Abstract
In anaplastic large-cell lymphoma (ALCL), the (2;5) chromosomal translocation creates a fusion gene encoding the 80-kD NPM-ALK hybrid protein. This report describes three new monoclonal antibodies, two of which recognize, by Western blotting, the N-terminal portion of NPM present in the NPM-ALK fusion protein and also in two other NPM fusion proteins (NPM-RAR and NPM-MLF1). The third antibody recognizes the C-terminal portion (deleted in NPM-ALK) and reacts only with wild-type NPM. The three antibodies immunostain wild-type NPM (in paraffin-embedded normal tissue samples) in cell nuclei and in the cytoplasm of mitotic cells. Cerebral neurones, exceptionally, show diffuse cytoplasmic labeling. In contrast to normal tissues, the two antibodies against the N-terminal portion of NPM labeled the cytoplasm of neoplastic cells, in four ALK-positive ALCL, reflecting their reactivity with NPM-ALK fusion protein, whereas the antibody to the C-terminal NPM epitope labeled only cell nuclei. Immunocytochemical labeling with these antibodies can therefore confirm that an ALK-positive lymphoma expresses NPM-ALK (rather than a variant ALK-fusion protein) and may also provide evidence for chromosomal anomalies involving the NPM gene other than the classical (2;5) translocation.
IN 1984, A LARGE-CELL lymphoma, encountered most frequently in children and young adults, was defined on the basis of its distinctive morphology and expression of CD30 (Ki-1).1 This tumor, which came to be generally known as anaplastic large-cell lymphoma (ALCL) or Ki-1 lymphoma, was subsequently found to be associated with a (2;5) chromosomal translocation.2 3
In 1994, Morris et al4 showed that this genetic anomaly juxtaposes part of the nucleophosmin (NPM) gene on chromosome 5 to a portion of the ALK receptor tyrosine kinase gene on chromosome 2, encoding its entire intracytoplasmic region. Cross-linking of the resultant 80-kD NPM-ALK hybrid protein (via motifs in the NPM moiety) activates its kinase activity,5,6 and this appears to contribute directly to neoplastic transformation, because transfection of murine hematopoietic cells with the NPM-ALKgene induces a transplantable lymphoid tumor.7
The NPM gene is also involved in other neoplasia-associated genetic abnormalities.8 In cases of acute promyelocytic leukemia carrying the (5;17)(q33;q12) translocation, the same N-terminal portion as that present in the NPM-ALK kinase fuses to part of the RARα gene product.9,10 TheNPM gene can also fuse to the MLF1 gene in rare cases of acute leukemia and myelodysplasia that carry a (3;5)(q25.1;q34-35) chromosomal translocation.11,12 The resultant fusion protein contains a longer sequence of the N-terminal portion of NPM than is present in NPM-ALK (175 v 117 amino acids), and this extra region contains a nuclear localization signal and acid residue clusters capable of interacting with other proteins.13
The NPM-ALK fusion gene can be detected in tissue samples by the reverse transcriptase-polymerase chain reaction (RT-PCR) technique. However, this technique is potentially prone to artefact, a problem that may account for discrepancies in the literature over the frequency of the NPM-ALK gene in different categories of lymphoid neoplasia14 and also for the controversial but unconfirmed claim that it is frequently present in Hodgkin’s disease.15-18 Furthermore, RT-PCR detection of theNPM-ALK gene is best performed on fresh tissue and in the past this has restricted the number of cases that could be studied.
The production of antibodies specific for the cytoplasmic portion of the ALK protein therefore represented a significant advance in the detection of NPM-ALK protein.19-22 Normal lymphoid tissue does not contain detectable ALK protein and a positive immunocytochemical staining reaction usually indicates that a lymphoma expresses the NPM-ALK fusion protein as a result of the t(2;5) anomaly.
Extensive immunohistochemical studies have now been performed using anti-ALK antibodies,21-26 and a clearer perception has emerged of the pathologic and clinical features of ALK-positive lymphomas and of their relation to other lymphoma types. In recent publications, the term “ALK-positive lymphoma” was proposed22 26 to distinguish these tumors from the heterogeneous group of neoplasms covered by the term ALCL.
However, there have been a few reports of cases in which ALK-positivity is caused by the presence of a protein other than the NPM-ALKgene product and it is of obvious interest to recognize them. One example is a rare large B-cell lymphoma expressing full-length ALK protein,27 which can be recognized on the basis of its unusual morphology and phenotype. It also shows a characteristic granular intracytoplasmic labeling pattern for ALK that differs from the diffuse cytoplasmic and nuclear labeling seen in classical ALK-positive ALCL.
A case of ALK-positive ALCL carrying a (1;2) translocation has been reported,6,21,28 and in this case, the ALK gene (at 2p23) was presumably linked to a gene on chromosome 1q25 rather than toNPM. Two other ALK-positive lymphomas with variants of the classical (2;5) translocation have been reported.25 In one case, fluorescence in situ hybridization (FISH) analysis showed a cryptic NPM-ALK fusion gene, but in the other it appeared that a gene located at 2q35 may have fused to ALK. Other cases of ALCL with possible novel chromosomal translocations have been reported,29-32 but they were not analyzed for ALK expression.
One possible way to confirm that an ALK-positive case carries the (2;5) translocation and to exclude variant ALK rearrangements would be to immunostain for the N-terminal and the C-terminal portions of NPM, because the former is present in NPM-ALK and the latter absent. In this report, we describe monoclonal anti-NPM antibodies with the required specificities that are suitable for Western blotting and that can distinguish, by this technique, between wild-type NPM and NPM-ALK (and also the NPM-RARα and NPM-MLF1 fusion proteins). They also label routinely processed paraffin-embedded tissue and give differential staining patterns that allow NPM-ALK–positive lymphomas to be specifically detected. They are therefore of potential value for exploring variants of the (2;5) translocation and for detecting tumors that contain novel NPM fusion proteins.
MATERIALS AND METHODS
Cells and Tissues
Cell lines were cultured at 37°C in RPMI 1640 medium containing 10% fetal calf serum (FCS; Life Technologies Ltd, Paisley, Scotland). Cytocentrifuge preparations of cell lines were made as previously described.33 Samples of human tonsil, obtained from one of the authors’ (D.Y.M.) laboratories, had been snap frozen and stored at −70°C.33 Other tissues obtained from the same source had been fixed in formalin before embedding in paraffin and comprised one sample each of tonsil, lymph node, thymus, spleen, kidney, liver, pancreas, lung, brain, prostate, and gut, and biopsies of 9 carcinomas (3 squamous cell and 2 basal cell carcinomas of skin and 4 ductal carcinomas of breast) and of 4 cases of ALCL. Additional routinely processed normal human tissue specimens (1 sample each of lymph node, spleen, muscle, and liver) were obtained from another of the authors’ (B.F.) laboratories, as were bone marrow biopsies that had been fixed in B5 fixative for 3 hours and decalcified in EDTA before paraffin-embedding. All routinely processed paraffin-embedded samples had been diagnosed by conventional histologic criteria and immunohistologic labeling for cell lineage markers. No differences in immunostaining were noted between material from different sources.
Normal mouse tissues (1 sample each of lymph node, spleen, bone marrow, muscle, and liver) were obtained in one of the authors’ (B.F.) laboratories and fixed in formalin for 24 hours or in B5 fixative for 2 hours before paraffin-embedding.
Recombinant Proteins
Proteins for immunization.
Three different recombinant proteins containing NPM were used to raise antibodies. The monoclonal antibody NA24 (anti–N-terminus) was raised to protein prepared in one of the authors’ (D.Y.M.) laboratories as follows: NPM-ALK cDNA plasmid in pBluescript was cut withPst I and the resulting 450-bp fragment, including the fusion point with ALK, was gel-purified and cloned into the PstI site of pBluescript SK− (Stratagene, La Jolla, CA) creating plasmid pAB192. This was then digested withEcoRI, and the 450-bp fragment was gel-purified and cloned into the EcoRI site of the vector pGEX1 (Pharmacia, Uppsala, Sweden). A glutathione S-transferase (GST) fusion protein, containing the N-terminus of NPM fused to 14 amino acids from ALK, was then expressed in Escherchia coli strain BL21 (DE3). Inclusion bodies were prepared for immunization as previously described,34 and soluble protein for the enzyme-linked immunosorbent assay (ELISA) was affinity purified using glutathione-Sepharose (Pharmacia) according to the manufacturer’s instructions.
The monoclonal antibody NPMa (anti–N-terminus) was raised against recombinant protein produced in another of the authors’ (P.-G.P.) laboratories by digesting NPM-ALK cDNA in pcDNA3 withEcoRI and Taq I. The resulting fragment was blunt ended with Klenow enzyme and then cloned in the Sma I site of pGEX-4T-3 (Pharmacia). The resulting GST-NPM-ALK fusion protein included the entire NPM-ALK coding sequence except for the first nine nucleotides of the NPM cloning sequence (which were lost during the subcloning procedure). This plasmid was transformed into E colistrain BL21 (DE3) and the recombinant GST-NPM-ALK fusion protein was prepared as described.34
The third recombinant antigen used to produce the antibody NPMc was also prepared in one of the authors’ (P.-G.P.) laboratories. TheNPM cDNA was isolated from a library made from the KG1 cell line and its identity was confirmed by DNA sequence analysis. The NPM coding sequence was then cloned into the Sma I site of the pGEX-4T-3 vector (Pharmacia). The resulting GST-NPM fusion protein included the entire NPM coding sequence, except for the first nine AA (which were lost during the subcloning procedure). This plasmid was transformed into E coli strain BL21 and the recombinant GST-NPM fusion protein was prepared as described previously.34
Eukaryotic expression of NPM proteins.
A cDNA fragment corresponding to the whole open reading frame of NPM-ALK protein was generated by PCR of total cDNA from the Karpas cell line, using oligonucleotide primers spanning from the NPM ATG to the ALK TGA triplets. The PCR product was cloned into the pCR2.1 TA cloning system (Invitrogen, San Diego, CA), checked by sequencing analysis, and subcloned into the eukaryotic expression plasmid pCDNA3 (Invitrogen). The NPM and MLF1full-length cDNAs were isolated by the PCR technique using total cDNA from the KG1 cell line as a template. RARα cDNA was already available in the laboratory.35
NPM-MLF1 and NPM-RARα chimeric cDNAs identical to the natural fusion genes were then constructed by recombinant PCR using the appropriate full-length cDNAs described above. The PCR-generated cDNAs were checked by sequencing and then cloned into the pCDNA3 expression plasmid. Aliquots of the CV1 cell line were transfected with the three plasmids.35 After 36 hours, the transfected cells were lysed in Laemmli buffer,36 and 30-μg aliquots of total lysate were analyzed by Western blotting.
Production of Monoclonal Antibodies
Monoclonal antibodies were raised by conventional techniques against recombinant proteins.37,38 Antibody NA24 was obtained by screening (by the ELISA technique) for supernatants that reacted with NPM-ALK-GST recombinant protein but not with GST. Supernatants were then screened by immunocytochemical labeling against the t(2;5)-positive SU-DHL-1 cell line.21
The fusions producing antibodies NPMa and NPMc were screened by an immuno-alkaline phosphatase technique (APAAP)39 on paraffin sections of human tonsil and of a biopsy from a t(2;5)-positive ALCL known to express the NPM-ALK protein.
Anti-ALK Antibodies
Immunocytochemical Labeling
Acetone-fixed cryostat sections were stained by immunoperoxidase or APAAP techniques, as described previously.39 Sections of paraffin-embedded tissue on Superfrost Plus slides (Speci-Microsystems Ltd, Carshalton, Surrey, UK) were dewaxed and then subjected to microwave treatment as described previously.40 Antibodies were used as undiluted tissue culture supernatants.
Biochemical Characterization
RESULTS
Western Blotting With Anti-NPM Antibodies
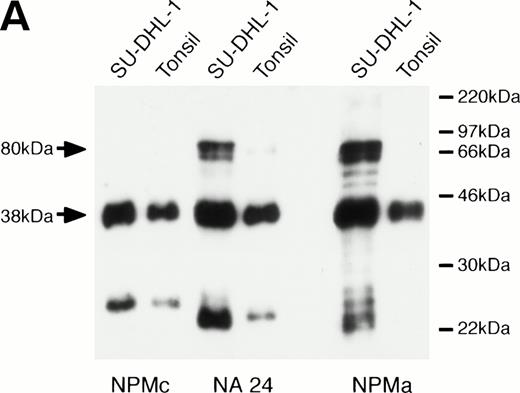
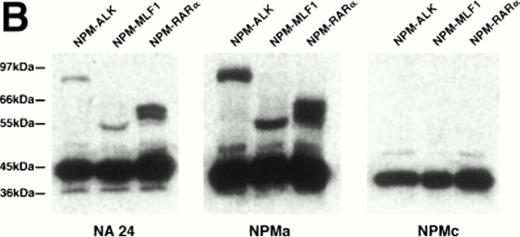
When the monoclonal antibody NPMc was tested by Western blotting, it detected a single band in extracts both of reactive lymphoid tissue (tonsil) and of the t(2,5)-positive cell line SU-DHL-1 (Fig 1A). In contrast, antibodies NA24 and NPMa both detected an additional band in the SU-DHL-1 extract, but not in the extract of normal tonsil. This band corresponded in size (80 kD) to the NPM-ALK fusion protein, and it was concluded that these two antibodies detect epitopes in the N-terminal portion of NPM conserved in the fusion protein, whereas NPMc detects an epitope encoded on the C-terminal side of the NPM breakpoint. The antibodies also showed differential reactivity by Western blotting against two recombinant fusion proteins, NPM-MLF1 and NPM-RARα (Fig 1B).
Western blotting reactivity of monoclonal anti-NPM antibodies. (A) Wild-type NPM (38 kD) is detected in lysates of both normal cells (tonsil) and of the t(2;5)-positive cell line SU-DHL-1 with each of the three antibodies. However, only the two antibodies to the N-terminal region (NA24 and NPMa) react with the hybrid NPM-ALK protein (80 kD). (B) The two antibodies to the N-terminal region (NA24 and NPMa) detect recombinant NPM-ALK and also recombinant NPM-MLF1 and NPM-RAR proteins. Antibody NPMc (against the C-terminal region) is unreactive with any of these fusion proteins.
Western blotting reactivity of monoclonal anti-NPM antibodies. (A) Wild-type NPM (38 kD) is detected in lysates of both normal cells (tonsil) and of the t(2;5)-positive cell line SU-DHL-1 with each of the three antibodies. However, only the two antibodies to the N-terminal region (NA24 and NPMa) react with the hybrid NPM-ALK protein (80 kD). (B) The two antibodies to the N-terminal region (NA24 and NPMa) detect recombinant NPM-ALK and also recombinant NPM-MLF1 and NPM-RAR proteins. Antibody NPMc (against the C-terminal region) is unreactive with any of these fusion proteins.
Immunocytochemical Staining
Normal tissues.
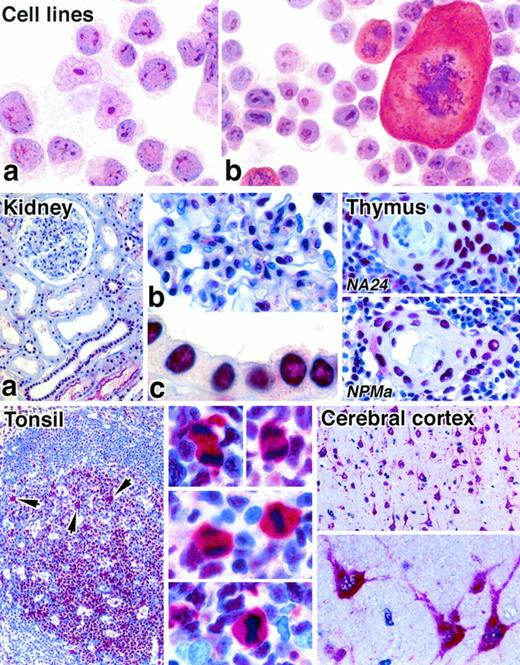
The anti-NPM antibody recognizing the C-terminus (antibody NPMc) stained nucleoli in cytospin preparations of cell lines (Fig 2) and in cryostat tissue sections. In contrast, the two antibodies against the N-terminus gave only weak or negative reactions on cytospin preparations and cryostat sections. However, all three antibodies gave strong nuclear labeling in both human and mouse tissues when paraffin-embedded tissue samples were tested (Fig 2), and their reactivity was essentially indistinguishable. The reactivity within nuclei was diffuse, although at higher antibody dilutions nucleolar labeling became evident. The intensity of nuclear staining varied from cell to cell within individual sections, regardless of the antibody used, and epithelial cells tended to give the most uniform labeling.
Immunostaining of cytocentrifuged cell lines and paraffin-embedded normal tissues for NPM (APAAP technique). Cell lines: The t(2;5)-positive cell line Karpas 299 (a) and a murine mastocytoma cell line (b) both show labeling of nucleoli with antibody to the C-terminal portion of NPM (antibody NPMc). Note the cytoplasmic labeling of a mitotic cell in (b). Kidney: (a) Many cells show nuclear labeling for nucleophosmin. (b) and (c) show high power views of, respectively, a glomerulus and tubular epithelium (antibody NA24). Thymus: Two different anti-NPM antibodies give essentially identical labeling patterns of cell nuclei in a Hassall’s corpuscle and in the surrounding medullary tissue. Tonsil: Nuclear labeling of lymphoid cells is seen. The arrows in the low power view (left) indicate scattered mitotic cells in which there is cytoplasmic labeling, seen at high power on the right (antibody NA24). Cerebral cortex: Neural cells show strong cytoplasmic staining, seen at low power (above) and high power (below) (antibody NA24).
Immunostaining of cytocentrifuged cell lines and paraffin-embedded normal tissues for NPM (APAAP technique). Cell lines: The t(2;5)-positive cell line Karpas 299 (a) and a murine mastocytoma cell line (b) both show labeling of nucleoli with antibody to the C-terminal portion of NPM (antibody NPMc). Note the cytoplasmic labeling of a mitotic cell in (b). Kidney: (a) Many cells show nuclear labeling for nucleophosmin. (b) and (c) show high power views of, respectively, a glomerulus and tubular epithelium (antibody NA24). Thymus: Two different anti-NPM antibodies give essentially identical labeling patterns of cell nuclei in a Hassall’s corpuscle and in the surrounding medullary tissue. Tonsil: Nuclear labeling of lymphoid cells is seen. The arrows in the low power view (left) indicate scattered mitotic cells in which there is cytoplasmic labeling, seen at high power on the right (antibody NA24). Cerebral cortex: Neural cells show strong cytoplasmic staining, seen at low power (above) and high power (below) (antibody NA24).
Diffuse cytoplasmic staining was noted with all three antibodies in human cerebral neuronal cells (Fig 2) and in 5% to 30% of megakaryocytes in both normal and reactive bone marrow samples. The only other normal cells that showed cytoplasmic labeling were mitotic cells, for example, in the germinal centers of reactive lymphoid tissue (Fig 2). Rare scattered nonmitotic cells also showed cytoplasmic staining, and these often lay adjacent to each other. The nuclei of erythroid precursors were frequently unstained with the anti-NPM antibodies.
Neoplastic tissues.
A number of human neoplasms were investigated with the anti-NPM antibodies (see Materials and Methods). The immunostaining pattern of carcinomas with each of the antibodies was essentially identical to that seen in normal tissues, ie, ubiquitous labeling of nuclei, and diffuse cytoplasmic labeling of scattered mitotic and nonmitotic cells (Fig 3).
In a squamous cell carcinoma, the nuclei of all cells are labeled (antibody NA24 APAAP technique). Occasional mitotic cells (arrows) show diffuse cytoplasmic labeling.
In a squamous cell carcinoma, the nuclei of all cells are labeled (antibody NA24 APAAP technique). Occasional mitotic cells (arrows) show diffuse cytoplasmic labeling.
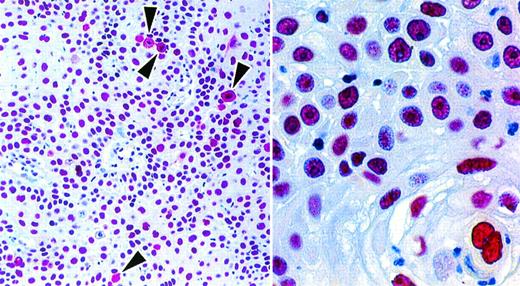
Four cases that fulfilled the conventional criteria for ALK-positive lymphoma22 26 (and that showed nuclear and cytoplasmic immunostaining with each of the 2 anti-ALK antibodies) were tested with the anti-NPM antibodies. The two anti–N-terminal antibodies NA24 and NPMa (which react with both wild-type NPM and NPM-ALK) stained, as expected, the nuclei of neoplastic cells, but also gave diffuse cytoplasmic labeling (Figs 4 and5). This was in contrast to the immunostaining pattern of antibody NPMc, recognizing the C-terminal region, which (apart from the expected cytoplasmic staining of scattered mitotic and nonmitotic cells) was restricted to cell nuclei (Figs 4 and 5).
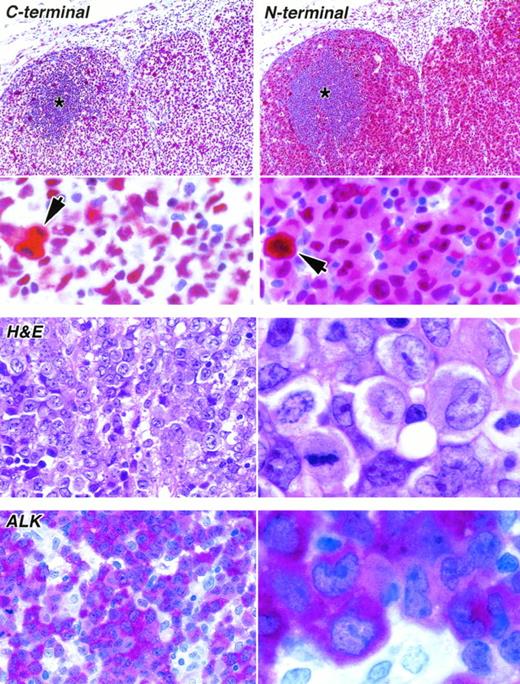
Staining of paraffin sections of an ALK-positive ALCL (APAAP technique). (Upper) The reactivity of the same area of lymph node with antibodies to the C-terminal epitope (antibody NPMc) and to the N-terminal region (antibody NA24) is seen at low power (above) and high power (below). The tumor cells surround a residual area of normal B cells (asterisks). A clear difference between the labeling patterns of the two antibodies is seen even at low magnification, accounted for by the reactivity of the anti–N-terminal antibody with tumor cell cytoplasm. Scattered mitotic cells showing diffuse cytoplasmic labeling are seen in both sections (arrowed) and can be picked out in the low-power view of the immunostaining for the C-terminus. (Lower) The appearance of the tumor in sections stained by hematoxylin and eosin and for ALK protein is shown at low power (left) and high power (right).
Staining of paraffin sections of an ALK-positive ALCL (APAAP technique). (Upper) The reactivity of the same area of lymph node with antibodies to the C-terminal epitope (antibody NPMc) and to the N-terminal region (antibody NA24) is seen at low power (above) and high power (below). The tumor cells surround a residual area of normal B cells (asterisks). A clear difference between the labeling patterns of the two antibodies is seen even at low magnification, accounted for by the reactivity of the anti–N-terminal antibody with tumor cell cytoplasm. Scattered mitotic cells showing diffuse cytoplasmic labeling are seen in both sections (arrowed) and can be picked out in the low-power view of the immunostaining for the C-terminus. (Lower) The appearance of the tumor in sections stained by hematoxylin and eosin and for ALK protein is shown at low power (left) and high power (right).
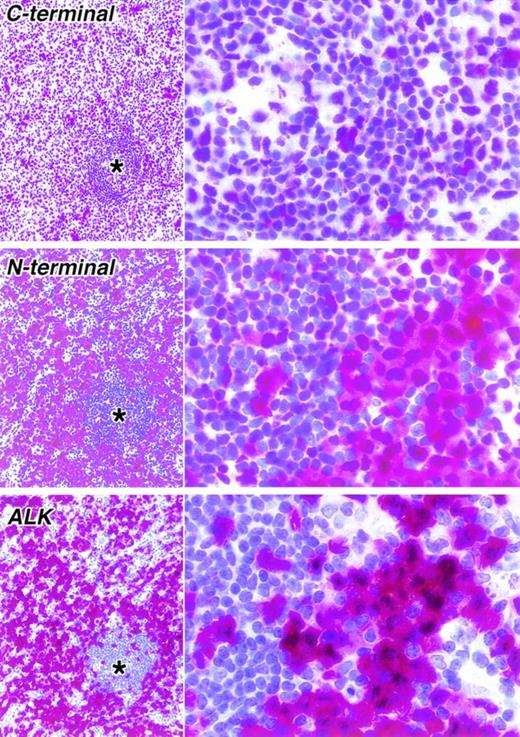
Staining of paraffin sections of a second ALK-positive ALCL seen at low (left) and high (right) power (APAAP technique). As in the case shown in Fig 4, a clear difference is seen between the immunostaining for the C-terminal epitope (antibody NPMc) and an N-terminal epitope (antibody NPMa). Immunostaining for ALK is also shown. In the lower power view, the corresponding areas in three sections are shown. The asterisks indicate residual normal lymphoid tissue surrounded by tumor cells.
Staining of paraffin sections of a second ALK-positive ALCL seen at low (left) and high (right) power (APAAP technique). As in the case shown in Fig 4, a clear difference is seen between the immunostaining for the C-terminal epitope (antibody NPMc) and an N-terminal epitope (antibody NPMa). Immunostaining for ALK is also shown. In the lower power view, the corresponding areas in three sections are shown. The asterisks indicate residual normal lymphoid tissue surrounded by tumor cells.
DISCUSSION
Nucleophosmin was first identified more than 15 years ago as B23, an acidic 37-kD phosphoprotein associated with cell nucleoli.41 Subsequently, the nuclear matrix protein numatrin, which had been independently identified, was shown to be identical to B23/nucleophosmin.42 It was evident from the earliest studies, and confirmed by immunoelectron microscopy,43 that nucleophosmin is associated with the nucleolus,44 although it was also recognized that, under certain conditions (eg, treatment with cytotoxic drugs), it may enter the nucleoplasm.45-47 However, it was reported in 1989 by Borer et al48 that nucleophosmin shuttles continuously between the cytoplasm and the nucleolus and that this reflects its role as a carrier of newly synthesized ribosomal proteins into the nucleolus.
Both polyclonal and monoclonal antibodies to nucleophosmin have been reported, but their main use has been in biochemical or immunocytochemical analysis of cell lines.41,42,44-47,49Only a few studies of the immunocytochemical distribution of nucleophosmin in human tissues have been reported, and these have confirmed its ubiquitous expression in both normal and neoplastic cells.50 51
In the present report, we describe three monoclonal antibodies that detect NPM in paraffin-embedded tissue samples and confirm its ubiquitous distribution within cell nuclei. Most previous immunocytochemical studies have emphasized its nucleolar localization, and it has been claimed that in colorectal tumors a change from a nucleolar to a diffuse nuclear pattern is associated with adenoma/carcinoma progression.51 However, in our experience, nucleolar labeling was not obvious, but this probably reflects NPM diffusion during tissue fixation, because a comparable difference between cryostat and paraffin-embedded sections has been noted previously.50 We also found that nucleophosmin is present diffusely within the cytoplasm of mitotic cells, as has been observed in cultured cells,52,53 and in a previous immunohistologic study.51 We interpret the rare scattered cells in all normal tissues that show cytoplasmic staining as cells that have recently undergone mitosis (because such cells were often seen lying in pairs adjacent to each other).
One novel observation was the diffuse cytoplasmic labeling of cerebral neurones (Fig 2). There is evidence that phosphorylation of NPM is implicated in the initiation of mitosis,54 55 so that its unique distribution in neural cells, which are known to be nondividing, is of interest and merits further study.
Two of the antibodies (NA24 and NPMa) recognize the N-terminal portion of NPM, which is preserved in the fusion protein encoded by theNPM-ALK fusion gene, whereas the third (NPMc) is specific for the C-terminal region (absent from this hybrid gene product). Western blotting with polyclonal anti-NPM antisera has been used previously to demonstrate the differing molecular sizes of wild-type NPM and NPM-ALK fusion proteins,5 6 but this is the first study in which monoclonal antibodies against different regions of the NPM molecule have been used to distinguish between these molecules.
The ALCLs tested in this study showed diffuse cytoplasmic staining with antibody against the N-terminal NPM sequence present in NPM-ALK, whereas the cytoplasm of the same cells was unstained with antibody to the C-terminal portion of NPM. The cytoplasmic staining for the N-terminal portion of NPM (preserved in NPM-ALK) mirrored the labeling pattern obtained with anti-ALK (Figs 4 and 5), and the immunostaining with antibodies to the N-terminal portion of NPM reactivity presumably represents reactivity with cytoplasmic NPM-ALK, as shown schematically in Fig 6.
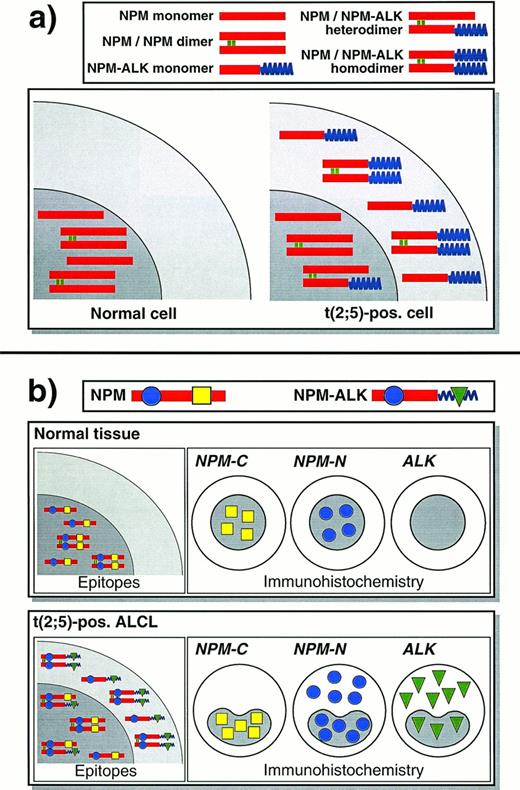
Schematic diagram of NPM and ALK proteins in normal cells and in t(2;5)-positive lymphoma cells and the resulting immunostaining patterns. This scheme assumes that NPM and NPM-ALK are present in both monomeric and dimeric forms in approximately equal numbers, and does not show larger oligomers that might form.5 It may thus represent an oversimplification of what occurs in vivo. (a) In a normal cell, NPM is confined to the nucleus. In a cell carrying the (2;5) translocation, NPM-ALK is found in the cytoplasm and other NPM-ALK that has heterodimerized with wild-type NPM has acquired nuclear localization. (b) Immunostaining for N- and C-terminal epitopes of NPM and for ALK show distinctive patterns, reflecting the localization of the proteins in the scheme shown above.
Schematic diagram of NPM and ALK proteins in normal cells and in t(2;5)-positive lymphoma cells and the resulting immunostaining patterns. This scheme assumes that NPM and NPM-ALK are present in both monomeric and dimeric forms in approximately equal numbers, and does not show larger oligomers that might form.5 It may thus represent an oversimplification of what occurs in vivo. (a) In a normal cell, NPM is confined to the nucleus. In a cell carrying the (2;5) translocation, NPM-ALK is found in the cytoplasm and other NPM-ALK that has heterodimerized with wild-type NPM has acquired nuclear localization. (b) Immunostaining for N- and C-terminal epitopes of NPM and for ALK show distinctive patterns, reflecting the localization of the proteins in the scheme shown above.
The N-terminal antibodies also detected the chimeric proteins encoded by the NPM-RARα and NPM-MLF1 fusion genes, which were created, respectively, by the (5;17) and the (3;5) chromosomal translocations.9,11 12 The first of these anomalies is occasionally found in acute promyelocytic leukemia and the other has been reported in rare cases of myelodysplasia/acute myeloid leukemia. Because of their rarity, we have not studied any fresh clinical samples carrying these genetic lesions, but it is possible that differential staining with anti–N-terminal and anti–C-terminal anti-NPM antibodies, of the type seen in ALCL (Figs 4 and 5), would be obtained. More importantly, these antibodies could be used in the immunocytochemical screening of human neoplasms for novel genetic rearrangements that involve the NPM gene. Even if such anomalies occur only rarely, they might identify novel fusion partners for NPM that are involved more widely in neoplasia.
Immunostaining with these anti-NPM antibodies may also offer, in the context of ALCL, a means of detecting genetic anomalies that fuse theALK gene to a gene other than NPM. In classical ALCLs carrying the (2;5) translocation, NPM-ALK protein is found not only in the cytoplasm, but also within the nucleus,6,21 reflecting heterodimer formation between NPM-ALK and wild-type NPM. Nuclear localization motifs are absent from NPM-ALK, but their presence in the wild-type protein causes the NPM/NPM-ALK heterodimers/oligomers to relocate to the nucleus.5,6 In contrast, if ALKfuses to a gene other than NPM the resultant hybrid protein is likely to be oncogenic if its kinase function is activated (eg, by cross-linking), but it would not show any tendency to move to the nucleus (unless, by chance, the partner protein was also nuclear-associated). This has been directly demonstrated using artificial constructs in which a cross-linking sequence (TPR) with no affinity for the cell nucleus was fused to ALK.5 6Expression of TPR-ALK in vivo and in vitro caused cell transformation, but the protein did not localize to the nucleus.
A (1;2) translocation, which is presumed to fuse an unknown gene on chromosome 1 to the ALK gene on chromosome 2, has been reported in a single case of ALCL.6,21,28 As predicted, the neoplastic cells in this lymphoma expressed an ALK protein that was restricted to the cytoplasm.6 In two recent studies it has been noted that approximately 15% of ALK-positive large-cell lymphomas show this pattern,22 26 but little cytogenetic or RT-PCR data were available for these cases. It would be of interest to investigate biopsy samples from such cases to see if the predicted nuclear-restricted staining pattern is obtained with the anti–N-terminal NPM antibodies reported in this study. Cases could then be studied further by biochemical and molecular biological techniques, offering the possibility of identifying novel oncogenic sequences that may be implicated in other tumor types.
ACKNOWLEDGMENT
The SU-DHL-1 cell line was kindly provided by Dr M.L. Cleary (Stanford, CA) and the NPM-ALK plasmid was kindly provided by Dr Stephan Morris (Memphis, TN).
Supported by the Leukaemia Research Fund of Great Britain and A.I.R.C (Associazione Italiana per la Ricerca sul Cancro).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests or requests for antibodies to David Y. Mason, MD, Hematology Department, John Radcliffe Hospital, Oxford, OX3 9DU, UK (antibody NA24) or to Brunangelo Falini, MD, Institute of Hematology, Policlinico Monteluce, 06100 Perugia, Italy (antibodies NPMa and NPMc).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal