Abstract
P-glycoprotein (P-gp), a transmembrane efflux pump encoded by theMDR1 gene, has been found to be expressed in many normal bone marrow and peripheral blood cells. Among normal leukocytes, CD3−CD16+ or CD3−CD56+ lymphocytes, ie, natural killer (NK) cells, express relatively high levels of P-gp, but little is known about P-gp in abnormally expanded NK cells. In this study, we examined the expression and activity of P-gp on NK cells derived from three normal donors, six patients with indolent NK cell-lineage granular lymphocyte-proliferative disorder (NK-GLPD), three patients with aggressive NK cell tumors (one NK cell leukemia and two nasal NK cell lymphoma), and two NK cell lines. By flow cytometric analysis using the monoclonal antibody (MoAb) MRK16 and rhodamine 123 dye (Rh123), P-gp expression and the efflux of Rh123 were found in all NK samples except one NK cell line. The Rh123 efflux of NK cells was inhibited by cyclosporin A (CsA) and its analogue PSC 833, but the aggressive NK tumor cells were less inhibited than were the other NK cells. The percent inhibition of efflux in the normal NK cells, indolent NK-GLPD cells and aggressive NK cell tumors was 81.8% ± 0.9%, 93.4% ± 3.1% and 36.9% ± 11.7%, respectively, by 1 μmol/L CsA, and 80.2% ± 3.6%, 91.7% ± 2.6% and 32.7% ± 10.1%, respectively, by 1 μmol/L PSC833. In reverse transcription-polymerase chain reaction (RT-PCR) analysis, the low inhibitory effect of P-gp modulators in aggressive NK cell tumors did not correlate to the expression level of MDR1 gene, multidrug resistance-associated protein gene, or human canalicular multispecific organic anion transporter gene. This phenomenon could be related to the presence of other transporters or to unknown cellular or membrane changes. Some patients with NK cell tumors have been reported to show a highly aggressive clinical course and to be refractory to chemotherapy, and this could be related to the expression of P-gp on NK cells. Our results suggest that, although the inhibitors for P-gp have been used in combination with chemotherapy in some hematologic tumors, these inhibitors may be less effective against aggressive NK cell tumors.
IN TUMOR CELLS, the development of resistance against multiple lipophilic cytotoxic drugs is a major impediment to cancer chemotherapy. Such multidrug resistance (MDR) is mediated by the multidrug transporter P-glycoprotein (P-gp), encoded by the MDR1 gene, which functions as an adenosine triphosphate (ATP)-dependent drug efflux pump of broad substrate specificity.1,2 P-gp is expressed not only in neoplastic cells, but also in various normal cells including epithelial cells of the gastrointestinal tract, adrenal gland, and biliary canaliculi.3,4 Among normal blood cells, CD34+ cells, CD56+ cells, and CD8+ cells have been shown to display high levels of P-gp expression.5-8 Natural killer (NK) cells usually express CD56 antigen, so it is expected that NK cells express P-gp. To date, however, P-gp expression on normal NK cell subsets has not been reported, to our knowledge, and only a few studies have been reported about the relationship between P-gp and NK cell tumors.9-12
Since the discovery of the reversal effect of verapamil on MDR,13 many agents that modulate P-gp function have been identified, including several calcium antagonists, calmodulin inhibitors, quinoline compounds, FK506, cyclosporin A (CsA), and its analogue PSC833.14-17 CsA and PSC833, with chemotherapeutic agents, have recently been used in the treatment of acute myeloid leukemia (AML), non-Hodgkin’s lymphoma, and multiple myeloma.18-21 There are no reports describing the use of these modulators in NK cell tumors to our knowledge.
In this study, to clarify whether the P-gp expression in NK cells is carried into the malignancy originating from that cell type, we analyzed the expression and function of P-gp on various NK cell samples, including normal and abnormally expanded NK cells. We also investigated the effect of the P-gp modulators CsA and PSC833 on these NK cells.
MATERIALS AND METHODS
Isolation of NK cells.
From three healthy donors, peripheral blood mononuclear cells (PBMC) were isolated from freshly drawn heparinized peripheral blood by Ficoll-Conray density gradient centrifugation. To purify NK cells, the PBMC were applied to columns of nylon-wool and incubated for 1 hour at 37°C. Nylon-wool nonadherent cells were eluted with warm medium and added to Percoll (Pharmacia Fine Chemicals, Uppsala, Sweden) density gradient solution mixed with 10% fetal calf serum (FCS) containing RPMI 1640 medium (GIBCO BRL, Grand Island, NY). The Percoll solutions, in volumes of 2 mL, were carefully layered into a 10-mL round-bottom test tube, starting with 40% Percoll (fraction 5) and graded by 2.5% concentration reductions to 30% Percoll on the top (fraction 1). After the lymphocytes were added to the Percoll solution, they were centrifuged at 650g for 20 minutes at room temperature. The lymphocytes from low-density fractions 1 and 2 were mixed and used as NK-rich lymphocytes. For the further purification of the NK cells, CD3+ and CD20+ cells were removed from NK-rich fractions by an immunomagnetic isolation technique. Patient NK cells were purified from PBMC and lymph nodes by an immunomagnetic isolation technique.
NK cell lines.
The NK cell line, NK92,22 was kindly provided by Dr Jiang-Hong Gong (University of British Columbia, Vancouver, Canada). The NK cell line, NKL,23 was kindly provided by Dr Michael J. Robertson (Harvard Medical School, Boston, MA). Both cell lines were grown in RPMI 1640 supplemented with 15% FCS, 500 U/mL of interleukin-2 (IL-2; Shionogi, Tokyo, Japan), 100 U/mL penicillin and 100 μg/mL streptomycin.
Flow cytometric analysis of P-gp and NK-related antigens.
The expression of P-gp was measured by immunofluorescence using a P-gp–reactive monoclonal antibody (MoAb), MRK16 (Kyowa Medex, Tokyo, Japan). NK cells were stained with a three-color immunofluorescence method as follows. First, 5 × 105 cells were reacted with 5 μg of MRK16 or IgG2a control antibody for 30 minutes at 4°C. After two washes with phosphate-buffered saline (PBS), the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated F(ab’)2 goat antimouse immunoglobulin (Ig). The cells were washed twice and blocked with mouse IgG, then stained with phycoerythrin-cyanine 5 (PC5)-conjugated anti-CD16 MoAb (Immunotech, Marseilles, France) and phycoerythrin (PE)-conjugated anti-CD56 MoAb (Leu-19; Becton Dickinson, San Jose, CA). The P-gp expression on NK cells was analyzed by flow cytometry (CYTORON ABOLUTE; Ortho Diagnostic System, Raritan, NJ), gating on CD16+ or CD56+ populations.
Measurement of Rh123 efflux.
The P-gp–mediated efflux in the NK cells was determined indirectly by measuring the retention of a fluorescent P-gp substrate, rhodamine 123 dye (Rh123; Sigma, St Louis, MO), as previously described.7Briefly, 5 × 105 cells were stained with 150 ng/mL Rh123 for 15 minutes at 37°C and, after two washes, they were incubated in 10 mL dye-free RPMI 1640 containing 10% FCS for 3 hours at 37°C with or without P-gp inhibitors, 1 μmol/L CsA (Sandoz Pharmaceuticals, Basel, Switzerland) or 1 μmol/L PSC833. PSC833 was a kind gift from Sandoz. After the 3-hour efflux period, the cells were labeled with PC5-conjugated anti-CD16 MoAb and PE-conjugated anti-CD56 MoAb, then washed, and immediately analyzed for fluorescence using the flow cytometer gating on CD16+ or CD56+populations. The mean channel fluorescence was measured for each sample at the beginning (T0 hour) and end (T3 hour) of the assay. The percent inhibition of efflux was calculated by incorporating the Rh123 fluorescence values into the following formula:
Analysis of mRNA expression and quantification of polymerase chain reaction (PCR) products.
The expression of MDR1 gene, multidrug resistance-associated protein (MRP), human canalicular multispecific organic anion transporter (cMOAT), and β2-microglobulin (β2m) were detected by the reverse transcription (RT)-PCR method as previously described.24-26 Total cellular RNA was extracted from the purified NK cells by using the guanidium thiocyanate method26 with Trizol (GIBCO BRL) according to the manufacturer’s protocol. cDNA was synthesized with 5 μg of total cellular RNA using Ready To Go You-Prime First Strand Beads (Amersham Pharmacia Biotech, Buckinghamshire, UK). cDNA derived from 50 ng of RNA was next subjected to PCR for 35 cycles in a final volume of 100 μL using 2.5 U Taq polymerase (Takara, Tokyo, Japan). The contents of reaction mixtures have been described previously.26 The PCR conditions are as follows: after an initial denaturation for 2 minutes at 94°C, each cycle consisted of 30 seconds at 94°C, 30 seconds at 55°C, and 60 seconds at 72°C. The sequences of the primers were: MDR1 forward 5′-CCCATCATTGCAATAGCAGG-3′ and reverse 5′-GTTCAAACTTCTGCTCCTGA-3′; MRP forward 5′-TGGGACTGGAATGTCACG-3′ and reverse 5′-AGGAATATGCCCCGACTTC-3′; cMOAT forward 5′-CTAATCTAGCCTACTCCTGC-3′ and reverse 5′-CTGCAGCTCTCTCTTCATGTGC-3′; and β2m forward 5′-ACCCCCACTGAAAAAGATGA-3′ and reverse 5′-ATCTTCAAACCTCCATGATG-3′. The PCR products were 167, 293, 275, and 120 bp long, corresponding to MDR1, MRP, cMOAT and β2m, respectively. PCR products were separated on a 2% agarose gel. The bands were visualized by ethidium bromide and photographed. For quantification of MDR1 gene, the density of the bands was analyzed using BIO-CAPT V97 (Vilber Lourmat, Marne La Vallee, France) and ZERO-Dscan (Scanalytics, Billerica, MA). Intensity of MDR1expression was depicted as a ratio of MDR1 and β2m. The cell lines, K562/ADM and HepG2, were used for positive controls, K562/ADM for MDR1 and β2m, and HepG2 for MRP and cMOAT. These cell lines were kindly provided by Dr Toshiko Motoji (Department of Hematology, Tokyo Women’s Medical College, Tokyo, Japan).
RESULTS
Characterization of the patients studied.
The clinical findings of nine patients in our study are summarized in Table 1. Patients 1 through 6 were diagnosed to have indolent NK cell-lineage granular lymphocyte-proliferative disorder (NK-GLPD), patient 7 had aggressive NK cell leukemia, and patients 8 and 9 had nasal NK cell lymphoma. Patients 1 through 6 and 8 are described elsewhere.27,28 Patient 9 showed leukemic change. The classification into indolent and aggressive disorders was principally based on their clinical course,27 but the phenotype of NK cells, age, the presence of fever, and hepatosplenomegaly or lymphadenopathy were somewhat helpful for the classification.27 29 NK cells were isolated from PBMC in patients 1 through 7 and 9 and from a metastatic lymph node in patient 8. None of the patients had received any treatment at the time when their PBMC or lymph node cells were isolated. Their surface phenotypes of PBMC or lymph node cells were CD16+ CD56+ in patients 1 through 4 and 8, CD16+ CD56− in patients 5 and 6, and CD16−CD56+ in patients 7 and 9. All four patients tested with an indolent clinical course, and only one of the three patients with aggressive clinical course had high NK cell-mediated cytotoxicity against K562 target cells. Patients 1 through 6 have been followed for at least 2 years and have exhibited a stable clinical course without any treatment. Patients 7 through 9 had a progressive clinical course and died within 6 months despite the administration of combination chemotherapy.
Patient Characteristics and Clinical Data
| Patient No. . | Age and Sex . | Percentage of Positive Cells* . | TCR β . | NK CellMediated Cytolysis† . | Clinical Course . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD2 . | CD3 . | CD4 . | CD8 . | CD16 . | CD56 . | |||||
| 1 | 62F | 98 | 3 | 2 | 90 | 94 | 95 | G | 81 | I |
| 2 | 51F | 92 | 21 | 15 | 18 | 52 | 76 | G | 63 | I |
| 3 | 34M | ND | 35 | 17 | 41 | 33 | 51 | G | ND | I |
| 4 | 10M | 89 | 20 | 12 | 8 | 64 | 58 | G | ND | I |
| 5 | 51F | 89 | 7 | 6 | 8 | 89 | 8 | G | 77 | I |
| 6 | 66M | 92 | 18 | 17 | 14 | 78 | 6 | G | 72 | I |
| 7 | 28M | 93 | 10 | 5 | 3 | 1 | 96 | G | 1 | A |
| 8 | 57M | 98 | 4 | 5 | 3 | 59 | 89 | G | 71 | A |
| 9 | 67F | 87 | 6 | 3 | 4 | 10 | 80 | G | 0 | A |
| Patient No. . | Age and Sex . | Percentage of Positive Cells* . | TCR β . | NK CellMediated Cytolysis† . | Clinical Course . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD2 . | CD3 . | CD4 . | CD8 . | CD16 . | CD56 . | |||||
| 1 | 62F | 98 | 3 | 2 | 90 | 94 | 95 | G | 81 | I |
| 2 | 51F | 92 | 21 | 15 | 18 | 52 | 76 | G | 63 | I |
| 3 | 34M | ND | 35 | 17 | 41 | 33 | 51 | G | ND | I |
| 4 | 10M | 89 | 20 | 12 | 8 | 64 | 58 | G | ND | I |
| 5 | 51F | 89 | 7 | 6 | 8 | 89 | 8 | G | 77 | I |
| 6 | 66M | 92 | 18 | 17 | 14 | 78 | 6 | G | 72 | I |
| 7 | 28M | 93 | 10 | 5 | 3 | 1 | 96 | G | 1 | A |
| 8 | 57M | 98 | 4 | 5 | 3 | 59 | 89 | G | 71 | A |
| 9 | 67F | 87 | 6 | 3 | 4 | 10 | 80 | G | 0 | A |
Patients 1 to 6 had indolent NK cell-lineage granular lymphocyte-proliferative disorder (NK-GLPD), patient 7 had aggressive NK cell leukemia, and patients 8 and 9 had nasal NK cell lymphoma.
Abbreviations: ND, not done; G, germ-line configuration; I, indolent; A, aggressive.
Surface phenotypes of PBMC (patients 1 through 7 and 9) or lymph node (patient 8) are shown in percentages.
Cytotoxicity was assayed against K562 target cells at an effector-to-target cell ratio of 20:1 with 4 hours of incubation.
Expression and function of P-gp.
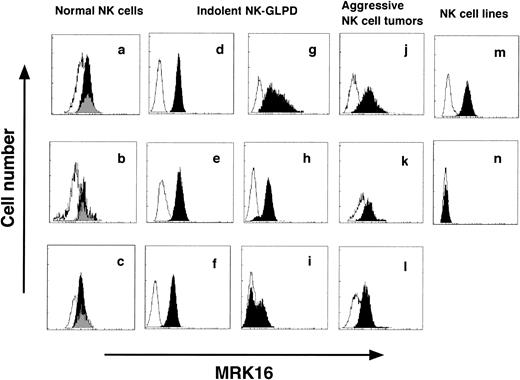
P-gp was expressed on all of the specimens tested except for one NK-cell line, NKL (Fig 1). Two populations, P-gp–positive and -negative, were present in NK cells in patient 6 (Fig 1i). In normal blood NK cells, it is said that three subsets are present, CD16bright/CD56dim, CD16dim/CD56bright, and CD16neg/CD56bright.30 Because the subset of CD16dim/CD56bright was a small population, P-gp was measured in the following two subsets, CD16+CD56+ and CD16−CD56+. In all three normal donors, no difference was found in P-gp expression between these two subsets (Fig1a through c).
P-gp expression on NK cells. (a through c) are NK cells from normal donors, (d through l) are those from patients, and (m) and (n) are NK cell lines. (d through l) correspond to patients 1 through 9 of Table 1, respectively. (m) is the cell line NK92, and (n) is the cell line NKL. Dark zone shows the results with MRK16 MoAb, and clear zone shows the results with control IgG2a MoAb. In normal NK cells (a through c), P-gp was measured in two subsets, CD16+CD56+ (dark zone) and CD16−CD56+ (gray zone).
P-gp expression on NK cells. (a through c) are NK cells from normal donors, (d through l) are those from patients, and (m) and (n) are NK cell lines. (d through l) correspond to patients 1 through 9 of Table 1, respectively. (m) is the cell line NK92, and (n) is the cell line NKL. Dark zone shows the results with MRK16 MoAb, and clear zone shows the results with control IgG2a MoAb. In normal NK cells (a through c), P-gp was measured in two subsets, CD16+CD56+ (dark zone) and CD16−CD56+ (gray zone).
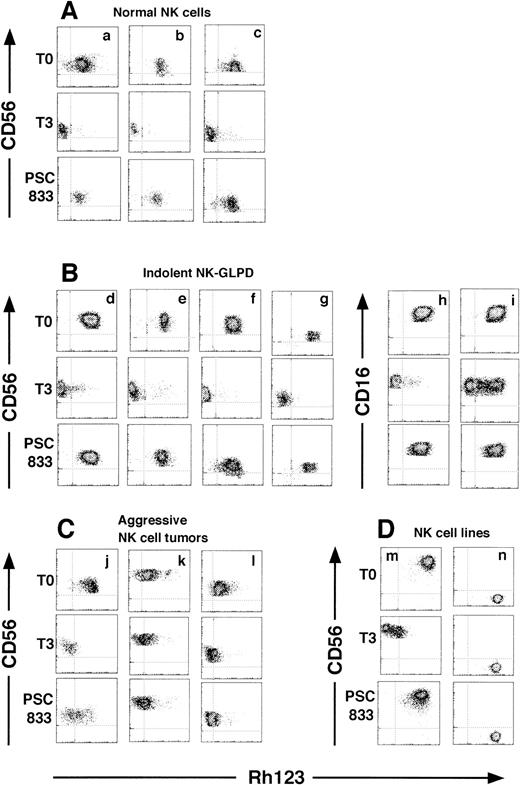
P-gp function was measured by Rh123 efflux (Fig 2). After the 3-hour efflux period, P-gp–positive NK cells of all normal donors, indolent NK-GLPD, and aggressive NK cell tumors and those of the NK cell line extruded Rh123, but the P-gp–negative cell line, NKL, did not. Patient 6 had two populations of NK cells, with the presence or absence of efflux function (Fig 2i). In the three normal donors, no difference was found in Rh123 efflux between the subsets of CD16+CD56+ and CD16−CD56+ (data not shown).
Two-dimensional flow cytometric dot plots of a three-color flow cytometric assay of NK cells stained with CD16 and CD56 after Rh123 efflux, gating on CD16+ or CD56+ populations. (A, B, C, and D) are NK cells from the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and NK cell lines, respectively. (d through l) correspond to patients 1 through 9 of Table 1, respectively. (m) is the cell line NK92, and (n) is the cell line NKL. Dot density maps stained with Rh123 (T0), after the 3-hour efflux period without inhibitors (T3), and with 1 μmol/L PSC833 (PSC833). The results with 1 μmol/L CsA are similar to those with 1 μmol/L PSC833 and are omitted.
Two-dimensional flow cytometric dot plots of a three-color flow cytometric assay of NK cells stained with CD16 and CD56 after Rh123 efflux, gating on CD16+ or CD56+ populations. (A, B, C, and D) are NK cells from the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and NK cell lines, respectively. (d through l) correspond to patients 1 through 9 of Table 1, respectively. (m) is the cell line NK92, and (n) is the cell line NKL. Dot density maps stained with Rh123 (T0), after the 3-hour efflux period without inhibitors (T3), and with 1 μmol/L PSC833 (PSC833). The results with 1 μmol/L CsA are similar to those with 1 μmol/L PSC833 and are omitted.
Inhibition of P-gp function by CsA and PSC833.
Rh123 efflux was examined in the presence of the P-gp inhibitors, CsA and PSC833 (Fig 2 and Table 2). When 1 μmol/L CsA or PSC833 was added, the percent inhibition of efflux in the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and the cell line, NK92, were 81.8% ± 0.9%, 93.4% ± 3.1%, 36.9% ± 11.7% and 93.7%, respectively, by CsA and 80.2% ± 3.6%, 91.7% ± 2.6%, 32.7% ± 10.1% and 84.7%, respectively, by PSC833, indicating that the P-gp of aggressive NK cell tumors was less inhibited by CsA and PSC833 than were other NK cells.
Rh123 Efflux and Its Inhibition in NK Cells
| . | Mean Channel Fluorescence of Rh123 . | % Inhibition of Rh123 Efflux . | ||||
|---|---|---|---|---|---|---|
| T0 . | T3 . | T3/CsA . | T3/PSC833 . | CsA . | PSC833 . | |
| Normal NK cells (n = 3) | 123.2 ± 14.6 | 19.1 ± 3.1 | 104.3 ± 12.6 | 102.7 ± 14.6 | 81.8 ± 0.9 | 80.2 ± 3.6 |
| Indolent NK-GLPD (n = 6) | 151.3 ± 11.9 | 34.6 ± 32.0 | 143.1 ± 14.9 | 141.5 ± 13.3 | 93.4 ± 3.1 | 91.7 ± 2.6 |
| Aggressive NK cell tumors (n = 3) | 131.8 ± 9.4 | 39.6 ± 18.9 | 72.9 ± 13.7 | 68.4 ± 20.8 | 36.9 ± 11.7 | 32.7 ± 10.1 |
| Cell line (n = 1) | 203.0 | 40.0 | 192.7 | 178.1 | 93.7 | 84.7 |
| . | Mean Channel Fluorescence of Rh123 . | % Inhibition of Rh123 Efflux . | ||||
|---|---|---|---|---|---|---|
| T0 . | T3 . | T3/CsA . | T3/PSC833 . | CsA . | PSC833 . | |
| Normal NK cells (n = 3) | 123.2 ± 14.6 | 19.1 ± 3.1 | 104.3 ± 12.6 | 102.7 ± 14.6 | 81.8 ± 0.9 | 80.2 ± 3.6 |
| Indolent NK-GLPD (n = 6) | 151.3 ± 11.9 | 34.6 ± 32.0 | 143.1 ± 14.9 | 141.5 ± 13.3 | 93.4 ± 3.1 | 91.7 ± 2.6 |
| Aggressive NK cell tumors (n = 3) | 131.8 ± 9.4 | 39.6 ± 18.9 | 72.9 ± 13.7 | 68.4 ± 20.8 | 36.9 ± 11.7 | 32.7 ± 10.1 |
| Cell line (n = 1) | 203.0 | 40.0 | 192.7 | 178.1 | 93.7 | 84.7 |
The values of mean channel fluorescence are shown after staining with Rh123 (T0), and after 3 hour efflux without inhibitors (T3), with inhibitors of 1 μmol/L CsA (T3/CsA), and of 1 μmol/L PSC 833 (T3/PSC833). Percent inhibition of efflux was calculated by incorporating Rh123 fluorescence values into the following formula: 100 (T3/CsA or T3/PSC833 − T0)/(T3 − T0).
Quantification of MDR1 gene.
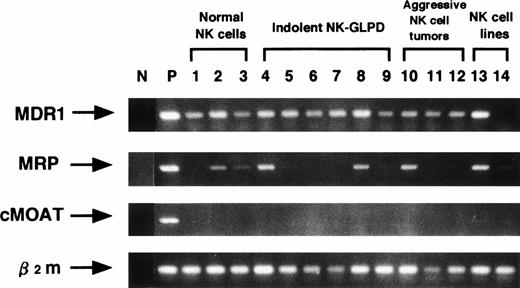
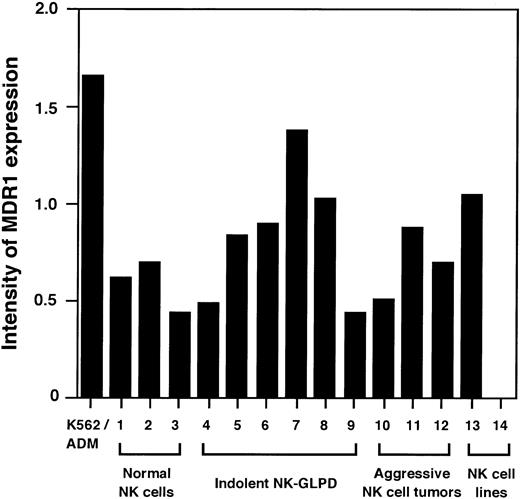
Next, quantitative RT-PCR for MDR1 gene was performed to examine whether the different effect of CsA and PSC833 on aggressive NK cell tumors was due to a higher MDR1 gene expression.MDR1 mRNA was detected on all the specimens tested except for one NK-cell line, NKL (Fig 3), but the aggressive NK cell tumors did not show higher MDR1 expression than other NK cells (Fig 4).
Analysis of mRNA expression of MDR1, MRP, cMOAT and β2m genes using RT-PCR. Lane (N) is negative control, without cDNA, and lane (P) is positive controls, K562/ADM for MDR1 and β2m, HepG2 for MRP and cMOAT. Lanes 1 through 3, 4 through 9, 10 through 12 and 13 through 14 are NK cells from the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and NK cell lines, respectively. Lanes 4 through 12 correspond to patients 1 through 9 of Table 1, respectively. Lane 13 is the cell line NK92, and lane 14 is the cell line NKL.
Analysis of mRNA expression of MDR1, MRP, cMOAT and β2m genes using RT-PCR. Lane (N) is negative control, without cDNA, and lane (P) is positive controls, K562/ADM for MDR1 and β2m, HepG2 for MRP and cMOAT. Lanes 1 through 3, 4 through 9, 10 through 12 and 13 through 14 are NK cells from the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and NK cell lines, respectively. Lanes 4 through 12 correspond to patients 1 through 9 of Table 1, respectively. Lane 13 is the cell line NK92, and lane 14 is the cell line NKL.
Quantitative RT-PCR analysis for MDR1 gene. Intensity of MDR1 expression is shown as a ratio of the density of the bands for MDR1 and β2m. Lanes 1 through 3, 4 through 9, 10 through 12, and 13 and 14 are NK cells from the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and NK cell lines, respectively. Lanes 4 through 12 correspond to patients 1 through 9 of Table 1, respectively. Lane 13 is the cell line NK92, and lane 14 is the cell line NKL.
Quantitative RT-PCR analysis for MDR1 gene. Intensity of MDR1 expression is shown as a ratio of the density of the bands for MDR1 and β2m. Lanes 1 through 3, 4 through 9, 10 through 12, and 13 and 14 are NK cells from the normal donors, indolent NK-GLPD, aggressive NK cell tumors, and NK cell lines, respectively. Lanes 4 through 12 correspond to patients 1 through 9 of Table 1, respectively. Lane 13 is the cell line NK92, and lane 14 is the cell line NKL.
MRP and cMOAT expression.
To determine the presence of other non-P–gp transport mechanisms, MRP and cMOAT were analyzed by RT-PCR (Fig 3). MRP was detected in NK cells in two of the three normal donors, two of the six indolent NK-GLPD, one of the three aggressive NK cell tumors, and both NK cell lines. cMOAT was not detected in all of the NK cell samples tested. Thus, the low inhibitory effect of P-gp modulators did not correlate with the expression of MRP or cMOAT.
DISCUSSION
In this study, we examined the 14 NK cell samples (three normal, nine abnormal, two cell lines), and all except one (the cell line, NKL) expressed P-gp. In normal blood cells, as we and other investigators have pointed out,6-8 CD56+ cells highly express P-gp, but P-gp expression on NK cell subsets or their precursors has not been reported.
Using human colon carcinoma and neuroblastoma cell lines, Mickley et al31 and Bates et al32 found that the expression of MDR1 gene was upregulated with differentiation. NK cells are generated from CD34+ hematopoietic stem cells under appropriate conditions in vitro and in vivo.33Chaudhary et al5 have reported that CD34+ cells express P-gp, but MDR1 expression in normal NK developmental pathway is still an enigma. In normal adult NK cells, it is said that three subsets are present in the peripheral blood, ie, CD16bright/CD56dim, CD16dim/CD56bright, and CD16neg/CD56bright, and NK cells seem to differentiate inversely from the last to the first.30 We investigated the differences of P-gp expression and Rh123 efflux among the two subsets CD16+CD56+ and CD16−CD56+. In all three normal donors tested, no difference was found between these two subsets (Fig 1 and data not shown). In this view, it thus seemed that there was no relationship between the differentiation and the P-gp expression in the late stage of NK cell development.
Many patients with NK-GLPD have a stable clinical course, while some have an aggressive clinical course.27 29 Among six patients with indolent NK-GLPD, the phenotype of the NK cells in patients 1 through 4 was CD16+CD56+. These NK cells were considered as mature NK cells and exhibited the same characteristics with normal mature NK cells in terms of P-gp expression and function. The phenotype of the NK cells in patients 5 and 6 was CD16+CD56−, and this phenotype is unusual in NK cells. Two populations, P-gp–positive and P-gp–negative, existed in NK cells in patient 6. P-gp–negative NK cells may exist in some particular population of NK cells.
According to their clinical course, three patients were classified as having aggressive NK cell tumors. As described before,28 we considered the NK cells of case 8 as activated mature NK cells rather than immature NK cells: these NK cells expressed activation markers such as HLA-DR, CD71, CD45RO, CD30 and IL-2Rα, and strong NK activity. On the other hand, NK cells of patients 7 and 9 did not express CD16 antigen, nor did NK activity, suggesting that they were derived from immature NK cells. The MDR1 gene expression level of them was not particularly higher or lower than that of other NK cells. However, because the sample size was too small, it will be difficult to discuss the relationship between NK cell differentiation and P-gp expression based on the present data.
Although the NK cell samples showed various degrees of P-gp expression, all P-gp–positive NK cells functionally extruded Rh123. The discordance between P-gp expression and functional Rh123 efflux, with the failure of Rh123 efflux despite P-gp expression, has been reported in some AML cases,34 but the P-gp–positive NK cells observed in our study and others seemed to be always functional. Several investigators have described the relationship between P-gp expression and NK cell-mediated cytolysis.35-38 Klimecki et al38 suggested that P-gp in NK cells functions as a protective mechanism to remove perforin monomers from the NK cell plasma membrane, or that P-gp is a transporter of cytotoxic factors, which is involved in the killing process. In fact, evidence exists that P-gp participates in the transport of cytokines (IL-2, IL-4, and interferon-γ) in normal peripheral T lymphocytes.39 Our finding of P-gp expression and absent cytotoxicity in patients 7 and 9, however, does not support previous findings of a strong association between P-gp and NK cell function.
In preliminary experiments, the concentrations of CsA and PSC833 in the culture were changed, and 1 μmol/L of CsA and PSC833 was found to efficiently inhibit P-gp function in the cell line, NK92, and in NK cells from one of the indolent NK-GLPD patients (data not shown). We therefore used 1 μmol/L CsA and PSC833 in the present study. The P-gp function in normal NK cells, indolent NK-GLPD cells, and the cell line, NK92, was efficiently inhibited by 1 μmol/L CsA and PSC833, but interestingly, P-gp function in the aggressive NK cell tumors could not be inhibited as in the other NK cells (Fig 2 and Table 2). After increasing the concentration of CsA and PSC833, we examined the Rh123 efflux in two of the three cases of aggressive NK cell tumors, patients 8 and 9. When 10 μmol/L CsA or PSC833 was added, the percent inhibition of efflux was increased from 33% to 50% by CsA and from 22% to 65% by PSC833 in patient 8 and was increased from 50% to 65% by CsA and from 42% to 67% by PSC833 in patient 9 (data not shown). Thus, the aggressive NK cell tumors could not be inhibited sufficiently by even 10 μmol/L CsA and PSC833 and seem to be resistant to CsA and PSC833. The cell line, NK92, was established from a patient with an aggressive NK cell lymphoma, and the P-gp function of this cell line was, in contrast, efficiently inhibited by 1 μmol/l CsA and PSC833. It will be intriguing to examine the P-gp function of original NK cells from this patient.
Several explanatory possibilities will be pointed out regarding the question of why the P-gp function in the aggressive NK-cell tumors was not sufficiently inhibited by CsA or PSC833. First, high MDR1gene expression in aggressive NK cell tumors was expected. However, aggressive NK cell tumors did not show higher MDR1 expression than other NK cells (Fig 4). Second, a mutant P-gp must be considered. Chen et al40 described a P-gp–positive human sarcoma cell line, which was not modulated by CsA or PSC833. This cell line had a mutant MDR1 gene, which results in the deletion of the amino acid phenylalanine at position 335 of P-gp (Phe335), and they suggested that Phe335 was an important binding site on P-gp for CsA and PSC833. We therefore investigated the sequence of DNA from base pairs 1404-1501 containing codon 335 by PCR in patients 8 and 9, but a deletion of Phe335 was not found (data not shown). Last, MDR mechanisms other than P-gp, such as MRP41 and cMOAT,42 may participate in Rh123 efflux. MRP and cMOAT also belong to the ATP binding cassette of drug transporter proteins.41,42MRP was detected in one of the three aggressive NK cell tumors and also in several other NK cells. cMOAT was not detected in all of the NK cell samples tested. Our Rh123 assay results therefore seem to be unrelated to MRP and cMOAT, but the involvement of other transporter proteins, such as MRP homologues (MRP3, MRP4, and MRP5)43 and lung resistance-related protein,44 cannot be ruled out.
In summary, almost all NK cells expressed P-gp, and their P-gp was functional. Some patients with NK cell tumors have been reported to show a highly aggressive clinical course and to be refractory to chemotherapy, and this could be related to the expression of P-gp in NK cells. When chemotherapy is required for NK cell tumors, it may be necessary to consider using anticancer drugs that are not a substrate for P-gp. For the purpose of P-gp modulation, CsA and PSC833 have been used in combination with chemotherapy in acute leukemia, multiple myeloma, and malignant lymphoma,18-21 and this is an alternative treatment for NK cell tumors, but our results suggest that these inhibitors may be less effective in aggressive NK cell tumors due to the presence of other transporters, or to unknown cellular or membrane changes. Further studies are required to elucidate the relationship between P-gp and NK cells.
ACKNOWLEDGMENT
We thank Dr Jiang-Hong Gong (University of British Columbia, Vancouver, Canada), Dr Michael J. Robertson (Harvard Medical School, Boston, MA), and Dr Toshiko Motoji (Department of Hematology, Tokyo Women’s Medical College, Tokyo, Japan) for cell lines. We thank Dr Katsuhiko Kitsugi (Ortho Clinical Diagnostics, Tokyo, Japan) for technical advice.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Motoki Egashira, MD, Department of Hematology, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal