVB7.1- but not HSVlac-infected stimulator cells (Fig 3). The B7.1-dependent T-cell proliferative response observed with the HSVB7.1-infected EL4 cells was comparable to that seen with the retrovirally transduced control stimulator cells EL4-B7.1 or CHO-B7.1.
In vitro murine T-cell proliferation using HSVB7.1 amplicon-infected CHO or EL4 cells. Splenocytes from adult C57BL/6 mice were harvested and T cells were enriched as described in Materials and Methods. Purified T cells were cultured in triplicate wells with HSVB7.1-infected CHO or EL4 cells. CHO-B7.1 or EL4-B7.1 cells stably transduced with a retroviral vector expressing human B7.1 were used as positive controls. HSVlac-infected or parental EL4 or CHO cells were used as negative controls. Antimurine CD3 antibody (2C11) at a final 1:50 dilution or PMA (10 ng/mL) with Ionophore (0.1 ng/mL) was added as indicated. After 72 hours, the cells were pulsed with3H-thymidine and harvested and incorporated radioactivity was measured as described in Materials and Methods. The results are represented as the mean counts per minute (cpm) from triplicate cultures ± standard deviation.
In vitro murine T-cell proliferation using HSVB7.1 amplicon-infected CHO or EL4 cells. Splenocytes from adult C57BL/6 mice were harvested and T cells were enriched as described in Materials and Methods. Purified T cells were cultured in triplicate wells with HSVB7.1-infected CHO or EL4 cells. CHO-B7.1 or EL4-B7.1 cells stably transduced with a retroviral vector expressing human B7.1 were used as positive controls. HSVlac-infected or parental EL4 or CHO cells were used as negative controls. Antimurine CD3 antibody (2C11) at a final 1:50 dilution or PMA (10 ng/mL) with Ionophore (0.1 ng/mL) was added as indicated. After 72 hours, the cells were pulsed with3H-thymidine and harvested and incorporated radioactivity was measured as described in Materials and Methods. The results are represented as the mean counts per minute (cpm) from triplicate cultures ± standard deviation.
Expression and Bioactivity of RANTES from HSVrantes Amplicon-Infected Cells
To monitor the production of soluble RANTES from HSVrantes-transduced cells, EL4 cells were infected with HSVrantes or HSVlac at an MOI of 0.5. Conditioned media from culture supernatants were collected 24 hours later and analyzed for RANTES by quantitative ELISA. In uninfected EL4 cells or cells transduced with HSVlac, no detectable RANTES secretion was observed in culture supernatants. Cells infected with HSVrantes at an MOI of 0.5 produced 3.1 ng of RANTES/mL/24 hours/106 cells. The observed levels of RANTES were higher than those measured in pooled G418 selected retrovirally transduced EL4-RANTES cells that secreted RANTES at a concentration of 1.45 ng/mL/24 hours/106 cells.
To test for the bioactivity of soluble RANTES from HSVrantes-infected EL4 cells, culture supernatant from HSVrantes-transduced cells was tested in a murine T-cell migration assay using a transwell chamber. Culture supernatant from HSVlac-transduced cells was used as a negative control. Recombinant human RANTES was used as a positive control. Control migration of murine T cells was also tested in response to diluent medium alone. Observed migration in response to HSVrantes-infected EL4 culture supernatant was significantly higher than that seen with the HSVlac or the control medium and comparable to that observed with corresponding dilutions of recombinant RANTES (Table 1). This demonstrated that soluble RANTES secreted by the HSVrantes-infected EL4 cells is capable of eliciting a chemotactic response in murine T cells.
Murine T-Cell Migration in Response to Soluble RANTES
| RANTES (ng/mL) . | No. of Migrating Cells . | |
|---|---|---|
| Recombinant RANTES . | HSVrantes . | |
| 0.1 | 16,041 ± 1,214 | 22,666 ± 1,527* |
| 1.0 | 26,833 ± 2,173* | 29,108 ± 4,741† |
| 10 | 26,666 ± 1,154† | 20,250 ± 1,299* |
| No. of Migrating Cells | ||
| HSVlac | Control | |
| Undiluted ND | 15,000 ± 1,500 | 11,916 ± 938 |
| 1:3 diluted ND | 11,500 ± 866 | |
| 1:9 diluted ND | 10,000 ± 1,732 | |
| RANTES (ng/mL) . | No. of Migrating Cells . | |
|---|---|---|
| Recombinant RANTES . | HSVrantes . | |
| 0.1 | 16,041 ± 1,214 | 22,666 ± 1,527* |
| 1.0 | 26,833 ± 2,173* | 29,108 ± 4,741† |
| 10 | 26,666 ± 1,154† | 20,250 ± 1,299* |
| No. of Migrating Cells | ||
| HSVlac | Control | |
| Undiluted ND | 15,000 ± 1,500 | 11,916 ± 938 |
| 1:3 diluted ND | 11,500 ± 866 | |
| 1:9 diluted ND | 10,000 ± 1,732 | |
Murine T cells (105) in X-Vivo 10 medium were placed in the upper well of a transwell chamber. Samples were either recombinant RANTES, HSVrantes-infected EL4 culture supernatant, or HSVlac-infected EL4 supernatant. All samples were diluted in X-Vivo 10 medium to get 10, 1, or 0.1 ng/mL of soluble RANTES and were added to the lower wells. Control wells contained only the X-Vivo 10 medium. The transwell chambers were incubated at 37°C for 4 hours, and cells that migrated to the lower wells were counted using a hemocytometer. The results are represented as the mean ± SD of the number of cells in the lower chamber in triplicate wells. P values were compared with the HSVlac control using the Student’s t-test.
Abbreviation: ND, not detected.
P ≤ .003.
P < .001.
Growth of HSV Amplicon-Transduced EL4 Cells in Mice
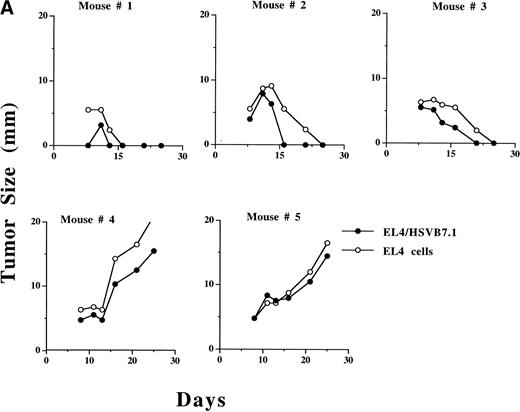
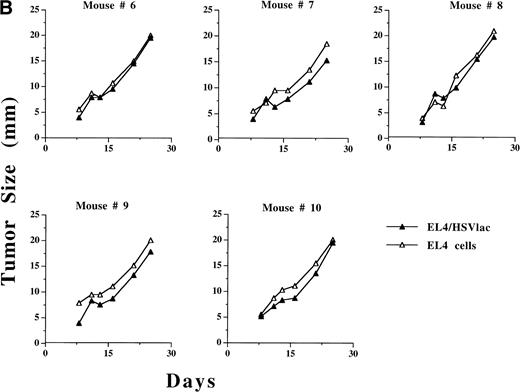
In preliminary experiments, the growth of ex vivo HSV amplicon-infected EL4 cells was measured in adult C57BL6 mice. After transduction with the HSV amplicon virus at an MOI of 1, 106 viable infected cells were inoculated SC in C57BL6 mice and tumor size was measured every 2 to 3 days. The results are summarized in Table 2. On day 20, complete regression of tumor was noted in 3 of 6 mice inoculated with HSVB7.1-infected EL4 cells, whereas 2 of 6 mice inoculated with HSVrantes-infected EL4 cells showed initial tumor growth followed by complete regression. When EL4 cells were infected with both HSVB7.1 and HSVrantes, 5 of 6 mice showed complete regression after initial tumor growth. Although some mice demonstrated initial tumor growth before regression, regression generally began within 7 to 10 days of tumor inoculation, suggesting a gradually increasing antitumor response. Contralateral nontransduced EL4 cells showed a delay in regression of 2 to 3 days relative to transduced cells (Fig 4A). Control EL4 cells or EL4 cells infected with the HSVlac vector grew in 100% of the mice (6 of 6), whereas stably transduced EL4-B7.1 cells showed no evidence of tumor growth in 6 of 6 mice by day 20 (data not shown). These results suggest that HSVB7.1 or HSVrantes amplicon-infected cells may have been rejected due to a tumor-specific immune response.
Tumor Growth of EL4 Cells Infected Ex Vivo With HSV Amplicons
| HSV Amplicon . | No. of Mice With Tumor/ No. of Mice Inoculated . |
|---|---|
| HSV-B7.1 | 3/6 (P = .182) |
| HSVrantes | 4/6 (P = .455) |
| HSV-B7.1 and HSVrantes | 1/6 (P = .015) |
| HSVlac | 6/6 |
| HSV Amplicon . | No. of Mice With Tumor/ No. of Mice Inoculated . |
|---|---|
| HSV-B7.1 | 3/6 (P = .182) |
| HSVrantes | 4/6 (P = .455) |
| HSV-B7.1 and HSVrantes | 1/6 (P = .015) |
| HSVlac | 6/6 |
EL4 cells were infected in vitro with HSV amplicon virus at an estimated MOI of 1 and maintained in culture for 8 hours. Viable HSV amplicon-infected EL4 cells (106) were inoculated SC in mice and tumor presence was recorded at 1 month. Statistical analysis using Fisher’s exact test and the P value for each group compared with the control group (HSVlac) is shown in parentheses.
Growth of HSVB7.1 or HSVlac amplicon-transduced EL4 cells and parental EL4 cells injected contralaterally in C57BL/6 mice. EL4 cells were infected ex vivo at an estimated MOI of 1 with either HSVB7.1 (A; mice no. 1 through 5) or HSVlac virus (B; mice no. 6 through 10). Viable transduced EL4 cells (106) were implanted SC on one side of the hind limb of the C57BL/6 mice, and parental (nontransduced) EL4 cells (106 cells/per mouse) were implanted on the contralateral hind limb. Tumor diameter was measured and expressed in millimeters. Tumor size in individual animals is shown.
Growth of HSVB7.1 or HSVlac amplicon-transduced EL4 cells and parental EL4 cells injected contralaterally in C57BL/6 mice. EL4 cells were infected ex vivo at an estimated MOI of 1 with either HSVB7.1 (A; mice no. 1 through 5) or HSVlac virus (B; mice no. 6 through 10). Viable transduced EL4 cells (106) were implanted SC on one side of the hind limb of the C57BL/6 mice, and parental (nontransduced) EL4 cells (106 cells/per mouse) were implanted on the contralateral hind limb. Tumor diameter was measured and expressed in millimeters. Tumor size in individual animals is shown.
We next evaluated whether inoculation of HSV vector-transduced cells would inhibit growth of concurrent contralaterally inoculated parental nontransduced EL4 cells. In 3 of 5 mice, regression of ex vivoHSVB7.1-infected EL4 tumor was concordant with regression of a contralaterally implanted EL4 cell (Fig 4A). Both HSVlac-infected EL4 cells and contralateral parental EL4 cells developed into tumor in 5 of 5 animals studied (Fig 4B). These data suggest that systemic tumor-specific immunity to parental EL4 cells had developed in a subset of mice inoculated with HSVB7.1-transduced EL4 cells.
Intratumoral Delivery of the HSV Amplicon Vectors
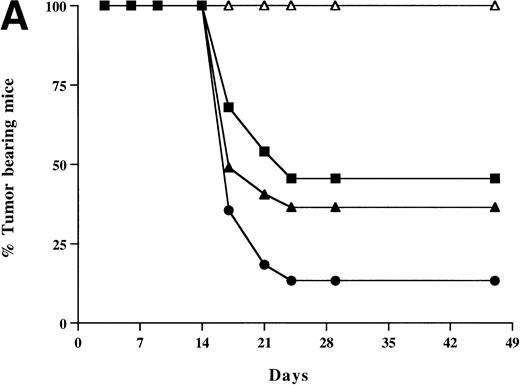
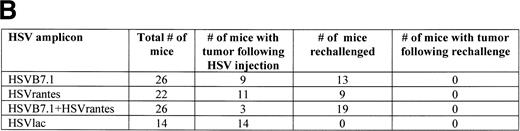
To test the effect of HSVB7.1 and HSVrantes on growth of established tumors in mice, 106 viable EL4 cells were inoculated SC in the hind limb of mice, and tumors were allowed to grow to a diameter of 5 to 6 mm (6 to 7 days). On days 7 and 14, HSV amplicons were delivered intratumorally (10 to 12 mice/group) and tumor size was measured every 2 to 3 days (Fig 5). In three combined experiments, complete tumor regression was observed in 17 of 26 (65%) mice injected with HSVB7.1 vector alone, in 11 of 22 (50%) mice injected with HSVrantes, and in 23 of 26 (88%) mice injected with the combination of HSVB7.1 and HSVrantes (Fig 5). Although additional tumor growth was initially observed, rejection of both HSV amplicon-injected and noninjected tumor generally occurred within 7 to 14 days. A modest lag was observed in rejection of contralateral tumor in some mice, suggesting that gradual amplification of the systemic response was necessary. To determine whether regression of tumor correlated with the development of T-cell memory, mice manifesting complete tumor regression after 1 month were rechallenged with 106 viable EL4 cells in the hind limb contralateral to the primary inoculation. All mice rechallenged with EL4 cells showed no evidence of tumor growth at 1 month after rechallenge (Fig 5), indicating that systemic immunity had been established by the antecedent direct intratumoral delivery of HSVB7.1 and/or HSVrantes into pre-established tumors. Some mice were observed for 2 to 3 months, and no relapses were observed (data not shown).
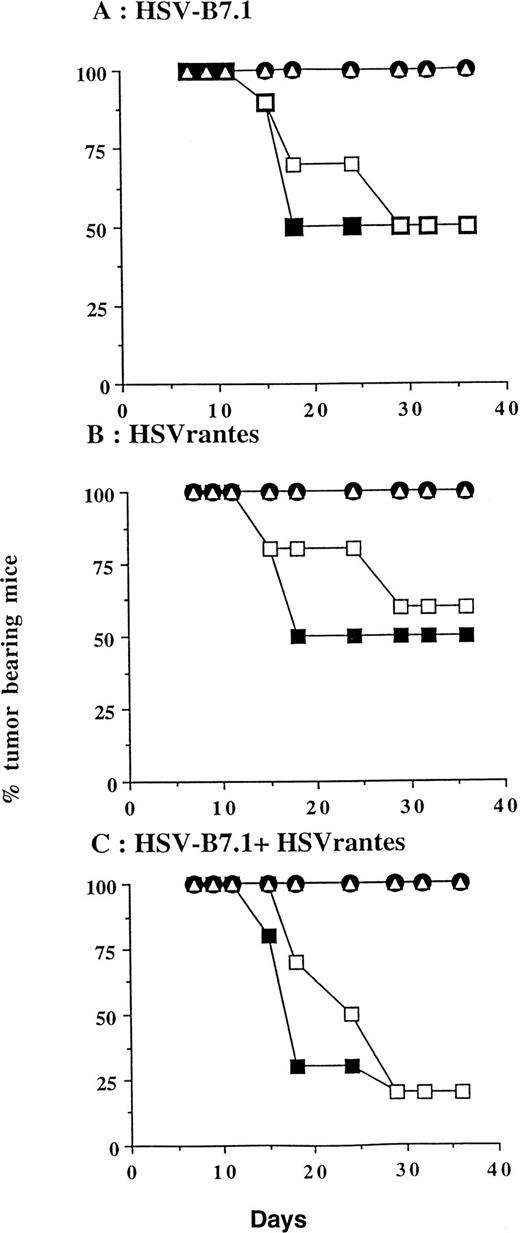
Tumor incidence in mice after inoculation of HSV amplicon vector into pre-established EL4 tumor. (A) Viable EL4 cells (106) were implanted SC on one side of the hind limb of the C57BL/6 mice (8 weeks old). Tumors were allowed to develop to a size of 5 to 6 mm in diameter. HSV amplicon virus (2 × 106amplicon-containing particles) was inoculated on day 7 and again on day 14, and tumor growth was monitored every 3 days. The graph represents the percentage of tumor-bearing mice over time. (▴) HSV-B7.1; (▪) HSVrantes; (•) HSV-B7.1 + HSVrantes; (▵) HSVlac. The number of animals used in each group is shown in (B). Results of three separate experiments are pooled. Mice in which primary tumor had regressed were selected for rechallenge with 106 viable EL4 cells and tumor growth was observed for another month. Mice were killed when the tumor diameter exceeded 22 mm. (C) Statistical analysis was performed using Fisher’s exact test comparing all four arms to each other.
Tumor incidence in mice after inoculation of HSV amplicon vector into pre-established EL4 tumor. (A) Viable EL4 cells (106) were implanted SC on one side of the hind limb of the C57BL/6 mice (8 weeks old). Tumors were allowed to develop to a size of 5 to 6 mm in diameter. HSV amplicon virus (2 × 106amplicon-containing particles) was inoculated on day 7 and again on day 14, and tumor growth was monitored every 3 days. The graph represents the percentage of tumor-bearing mice over time. (▴) HSV-B7.1; (▪) HSVrantes; (•) HSV-B7.1 + HSVrantes; (▵) HSVlac. The number of animals used in each group is shown in (B). Results of three separate experiments are pooled. Mice in which primary tumor had regressed were selected for rechallenge with 106 viable EL4 cells and tumor growth was observed for another month. Mice were killed when the tumor diameter exceeded 22 mm. (C) Statistical analysis was performed using Fisher’s exact test comparing all four arms to each other.
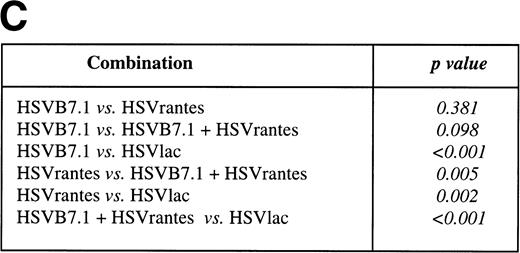
In a separate experiment, we tested whether the delivery of HSV amplicon into pre-established tumor resulted in induction of systemic immunity against a contralateral tumor inoculated at the same time on the left hind limb. Viable EL4 cells (106) were inoculated SC bilaterally on day 0, and HSV amplicon vectors were injected on days 7 and 14 to the tumor established on the right hind limb (10 mice/group; Fig 6). Tumor growth on both hind limbs was measured every 3 to 4 days. Complete tumor regression was seen in 5 of 10 mice (50%, P = .0325) inoculated with HSV-B7.1, 5 of 10 (50%, P = .0325) with HSVrantes, and 8 of 10 (80% P = .0007) in combined HSV-B7.1 and HSVrantes-treated animals. The P value is calculated relative to the HSVlac control group. In animals treated with HSVB7.1 alone (5 of 5, P= .0325) or with the combination of HSVB7.1 and HSVrantes (8 of 8,P = .0007), regression of the contralateral untreated tumor was consistently observed along with the treated tumor. In mice injected with the HSVrantes vector alone, of the 5 mice in which the injected tumor regressed, only 4 of 5 contralateral tumors regressed completely (P = .1734). The P value is calculated relative to the untreated tumor in HSVlac control group. In one of 5 HSVrantes-treated animals, the contralateral tumor grew at a reduced rate relative to control untreated animals. In the HSVlac-treated animals, 10 of 10 animals demonstrated tumor growth on both sides. We noted that HSVlac-treated tumors grew at a slightly reduced rate compared with control untreated tumors. Using a two-sided log rank test, a statistically significant effect was seen for both the treated and untreated contralateral tumor after treatment with HSVB7.1 and/or HSVrantes compared with the control HSVlac-injected group. Although an increased number of animals were tumor free after the combined use of HSVB7.1 and HSVrantes, there was no statistical significance compared with treatment with HSVB7.1 (P = .2823) or HSVrantes alone (P = .3223). This demonstrates that systemic immunity generated as a result of intratumoral HSV amplicon injection could prevent development of contralateral noninjected tumor.
Tumor incidence in mice inoculated with HSV amplicon into pre-established EL4 tumor and growth of parental EL4 cells contralaterally. Viable EL4 cells (106) were implanted SC on both hind limbs of 8-week-old C57BL6 mice. Tumors were allowed to develop to a size of 5 to 6 mm diameter. HSV amplicon virus (2 × 106 amplicon-containing virus particles) was injected into the right tumor on days 7 and 14, and growth of the HSV amplicon-treated (▪) and untreated EL4 tumor (□) was monitored every 3 days. Growth of HSVlac-treated tumor (•) or contralateral untreated EL4 tumor (▵) are also shown. Each experimental group consisted of 10 mice. The graph represents the percentage of tumor-bearing mice over time. Mice were killed when the tumor diameter exceeded 22 mm.
Tumor incidence in mice inoculated with HSV amplicon into pre-established EL4 tumor and growth of parental EL4 cells contralaterally. Viable EL4 cells (106) were implanted SC on both hind limbs of 8-week-old C57BL6 mice. Tumors were allowed to develop to a size of 5 to 6 mm diameter. HSV amplicon virus (2 × 106 amplicon-containing virus particles) was injected into the right tumor on days 7 and 14, and growth of the HSV amplicon-treated (▪) and untreated EL4 tumor (□) was monitored every 3 days. Growth of HSVlac-treated tumor (•) or contralateral untreated EL4 tumor (▵) are also shown. Each experimental group consisted of 10 mice. The graph represents the percentage of tumor-bearing mice over time. Mice were killed when the tumor diameter exceeded 22 mm.
CTL Response
To examine the induction of CTL responses in mice transduced intratumorally with the HSV amplicon vectors, splenocytes from these mice or from mice rechallenged with EL4 cells after primary tumor regression were cocultured in vitro along with irradiated stimulator EL4 cells for 6 to 7 days. Such in vitro boosted splenocytes were used at different effector to target ratios and 51Cr release from a fixed number of labeled EL4 cells counted as a measure of CTL activity. Significant CTL activity was seen in splenocytes from mice receiving HSVB7.1 or HSVrantes alone or in combination (Fig 7A). CTL responses were only seen in mice in which EL4 tumor regressed after direct delivery of the HSVB7.1 and/or HSVrantes amplicons into pre-established tumor. Little or no lytic activity was seen with splenocytes from mice treated with the HSVlac amplicon. Consistent with the absence of secondary tumor growth upon rechallenge, CTL activity was also observed in mice which had been rechallenged with the parental EL4 cells (data not shown).
(A) CTL activity in mice splenocytes after intratumoral inoculation with HSV amplicon vector. EL4 cells were inoculated SC in mice and tumors allowed to grow to 5 to 6 mm in diameter over 6 to 7 days. On days 7 and 14, HSV amplicon virus was injected as indicated directly into the tumors. Spleens were harvested 1 month later and splenocytes were cultured in vitro in the presence of irradiated EL4 cells. After 6 days in culture, CTL activity was measured by release of51Cr from labeled EL4 cells. CTL activity of splenocytes harvested from individual mice intratumorally injected with HSVB7.1 (▴, ▵, ▾) HSVrantes (▪, ⊞, □) HSVB7.1 and HSVrantes (•, ⊕, ○), or HSVlac (*, ×) are shown. Data are expressed as the percentage of specific lysis versus E:T ratio. (B) Antibody blocking of the CTL activity with anti-CD4, anti-CD8, anti–Thy-1, or anti–T-cell cocktail (anti-CD4, anti-CD8, and anti–Thy-1) antibodies. Splenocytes with known CTL activity (harvested from HSV-B7.1+ HSVrantes inoculated mouse no. 26 [A]) were used in an antibody blocking assay. No antibody (▪), anti-CD4 (GK1.5, □), anti-CD8 (3.155, ○), or anti–T-cell antibody cocktail (anti-CD4, anti-CD8, and anti–Thy-1, ▵) were added to the CTL assay at the indicated effector:target ratios as described in Materials and Methods. In a separate experiment, Yac-1 cells were used as target cells (*) for measuring NK activity. Data are represented as the percentage of specific lysis versus E:T ratio.
(A) CTL activity in mice splenocytes after intratumoral inoculation with HSV amplicon vector. EL4 cells were inoculated SC in mice and tumors allowed to grow to 5 to 6 mm in diameter over 6 to 7 days. On days 7 and 14, HSV amplicon virus was injected as indicated directly into the tumors. Spleens were harvested 1 month later and splenocytes were cultured in vitro in the presence of irradiated EL4 cells. After 6 days in culture, CTL activity was measured by release of51Cr from labeled EL4 cells. CTL activity of splenocytes harvested from individual mice intratumorally injected with HSVB7.1 (▴, ▵, ▾) HSVrantes (▪, ⊞, □) HSVB7.1 and HSVrantes (•, ⊕, ○), or HSVlac (*, ×) are shown. Data are expressed as the percentage of specific lysis versus E:T ratio. (B) Antibody blocking of the CTL activity with anti-CD4, anti-CD8, anti–Thy-1, or anti–T-cell cocktail (anti-CD4, anti-CD8, and anti–Thy-1) antibodies. Splenocytes with known CTL activity (harvested from HSV-B7.1+ HSVrantes inoculated mouse no. 26 [A]) were used in an antibody blocking assay. No antibody (▪), anti-CD4 (GK1.5, □), anti-CD8 (3.155, ○), or anti–T-cell antibody cocktail (anti-CD4, anti-CD8, and anti–Thy-1, ▵) were added to the CTL assay at the indicated effector:target ratios as described in Materials and Methods. In a separate experiment, Yac-1 cells were used as target cells (*) for measuring NK activity. Data are represented as the percentage of specific lysis versus E:T ratio.
Monoclonal antibodies recognizing murine CD4, CD8, or Thy-1 markers were used to further characterize the CTLs in the effector population. CTL assays were performed using effector cells from mouse no. 26 (HSVB7.1 and HSVrantes-treated with complete tumor regression) in the presence of antibodies to either CD4, CD8, or Thy-1. Lysis was markedly inhibited in the presence of either an anti–T-cell monoclonal antibody cocktail (CD4,CD8, and Thy-1) or anti-CD8 antibody, but not by anti-CD4 antibody (Fig 7B). To test whether NK cell lysis activity was present,51Cr-labeled Yac-1 cells were used as alternative targets in a CTL assay. Low levels (∼20%) of NK activity were detected at the highest effector:target ratio (100:1) but not at lower effector:target ratios (Fig 7B). Therefore, we conclude that the predominant effector population consisted of CD8+ CTLs.
DISCUSSION
Eradication of minimal residual disease remains a central problem in the treatment of hematologic malignancies. In this report, we describe complete eradication of pre-established lymphoid tumors in immunocompetent mice using HSV amplicon-mediated delivery of the T-cell costimulatory ligand CD80 and the chemokine RANTES.
With rare exception, expression of CD80 and/or CD86 is low to absent on tumors of both hematopoietic and nonhematopoietic origin.24 Expression of either CD80 or CD86 through gene transfer has therefore been used to generate tumor-specific immunity.25-28 In several studies, transfection of either CD80 or CD86 into immunogenic tumors resulted in the generation and establishment of antitumor immunity that offered long-term protection against challenge with B7-negative parental tumor cells.25-28 CD80 expression using retroviral vectors has been shown to confer immune protection and memory against EL4 cells as well as other tumors, including myelocytic leukemia and adenocarcinoma.13,14,25,26 Most of these studies involved primary immunization with CD80 transduced cells followed by rechallenge with the parental tumor cells. In a study with a bcr/abl-transformed myeloid leukemic cell line, a single exposure of mice to CD80 transduced leukemic cells conferred protective immunity.29In addition, a modest challenge using a lethal dose of parental leukemic cells could be rejected if mice were repeatedly immunized with ex vivo CD80-transduced cells starting 1 day after inoculation of the tumor challenge. However, if hyperimmunization was delayed greater than 3 days, protective effects were not seen. A similar requirement for repeated inoculation was demonstrated for M1 leukemic cells.30 In another study using both CD80- and IL-2–transduced cells, prolonged survival and delayed growth of NC tumor was observed after implantation of transduced cells on days 7, 18, and 33 posttumor implantation.7 These results suggested that vigorous and repeated immunization with cells expressing CD80 alone or with IL-2 may be required for an immune response to parental tumor. In the present study, we demonstrate that intratumoral inoculation with HSVrantes and/or HSV B7.1 into well-established tumors could produce sustained antitumor immunity. Our data suggest that direct viral inoculation in vivo into preexisting tumor may be similar to hyperimmunization and that the levels of CD80/chemokine expression after direct HSV amplicon vector injection were adequate to confer protection.
Although costimulation of T cells through the CD80-CD28 pathway has been shown to induce the synthesis of the chemokine macrophage inflammatory protein 1α (MIP1α), other C-C chemokines, such as MIP1β, lymphotactin, or RANTES, are not induced.31 Therefore, combined expression of CD80 and RANTES may potentiate different pathways than those induced by CD80 alone. RANTES may also enhance response to CD80 costimulation and may directly elicit an antitumor response at high concentrations in a murine sarcoma model.11 The in vitro levels of RANTES observed in this study were similar to those seen with other molecules, such as interferon-γ (IFN-γ), GM-CSF, or nerve growth factor (NGF), expressed through the use of HSV amplicons.19,23 32 It is possible that higher concentrations of RANTES could be achieved in vivo if higher virion doses were administered.
Although either HSVB7.1 or HSVrantes conferred partial protection, combined use of both vectors resulted in eradication of about 88% of established tumors. It has been reported that expression of IL-2 in combination with CD80 expression may be effective in reversing tumor-induced tolerance in follicular lymphomas.6 Examining a murine B-cell lymphoblastic leukemia model, Dilloo et al33 reported that CD80 induction using CD40 ligand or IL-2 treatment alone showed partial protection from tumor growth, whereas enhanced response was seen using a combination of CD40 ligand and IL-2. In the NC adenocarcinoma model, neither CD80 nor IL-2 alone had any significant effect on NC tumorigenicity. However, combined expression of CD80 and IL-2 substantially decreased NC tumorigenicity in mice.7 We are currently testing whether combined HSV amplicon delivery of cytokines such as IL-2, IFN-γ, or other chemokines may further enhance the observed protective response in the EL4 T-lymphoma model. Other investigators have demonstrated that recruitment using the chemokine lymphotactin, coupled with IL-2 stimulation, may be superior to the use of either alone in eliciting an antitumor response.34 Although RANTES is known to predominantly attract memory T cells, attraction of naive T cells or other T-cell subpopulations using other chemokines such as the recently characterized DC-CK135 or lymphotactin34 may further potentiate an antileukemic response. Nevertheless, these results suggest that expression of RANTES alone or in combination with CD80 is a useful strategy for eliciting an immune response to pre-established lymphoma.
We were successfully able to eradicate pre-established EL4 tumors in mice by intratumoral inoculation of HSVB7.1 and/or HSVrantes amplicon virus. If these two are combined and inoculated, an increased number of animals reject the tumor. We have also demonstrated that systemic tumor-specific immunity can be established, because mice that reject the HSV amplicon-injected EL4 tumor also rejected a contralateral challenge with parental tumor. A short delay in onset of tumor regression in contralateral uninjected tumors was seen, suggesting that 10 to 14 days were necessary for adequate amplification of a systemic antitumor response to facilitate rejection. We consistently observed that mice that had rejected the tumor maintain a tumor-specific memory T-cell response, because 100% of such mice were protected upon rechallenge with parental tumor cells. Tumor regression also correlated with the development of tumor cell-specific CTL responses in mice. Expression of CD80 in EL4 cells has been known to be immunogenic in C57BL/6 mice and this immunity was shown to be mediated by CD8+ T cells.25 Control animals that were mock-treated or inoculated with HSVlac amplicon virus did not show any significant CTL responses and appeared tolerized to the tumor. Because murine T cells respond to costimulation by human CD80 (Fig 3) and murine T cells migrate in vitro in response to human RANTES (Table1), we chose to use the human cDNA for the experiments described above. Furthermore, the CTL response observed to contralateral EL4 cells using human CD80 and RANTES cDNA suggests that systemic immunity did not depend on response to xenogeneic or HSV viral antigens. It has been reported by Townsend et al28 that specificity and longevity of antitumor responses induced by CD80-transfected tumor cells are maintained for more than 90 days, and other reports have detected CTL activity for up to 6 months.13 We measured CTL activity at different times up to 2 months and significant levels of CTL activity were sustained in mice that rejected tumors after the intratumoral delivery of HSV amplicon virus (data not shown). After rechallenge with the parental EL4 tumor cells, we observed an increase in CTL activity most likely mediated by memory T cells (data not shown). In addition, the observed CTL activity was mediated predominantly by the CD8+ CTLs.
Gene therapy strategies have involved numerous approaches. Among the viral vectors, adenovirus, adeno-associated virus, and retroviruses have been extensively analyzed. Other investigators have observed regression of established tumors using adenovirus vectors engineered to express IL-2.36 Limitations with the use of retroviruses have been the lower achievable titers and inability to transduce nondividing cells. HSV vectors have several advantages, including moderately high titer, robust levels of expression, increased packaging capability (150 kb), and transduction of multiple copies of the gene of interest. Dilloo et al37 used replication defective recombinant HSV vectors derived from HSV-2 to transduce the GM-CSF gene to elicit an antileukemic response against the A20 murine leukemia cell line in vivo. These investigators also report ability to withstand challenge with a lower number (105) of leukemic cells when followed by several subcutaneous injections of an equal number of cells transduced ex vivo with HSV vectors expressing GM-CSF.37 Amplicon vectors have several advantages relative to replication defective HSV as recombination is not necessary for vector generation. Use of amplicons confers the ability to deliver multiple genes per infectious event. Amplicon vectors should in theory be less prone to recombination and regeneration of infectious HSV virus due to the absence of the viral genome. Further studies with HSV amplicons will define the potential utility of this vector in humans in the setting of established HSV-specific immunity and pre-existing malignancy.
ACKNOWLEDGMENT
The authors sincerely acknowledge the antibodies for Thy-1, CD3, CD4, and CD8 received from Dr Edith Lord and Dr Richard Phipps (University of Rochester Cancer Center, Rochester, NY). Dr Richard Raubertas (Department of Biostatistics, University of Rochester Medical Center) is acknowledged for his help in statistical analysis. Karen Rosell from the Hematology-Oncology Unit was very helpful in the chemotaxis assay. The author M.K. dedicates this manuscript to the memory of Somnath Ghosh, MD, PhD, who had been a guiding beacon during his PhD research work.
Supported by National Institutes of Health (NIH) Grant No. AI07285-10 to M.K., NIH Grant No. PO1 CA59326 and the University of Rochester Cancer Center Discovery Fund to J.D.R., and NIH Grants No. DK 53160 and NS36420 to H.J.F.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Joseph D. Rosenblatt, MD, University of Rochester Medical Center, Box 704, 601 Elmwood Ave, Rochester, NY 14642; e-mail: Joe_Rosenblatt@urmc.rochester.edu.

![Fig. 7. (A) CTL activity in mice splenocytes after intratumoral inoculation with HSV amplicon vector. EL4 cells were inoculated SC in mice and tumors allowed to grow to 5 to 6 mm in diameter over 6 to 7 days. On days 7 and 14, HSV amplicon virus was injected as indicated directly into the tumors. Spleens were harvested 1 month later and splenocytes were cultured in vitro in the presence of irradiated EL4 cells. After 6 days in culture, CTL activity was measured by release of51Cr from labeled EL4 cells. CTL activity of splenocytes harvested from individual mice intratumorally injected with HSVB7.1 (▴, ▵, ▾) HSVrantes (▪, ⊞, □) HSVB7.1 and HSVrantes (•, ⊕, ○), or HSVlac (*, ×) are shown. Data are expressed as the percentage of specific lysis versus E:T ratio. (B) Antibody blocking of the CTL activity with anti-CD4, anti-CD8, anti–Thy-1, or anti–T-cell cocktail (anti-CD4, anti-CD8, and anti–Thy-1) antibodies. Splenocytes with known CTL activity (harvested from HSV-B7.1+ HSVrantes inoculated mouse no. 26 [A]) were used in an antibody blocking assay. No antibody (▪), anti-CD4 (GK1.5, □), anti-CD8 (3.155, ○), or anti–T-cell antibody cocktail (anti-CD4, anti-CD8, and anti–Thy-1, ▵) were added to the CTL assay at the indicated effector:target ratios as described in Materials and Methods. In a separate experiment, Yac-1 cells were used as target cells (*) for measuring NK activity. Data are represented as the percentage of specific lysis versus E:T ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.643/4/m_blod40224007ax.jpeg?Expires=1769307875&Signature=5LuCP-eX3rV3XvipRUwUhzj7kX5PeqXIT1lbXs8pVid-sKtECbxS9rgNT0k8etbz1Qi2OCqKlt2JXFENHi~Mgvy~XM~IZ4ElzujemHJuMSqC~RzOs-bV~Sn1bAOXLQ3gp7Pp20qmgaHHOCQN3TE5KT56Xbcq-Mn5tyCXCKFF0X1I4tzFjyMVpafMUDt2TMaZKacmfttCJbKBcXragixW0qWi4eFU9jMZU5WJqThhb6eNG5kR3tfzjCv-hyJesDFpZvuICK-ieeVLIOx2VhDSaqwLNQc4xxGTIupvtWiZnct-X21ERQX2MXg28U3q9QPZ9Wx~80pYrTeLc~Usw9xLNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. (A) CTL activity in mice splenocytes after intratumoral inoculation with HSV amplicon vector. EL4 cells were inoculated SC in mice and tumors allowed to grow to 5 to 6 mm in diameter over 6 to 7 days. On days 7 and 14, HSV amplicon virus was injected as indicated directly into the tumors. Spleens were harvested 1 month later and splenocytes were cultured in vitro in the presence of irradiated EL4 cells. After 6 days in culture, CTL activity was measured by release of51Cr from labeled EL4 cells. CTL activity of splenocytes harvested from individual mice intratumorally injected with HSVB7.1 (▴, ▵, ▾) HSVrantes (▪, ⊞, □) HSVB7.1 and HSVrantes (•, ⊕, ○), or HSVlac (*, ×) are shown. Data are expressed as the percentage of specific lysis versus E:T ratio. (B) Antibody blocking of the CTL activity with anti-CD4, anti-CD8, anti–Thy-1, or anti–T-cell cocktail (anti-CD4, anti-CD8, and anti–Thy-1) antibodies. Splenocytes with known CTL activity (harvested from HSV-B7.1+ HSVrantes inoculated mouse no. 26 [A]) were used in an antibody blocking assay. No antibody (▪), anti-CD4 (GK1.5, □), anti-CD8 (3.155, ○), or anti–T-cell antibody cocktail (anti-CD4, anti-CD8, and anti–Thy-1, ▵) were added to the CTL assay at the indicated effector:target ratios as described in Materials and Methods. In a separate experiment, Yac-1 cells were used as target cells (*) for measuring NK activity. Data are represented as the percentage of specific lysis versus E:T ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/2/10.1182_blood.v93.2.643/4/m_blod40224007bx.jpeg?Expires=1769307875&Signature=ymVm9KIXDvYs-9cKo3WaUKb49G4A51YLU5KcMykwfao3p~1JIqGcGl7f1VpLh4Ljv0a1gv2ZJMmhI4FJC38kP9ODh5cT5kStPI5Z6egf6PImqzQi0-N6ze5Z9YuYmXzu6927cv58PECwH~p3z4JvaXLZUzvLDn6quJ5WD7HTngEYXpsmqxw9-d66z70nKusEEUDbMOtHZAmSxKbIvqIy~b8NqxQFI7ms3ieShdfIrUSPdux0EGSyXN6c-4OpM1N5b1llFuqylKYR-Gm4Ar0Yp7oOIU6viIg~p68JPEbWKRpIxhnXRucxEpoeSSN5QUE9N0IwK3CrZLhNgXw8~MQ6mA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal