Abstract
Dendritic cells (DC) are highly efficient antigen-presenting cells (APC) that have an essential function in the development of immune responses against microbial pathogens and tumors. Although during the past few years our understanding of DC biology has remarkably increased, a precise characterization of the different DC subpopulations remains to be achieved with regard to their phenotype and lineage relationships. In this report, we have extensively studied the DC subpopulations present in the thymus, spleen, Peyer’s patches, lymph nodes (LN) and skin of the mouse. Thymus DC and 60% spleen DC have a lymphoid DC phenotype, ie, CD8+DEC-205high Mac-1low, whereas 40% spleen DC have a myeloid DC phenotype, ie, CD8−DEC-205low Mac-1high. Both CD8+and CD8− DC are leukocyte function-associated antigen-1 (LFA-1)high and highly adherent. Within Peyer’s patches the majority of DC correspond to the CD8+DEC-205high Mac-1lowlymphoid category. In the LN, together with CD8+ and CD8− DC, an additional nonadherent CD8intLFA-1int subpopulation with lymphoid DC characteristics is described. Finally, in the skin both epidermal Langerhans cells (LC) and dermal DC are CD8−DEC-205high Mac-1high , and do not express LFA-1. Interestingly, LC migration experiments indicate that LC underwent the upregulation of CD8 and LFA-1 upon migration to the LN, supporting the hypothesis that LC belong to the CD8+ lymphoid lineage.
DENDRITIC CELLS (DC) ARE antigen-presenting cells (APC) with a key function in the immune system as initiators of T-cell responses against microbial pathogens and tumors due to their capacity to stimulate naive T cells.1During the past few years, DC have become a very active area of research due to the possibility to use DC for antitumoral immunotherapy.1-4 In this sense, several reports in the murine system have described tumor regression mediated by the induction of antitumor CTL responses after transfer of DC pulsed with tumor antigens.4 These results provided a promising experimental basis for the development of clinical trials based on the antitumoral therapeutic potential of DC.3
Because DC are difficult to isolate in large numbers from lymphoid tissues, the majority of DC-mediated immunotherapy experiments have been performed using DC differentiated and expanded in vitro, following a variety of protocols differing in the DC precursor population and the cytokine combination employed.1 However, it is important to take into account that different DC subsets exist within lymphoid organs, and that DC generated in vitro from a defined precursor population may differ in their phenotype and more importantly in their APC potential, depending on the cytokines used. In this sense, to fully exploit the DC potential for immunotherapy, the best source of DC and experimental conditions to induce an optimal antitumor T-cell immune response have to be established. Consequently, a precise definition of the different DC subpopulations found in lymphoid and nonlymphoid tissues has to be achieved, with regard to both their T-cell stimulation capacity, and importantly their corresponding precursors. Experiments performed particularly in the murine system, have allowed the definition of two main DC subtypes that have been termed lymphoid and myeloid DC on the basis of their expression of the lymphoid and myeloid markers CD8 and Mac-1, respectively.5,6 In addition, functional differences between CD8+ and CD8− splenic DC concerning their T-cell stimulation potential, phagocytic activity, interleukin (IL)-12 secretion capacity and localization within the spleen, have been reported.7-10With regard to their progenitors, although thymic lymphoid DC have been shown to derive from lymphoid precursors both in the human and murine systems,11 the precursors of myeloid DC have not yet been precisely defined. Interestingly, a recent report analyzing the phenotype of mice homozygous for an Ikaros null mutation (Ikaros C−/− mice)12 in which TCRαβ T cells, CD8+ DC, and myeloid cells are produced, but neither B cells, nor natural killer (NK) cells, nor CD8− DC, suggest that CD8− DC may be related to the B-cell/NK-cell lineage rather than to the myeloid lineage. Besides, the correlation between skin Langerhans cells (LC), considered to be immature DC, and mature CD8+ and CD8− DC remains to be clarified. Therefore, additional studies are required to define the differential APC potential and the lineage relationships of the various DC subsets. In this context, to further understand the correlation between the different DC subtypes constituting the DC system, we have extensively studied the DC subpopulations present in the thymus, spleen, Peyer’s patches, lymph nodes (LN), and skin of the mouse, by analyzing their specific characteristics concerning their phenotype, as well as their adherence and migratory capacity.
MATERIALS AND METHODS
Animals.
BALB/c and C57BL/6 mice were purchased from IFFA Credo (L’Arbresle, France). In all experiments 5- to 7-week-old female mice were used.
Isolation of DC from the thymus, spleen, Peyer’s patches, and lymph nodes.
DC were purified from thymus, spleen, Peyer’s patches, and mesenteric and peripheral (auricular, axillar, and inguinal) LN, following an isolation protocol modified from our previously described method that avoids DC culture.13 14 Organs were cut into small fragments and then digested with collagenase A (0.5 mg/mL; Boehringer-Mannheim, Mannheim, Germany) and Dnase I (40 μg/mL, Boehringer-Mannheim) in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS) for 10 minutes at 37°C with continuous agitation. Digested fragments were filtered through a stainless-steel sieve, and cell suspensions washed twice in phosphate-buffered saline (PBS) solution supplemented with 5% FCS and 5 mmol/L EDTA (PBS-EDTA-FCS) containing 5 μg/mL Dnase I. The cells were then resuspended in cold isoosmotic Optiprep solution (Nyegaard Diagnostics, Oslo, Norway), pH 7.2, density 1.061 g/cm3, containing 5 mmol/L EDTA to dissociate DC-thymocyte complexes, and a low-density fraction, accounting approximately for 1% of the starting cell population, obtained by centrifugation at 1700g for 10 minutes, and washed twice in PBS-EDTA-FCS. T-lineage cells, B cells, macrophages and granulocytes were depleted by treating the recovered low-density cells for 50 minutes at 4°C with a monoclonal antibody (MoAb) mixture including anti-CD3 (clone KT3-1.1), anti-CD4 (clone GK1.5), anti-IL-2Rα (clone PC61.5), antimacrophage antigen F4/80 (clone C1.A3-1), and anti-granulocyte antigen Gr1 (clone RB6-8C5). The unwanted cells were then removed magnetically after incubation for 30 minutes at 4°C with a 1:1 mixture of antimouse Ig and antirat Ig coated magnetic beads, (Dynabeads, Dynal, Oslo, Norway) at a 7:1 bead-to-cell ratio. Flow cytometry analysis of the DC-enriched preparations obtained by this isolation method showed that they had a purity greater than 75% for the thymus, spleen, and Peyer’s patch DC preparations, and greater than 90% for the mesenteric and peripheral LN DC preparations, as assessed by their CD11c expression (data not shown). Subsequent phenotypic analysis of the different DC subsets was performed after gating for CD11c+ cells.
LN DC-adherence assay.
Mesenteric and peripheral (auricular, axillar, and inguinal) LN DC were purified as described, and incubated in RPMI 1640 medium with 5% FCS for 90 minutes at 37°C in 35-mm Petri dishes. After this culture time, the nonadherent cells were obtained by carefully collecting the floating cells. After washing the culture surface with warm RPMI medium with 5% FSC to remove the remaining nonadherent cells, adherent cells were collected by gentle pipetting.
Isolation of DC from the skin.
LC were obtained from epidermal sheets of mouse ears following a protocol modified from Schuler and Steinman.15 Briefly, ears were split with the aid of forceps into dorsal and ventral halves and incubated with 0.5% trypsin (Sigma, St Louis, MO) in PBS containing 5% FCS for 30 minutes at 37°C to allow the separation of the epidermal sheets from dermis. Trypsin treatment under these conditions did not affect the expression by LC of trypsin-sensitive markers, such as LFA-1 or L-selectin, as assessed by trypsin-treatment assays performed on mesenteric LN (data not shown), but DEC-205 was partially degraded, resulting in a reduced expression of this marker, as previously described.16 However, after overnight culture DEC-205 expression by LC was restored (Fig 2). Epidermal cell suspensions were obtained by filtering the trypsinized epidermal sheets through a stainless-steel sieve, washed in PBS with 5% FCS and a LC-enriched low-density fraction, accounting for 5% to 10% of the starting epidermal cell population, obtained by centrifugation in cold isoosmotic Optiprep solution as described above. This LC-enriched low-density cell fraction contained 20% to 30% LC, as assessed by flow-cytometry analysis of CD11c expression (data not shown). Subsequent phenotypic analysis of LC preparations was performed after gating for CD11c+ cells.
The comparative phenotypic analysis of epidermal LC and dermal DC shown in Fig 6 has been performed after purifying these two DC subpopulations in parallel following a method modified from the one previously described by Lenz et al.17 Briefly, whole ears were rinsed with 70% ethanol and the epidermal and dermal sheets were prepared as above and cultured for 24 hours in 24-well tissue culture plates in the presence of 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) (GM-CSF was kindly provided by Immunex Corp, Seattle, WA). After this culture period most LC and DC together with keratinocytes were released to the culture medium, and a high proportion of keratinocytes adhered to the plastic surface. The nonadherent cell fraction was then collected and the low-density fraction was obtained as described above. The epidermal low-density fraction obtained by this method contained 60% to 80% LC, whereas the dermal low-density fraction contained 30% to 40% DC, as assessed by flow-cytometry analysis (data not shown). Phenotypic analysis of both skin DC subsets was performed after gating for CD11c+cells. This method allows to perform in parallel the isolation of both epidermal LC and dermal DC, in contrast with the protocol described above, which is designed specifically for the isolation of epidermal LC but has the advantage over the latter of not involving a 37°C incubation step. In this sense, it is important to take into account that the culture period in the presence of GM-CSF included in the latter method determined some phenotypic variations, as discussed below.
LC migration assay.
BALB/c mice received 10 μL of 1% fluorescein isothiocyanate (FITC) (Sigma) dissolved in 1:1 acetone:dibutylphtalate (ADBP), on the dorsum of both ears following the protocol described by Cumberbatch et al.18 After 18 hours, 48 hours, or 5 days, the draining auricular LN DC were isolated as described.
Flow cytometry.
Phenotypic analysis of DC subpopulations was performed after triple staining with FITC-conjugated anti-CD11c (clone N418, hamster IgG), phycoerythrin (PE)-conjugated anti-CD8α (clone CT-CD8a, rat IgG2a; Caltag, San Francisco, CA) and biotin-conjugated anti–Mac-1 (clone M1/70, rat IgG2b), anti–DEC-205 (clone NLDC-145, rat IgG2a), anti–LFA-1α (clone FD441.8, rat IgG2b), anti-FcγRII/III (clone 2-4G2), anti–B7-2 (clone GL1, rat IgG2a; Pharmingen, San Diego, CA), anti-CD40 (clone FGK45, rat IgG), anti–L-selectin (clone MEL-14, rat IgG2a) or anti-macrophage antigen F4/80 (clone 31-A3-1, rat IgG2b) followed by streptavidin-tricolor (Caltag). Analysis of the phenotype of FITC+ cells in LC-migration assays was performed after double staining with PE-conjugated anti-CD8α and biotin-conjugated anti-CD11c, anti–Mac-1, anti–DEC-205, or anti–LFA-1α followed by streptavidin-tricolor. Ig isotype-matched control antibody stainings for rat IgG2a MoAbs were performed with anti-B220 (clone RA3-6B2, rat IgG2a) and shown in Fig 2. Equivalent background staining profiles were obtained with the nonreactive MoAbs anti-CD69 (clone H.1.2F3, hamster IgG) and anti–TCR-Vα11 (clone RR8.1, rat IgG2b), used as isotype-matched control antibodies for hamster IgG and rat IgG2b MoAbs, respectively (data not shown). All the staining steps were performed at 0° to 4°C in PBS containing 5 mmol/L EDTA and 2% FCS. Analysis was performed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) at the Flow Cytometry Laboratories of the Faculty of Biology and the Fundación Jiménez Dı́az (Madrid, Spain), using Lysys II and PC-Lysys softwares (Becton Dickinson).
RESULTS
DC have been characterized on the basis of their antigen presentation potential and their phenotype in a variety of lymphoid and nonlymphoid organs, using sophisticated isolation strategies. Although different DC subsets display specific functional and phenotypic features, they share a characteristic phenotypical profile: in the mouse, DC are considered to be MHC II+, CD11c+, B7.2+, CD40+, HSA+, CD3−, CD4−, B220−, Ig−, Gr1−.11 In addition, as shown in Fig 1 the differential expression of other cell surface molecules, such as CD8, Mac-1, DEC-205, and LFA-1 allows a precise definition of DC subpopulations in the thymus, spleen, Peyer’s patches, LN, and skin of BALB/c mice. Equivalent DC subpopulations can be defined in C57BL/6 mice, although minor differences in the proportion of the different splenic and LN DC subpopulations were observed (data not shown).
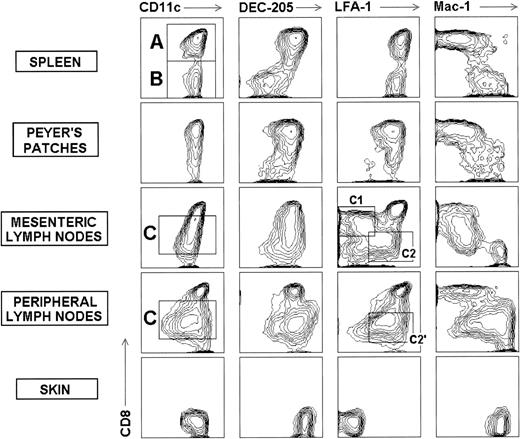
Definition of DC subpopulations from the spleen, Peyer’s patches, LN, and skin of the mouse. Contour plots show the CD8 versus CD11c, DEC-205, LFA-1, or Mac-1 profile of uncultured DC purified from the spleen, Peyer’s patches, mesenteric and peripheral LN, and skin of BALB/C mice, gated for CD11c+ cells. CD8+and CD8− DC subpopulations can be defined (A and B) in the spleen . In the Peyer’s patches most DC are of the CD8+ subset. In the LN an additional CD8intDC subset exists (C), which can be further subdivided on the basis of the CD8 versus LFA-1 expression: in the mesenteric LN, around 50% CD8int DC are CD8int-high LFA-1low(C1 cells) whereas the remaining 50% are CD8int-lowLFA-1int (C2 cells), whereas in peripheral LN, CD8int DC constitute a single population of CD8int-low LFA-1int cells (C2′ cells) (see text for details). Finally, epidermal LC constitute a single DC subpopulation with a distinctive phenotype. The CD8 versus DEC-205 contour plot for the skin corresponds to LC after overnight incubation because, as indicated in Materials and Methods, trypsin treatment used during LC isolation causes partial DEC-205 degradation, which can be restored on culture at 37°C (see also Fig 2). These data are representative of five to eight experiments with similar results.
Definition of DC subpopulations from the spleen, Peyer’s patches, LN, and skin of the mouse. Contour plots show the CD8 versus CD11c, DEC-205, LFA-1, or Mac-1 profile of uncultured DC purified from the spleen, Peyer’s patches, mesenteric and peripheral LN, and skin of BALB/C mice, gated for CD11c+ cells. CD8+and CD8− DC subpopulations can be defined (A and B) in the spleen . In the Peyer’s patches most DC are of the CD8+ subset. In the LN an additional CD8intDC subset exists (C), which can be further subdivided on the basis of the CD8 versus LFA-1 expression: in the mesenteric LN, around 50% CD8int DC are CD8int-high LFA-1low(C1 cells) whereas the remaining 50% are CD8int-lowLFA-1int (C2 cells), whereas in peripheral LN, CD8int DC constitute a single population of CD8int-low LFA-1int cells (C2′ cells) (see text for details). Finally, epidermal LC constitute a single DC subpopulation with a distinctive phenotype. The CD8 versus DEC-205 contour plot for the skin corresponds to LC after overnight incubation because, as indicated in Materials and Methods, trypsin treatment used during LC isolation causes partial DEC-205 degradation, which can be restored on culture at 37°C (see also Fig 2). These data are representative of five to eight experiments with similar results.
Spleen DC.
Spleen DC can be subdivided in two splenic DC subpopulations on the basis of CD8 expression (A and B, Fig 1). CD8+ and CD8− subsets represent approximately 60% and 40% of total splenic DC, respectively. CD8+ splenic DC have the same phenotype than thymic DC (not shown), ie, they are CD8+, express the endocytic receptor DEC-205 recognized by the MoAb NLDC-145, display low levels of the myeloid marker Mac-1, and have high levels of LFA-1, FcR, B7-2, and CD40 on their surface (Fig 2). On the other hand, CD8− splenic DC are DEC-205lowMac-1high, display high levels of LFA-1 and FcR, and express the costimulatory molecules B7-2 and CD40 at lower levels than CD8+ splenic DC. Our results concerning a differential CD40 expression by splenic CD8+ and CD8− DC are in agreement with Vremec and Shortman,6 although in this report the level of CD40 expression by both splenic DC subsets was lower than that described here, probably due to differences in the reagents used to detect this molecule by flow cytometry.
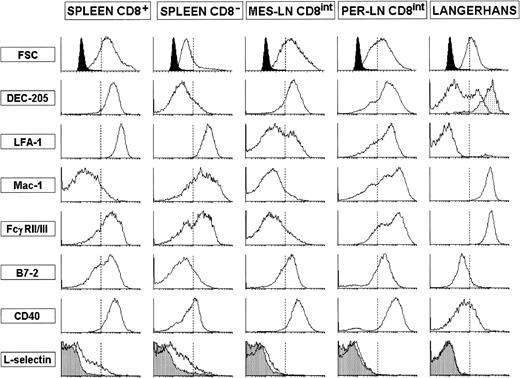
Phenotype of the major murine DC subpopulations. The histograms show the phenotype of the principal murine DC subpopulations defined in Fig 1. CD8+ and CD8− DC from the Peyer’s patches and LN have an almost identical phenotype than CD8+ and CD8- spleen DC respectively (not shown). The cells were analyzed after triple staining with FITC-conjugated anti-CD11c, PE-conjugated anti-CD8, and biotin-conjugated antibodies against the indicated markers followed by streptavidin-tricolor. The forward scatter (FSC) of the different DC subpopulations is compared with that of peripheral T cells (black profiles in the FSC histograms). Grey profiles in the L-selectin histograms represent the background staining with a nonreactive control MoAb (biotin-conjugated anti-B220, clone RA3-6B2) for each DC subpopulation. Details dealing with the different Ig isotype-matched control antibodies used are given in Materials and Methods. The dotted profile in the DEC-205 histogram for skin LC represents the DEC-205 expression after overnight incubation. The vertical dotted lines mark the lower limit defining the expression at high levels of the corresponding marker. These data are representative of five to eight experiments with similar results. MES-LN: mesenteric LN; PER-LN: peripheral LN.
Phenotype of the major murine DC subpopulations. The histograms show the phenotype of the principal murine DC subpopulations defined in Fig 1. CD8+ and CD8− DC from the Peyer’s patches and LN have an almost identical phenotype than CD8+ and CD8- spleen DC respectively (not shown). The cells were analyzed after triple staining with FITC-conjugated anti-CD11c, PE-conjugated anti-CD8, and biotin-conjugated antibodies against the indicated markers followed by streptavidin-tricolor. The forward scatter (FSC) of the different DC subpopulations is compared with that of peripheral T cells (black profiles in the FSC histograms). Grey profiles in the L-selectin histograms represent the background staining with a nonreactive control MoAb (biotin-conjugated anti-B220, clone RA3-6B2) for each DC subpopulation. Details dealing with the different Ig isotype-matched control antibodies used are given in Materials and Methods. The dotted profile in the DEC-205 histogram for skin LC represents the DEC-205 expression after overnight incubation. The vertical dotted lines mark the lower limit defining the expression at high levels of the corresponding marker. These data are representative of five to eight experiments with similar results. MES-LN: mesenteric LN; PER-LN: peripheral LN.
As discussed below, on the basis of their phenotypic, developmental, and functional characteristics, CD8+ and CD8− DC have been defined as lymphoid and myeloid DC, respectively.6
Peyer’s patch DC.
The majority (around 70%) of DC isolated from mouse Peyer’s patches belong to the CD8+ lymphoid category, and consequently express DEC-205 and LFA-1 at high levels and low levels of Mac-1 (Fig1). The rest of Peyer’s patch DC includes around 10% of CD8− myeloid DC and around 20% of DC displaying intermediate levels of CD8. These CD8int DC are similar to those found in the peripheral LN (see below).
LN DC.
As illustrated in Fig 1 and 2, mesenteric and peripheral LN DCs can be subdivided in three DC subpopulations. Apart from a CD8+DEC-205high Mac-1low lymphoid DC subpopulation and a CD8- DEC-205low Mac-1highmyeloid DC subpopulation equivalent to those described in the spleen, a third DC subpopulation displaying high levels of DEC-205, and from low to intermediate levels of CD8, can be defined (C, Fig 1). The expression of CD8 and DEC-205 by the CD8int LN DC subset supports the view that it belongs to the lymphoid category. In support of this hypothesis, in a recent report Inaba et al19 have shown that LN DC displaying intermediate levels of CD8 are functionally equivalent to the CD8+ splenic DC.
CD8+, CD8int, and CD8- LN DC subpopulations represent approximately 20%, 60%, and 20% respectively in the mesenteric LN, and 20%, 65%, and 15% in the peripheral LN. Forward scatter analysis showed that lymphoid DC, ie, CD8+ DC from the thymus, spleen, Peyer’s patches, and LN, as well as CD8int from LN, are bigger than myeloid DC, ie, CD8- DC from the spleen and LN (Fig 2).
The comparative analysis of CD8int DC from mesenteric and peripheral LN (Fig 1 and 2, Table 1) showed that mesenteric LN CD8int DC comprise two subpopulations defined on the basis of the CD8 versus LFA-1 expression (C1 and C2 in Fig 1). Around 50% are CD8int-high LFA-1low(C1 cells) whereas the remaining 50% are CD8int-lowLFA-1int (C2 cells). Both C1 and C2 cells are Mac-1low FcRlow. On the other hand, peripheral LN CD8int DC constitute a single population of CD8int-low LFA-1int cells (C2′ cells), similar to the C2 population described in the mesenteric LN, although in contrast to the latter, peripheral LN CD8int DC display intermediate to high levels of Mac-1 and FcR. With regard to C1 and C2 CD8int DC subpopulations described in the mesenteric LNs, their functional significance is currently being analyzed.
Phenotypic Characteristics of CD8int DC Present in the Mesenteric and Peripheral Lymph Nodes
| . | CD8 . | LFA-1 . | Mac-1 . | FcR . | DEC-205 . |
|---|---|---|---|---|---|
| Mesenteric LN | |||||
| C1 (50%) | int-high | low | low | low | high |
| C2 (50%) | int-low | int | low | low | high |
| Peripheral LN | |||||
| C2′ (100%) | int-low | int | int-high | int-high | high |
| . | CD8 . | LFA-1 . | Mac-1 . | FcR . | DEC-205 . |
|---|---|---|---|---|---|
| Mesenteric LN | |||||
| C1 (50%) | int-high | low | low | low | high |
| C2 (50%) | int-low | int | low | low | high |
| Peripheral LN | |||||
| C2′ (100%) | int-low | int | int-high | int-high | high |
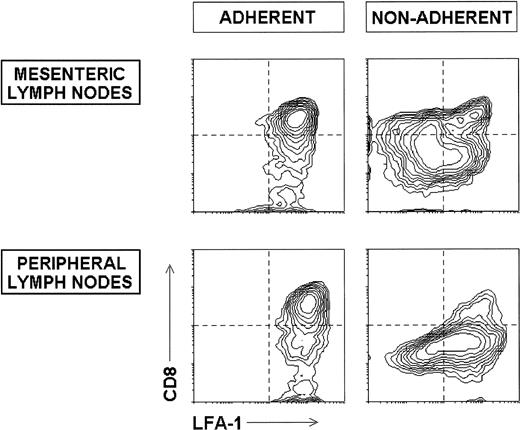
Interestingly, as shown in Fig 3, the analysis of the adherence capacity of DC purified from mesenteric or peripheral LN showed that both the CD8+ and CD8− DC subsets, which display high LFA-1 levels, were strongly adherent. On the other hand, CD8int DC from mesenteric LN (including the previously described C1 and C2 subpopulations), as well as CD8int DC from peripheral LN, were nonadherent. Interestingly, these CD8int nonadherent DC displayed low to intermediate levels of LFA-1. Additional adherence assays have shown that thymic DC, and both CD8+ and CD8− spleen DC, are adherent cells, whereas skin DC are nonadherent (data not shown).
Phenotype of adherent and nonadherent DC subsets from mesenteric and peripheral LN. Mesenteric and peripheral LN DC were purified as described, incubated for 90 minutes at 37°C, and the nonadherent and adherent fractions collected and analyzed for the expression of CD8 and LFA-1. The contour plots show the LFA-1 versus CD8 profiles of adherent and nonadherent DC after gating for CD11c+ cells. The dotted lines mark the lower limit defining the expression at high levels of the corresponding marker. These data are representative of four experiments with similar results.
Phenotype of adherent and nonadherent DC subsets from mesenteric and peripheral LN. Mesenteric and peripheral LN DC were purified as described, incubated for 90 minutes at 37°C, and the nonadherent and adherent fractions collected and analyzed for the expression of CD8 and LFA-1. The contour plots show the LFA-1 versus CD8 profiles of adherent and nonadherent DC after gating for CD11c+ cells. The dotted lines mark the lower limit defining the expression at high levels of the corresponding marker. These data are representative of four experiments with similar results.
Skin DC.
Epidermal DC, known as epidermal LC, constitute a single population with a distinctive phenotype (Fig 1). LC are CD11c+, express neither CD8 nor LFA-1, and display high levels of Mac-1 and FcR, but intermediate levels of B7-2 and CD40 (Fig 2). In addition after isolation, a majority of LC express low levels of DEC-205, but around 30% are DEC-205high. As previously reported16 and as indicated in Materials and Methods, DEC-205 was partially degraded by the trypsin treatment employed during the isolation process. However, after overnight culture at 37°C, LC underwent an upregulation of DEC-205, and all LC became DEC-205high, displaying this marker at an expression level comparable to that found on CD8+ DC from the thymus, spleen, or LN (Fig 1), according to Inaba et al.16Importantly, trypsin treatment did not affect either CD8 or LFA-1 expression, as shown by trypsin treatment assays performed in the same conditions on mesenteric LN and by the fact that some lymphocytes present in the epidermal LC preparations obtained after trypsin treatment, displayed CD8 and LFA-1 expression levels comparable to that of untreated peripheral LN lymphocytes (data not shown).
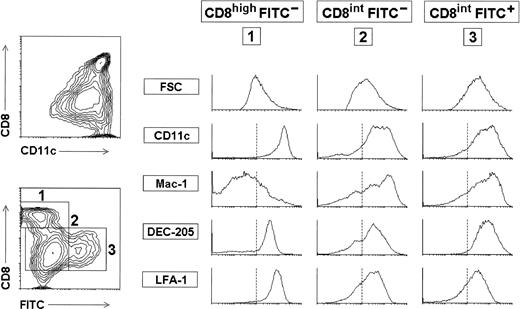
LC have been shown to represent immature DC that differentiate into mature DC when they migrate to the T-cell areas of the draining lymph nodes after an antigenic stimulation.20 Because most LN DC express intermediate to high levels of CD8 and are positive for DEC-205, it can be speculated that LC might represent immature DC of the lymphoid type, which acquire a mature lymphoid DC phenotype upon stimulation and migration. To test this hypothesis, we analyzed the phenotype of LC migrating to the peripheral draining LN after exposure to contact-sensitizing chemicals. For this purpose, BALB/c mice received 10 μL of 1% FITC in ADBP on the dorsum of both ears, following the method described by Cumberbatch et al.18After skin sensitization, the phenotype of the FITC+ LC that had migrated to the draining auricular LN was analyzed by purifying the DC from the draining LN. As illustrated in Fig 4, showing the phenotype of purified auricular LN DC 5 days after skin sensitization, FITC+cells were exclusively found within the CD8int DC subpopulation and represent around 20% of total DC from the draining LN. Similar results were obtained when the LN were analyzed 18 hours or 48 hours after skin sensitization (data not shown). FITC+DC had an almost identical phenotype than FITC−CD8int DC present in the auricular LN. No FITC+cells were found within the auricular T-cell or B-cell populations (data not shown). Consequently, these data suggest that at least some peripheral LN CD8int DC derive from LC that have migrated to the draining LN from the epidermis, and that LC migration was accompanied by the upregulation of CD8 and LFA-1, as illustrated in Fig 5.
Analysis of LC migrating to the draining LN after skin sensitization. BALB/c mice received 10μL of 1% FITC in ADBP on the dorsum of both ears and after 5 days the DC from the draining auricular LN were purified and analyzed. Contour plots show the CD11c versus CD8 profile of purified auricular LN DC, and the correlation between CD8 expression and FITC staining within these cells. FITC +cells were only found within the CD8 int DC subset and represented around 20% of total auricular LN DCs. Histograms show the phenotype of CD8 int FITC + DC (3) compared with that of CD8 high FITC - DC (1) and CD8int FITC - DC (2). The vertical dotted lines mark the lower limit defining the expression at high levels of the corresponding marker. These data are representative of four experiments with similar results.
Analysis of LC migrating to the draining LN after skin sensitization. BALB/c mice received 10μL of 1% FITC in ADBP on the dorsum of both ears and after 5 days the DC from the draining auricular LN were purified and analyzed. Contour plots show the CD11c versus CD8 profile of purified auricular LN DC, and the correlation between CD8 expression and FITC staining within these cells. FITC +cells were only found within the CD8 int DC subset and represented around 20% of total auricular LN DCs. Histograms show the phenotype of CD8 int FITC + DC (3) compared with that of CD8 high FITC - DC (1) and CD8int FITC - DC (2). The vertical dotted lines mark the lower limit defining the expression at high levels of the corresponding marker. These data are representative of four experiments with similar results.
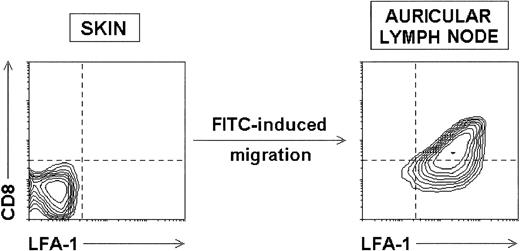
Compared phenotype of skin LC and LC after skin sensitization-induced migration to the draining LN. Contour plots represent the CD8 versus LFA-1 profiles of uncultured skin LC and FITC+ LC isolated from draining auricular LN 5 days after skin sensitization with FITC. Both CD8 and LFA-1 were upregulated by LC on FITC-induced migration.
Compared phenotype of skin LC and LC after skin sensitization-induced migration to the draining LN. Contour plots represent the CD8 versus LFA-1 profiles of uncultured skin LC and FITC+ LC isolated from draining auricular LN 5 days after skin sensitization with FITC. Both CD8 and LFA-1 were upregulated by LC on FITC-induced migration.
However, because a population of DC located in the dermis has been described,17 together with epidermal LC, within the human and mouse skin, in our experiments dermal DC could have also been induced to migrate after skin sensitization and could contribute to the FITC+ DC population found in the draining LN. In fact, it could be speculated that FITC+ CD8int DC derive from dermal DC, and not from epidermal LC as we have proposed above. Nevertheless, as it has been shown that skin sensitization induces epidermal LC migration to the LN,18,21-23 if only dermal DC but not epidermal LC were the precursors of FITC+CD8int cells found in the LN, we would also expect to find FITC+ CD8− DC corresponding to migrating FITC+ LC in the draining LN. However, our results clearly show that FITC+ DC found in the draining LN constitute a well-defined homogenous CD8int population. In addition, the compared phenotypic analysis of epidermal and dermal DC shown in Fig 6 indicates that in the mouse, both populations have an almost identical phenotype, according to Lenz et al,17 suggesting a close lineage relationship between them. More importantly, our data indicate that both skin DC subsets did not express either CD8 or LFA-1, and therefore they strongly support the rationale that CD8 and LFA-1 intermediate levels found on at least a proportion of FITC+ LN DC in LC migration experiments are the result of CD8 and LFA-1 upregulation by LC.
Comparative phenotypic analysis of epidermal LC and dermal DC. The histograms show the expression of the indicated markers by epidermal LC and dermal DC isolated from epidermal and dermal sheets respectively, after 24-hour culture as described. Note that ▩ represent the FSC. These data are representative of three experiments with similar results.
Comparative phenotypic analysis of epidermal LC and dermal DC. The histograms show the expression of the indicated markers by epidermal LC and dermal DC isolated from epidermal and dermal sheets respectively, after 24-hour culture as described. Note that ▩ represent the FSC. These data are representative of three experiments with similar results.
Note that the phenotype of epidermal LC performed on LC isolated from epidermal sheets after culture, basically coincides with the analysis performed on freshly isolated LC presented in Fig 2. However, in agreement with previous reports15,24 25 because the isolation method employed to isolate in parallel both skin DC subsets involves a 24-hour incubation step at 37°C, upon culture LC underwent the upregulation of CD40, B7-2, and MHC II and the downregulation of Mac-1 and FcR, but their expression of CD8 and LFA-1 remained unchanged (see Fig 2 and 6; data corresponding to B7-2 and MHC II are not shown).
DISCUSSION
Although a wide variety of DC subsets have been described in different lymphoid and nonlymphoid organs of the mouse,26 two main DC categories, ie, lymphoid and myeloid DC, can be characterized on the basis of their origin, phenotypic profile, and physiological properties. Lymphoid DC, such as thymic DC or CD8+ splenic DC, are defined as CD8+, DEC-205high, Mac-1low, whereas myeloid DC, such as CD8− splenic DC are CD8−, DEC-205low, Mac-1high. Evidence of the lymphoid origin of CD8+ DC derives from thymus reconstitution experiments showing that mouse thymic DC originate intrathymically from the CD4low lymphoid precursor population that has no myeloid potential.13 Later, Wu et al5 reported that CD8+ but not CD8- splenic DC are generated after intravenous transfer of CD4low lymphoid precursors. Additional experiments have shown the differential capacity of CD8+ and CD8− spleen DC to induce the stimulation of CD4+ and CD8+ peripheral T cells, to phagocytose zymosan particles, and to secrete IL-12.7-10 Importantly, attempts to generate in vitro CD8+ splenic DC from CD8− splenic DC have been unsuccessful so far, suggesting that the two splenic DC subpopulations belong, as proposed previously,5 to different cell lineages and do not represent different differentiation or maturation stages of a unique DC population. In this sense, it has been recently reported that in mice with a deletion at the C terminus of the Ikaros gene (Ikaros C −/−mice),12 CD8+ but not CD8−splenic DC were generated. Interestingly, these mice displayed some T-cell differentiation and had a normal development of myeloid cells but lacked B and NK cells, providing further evidence that CD8+ DCs are related to the T-cell lineage. Besides, these data might indicate that CD8− DC do not belong to the myeloid lineage but rather share some differentiation requirements with B and NK cells.
The data derived from skin sensitization-mediated LC-migration experiments showed that some CD8int DC present in the peripheral LN derive from LC that have migrated from the epidermis, and that LC migration involves CD8 and LFA-1 upregulation. In this sense, CD8int DC may represent recent immigrants reaching the LN as a consequence of an antigenic stimulation and/or a recirculation process. In this sense, differences in Mac-1 and FcR expression between mesenteric and peripheral LN CD8int-lowDC may reflect their respective provenance, because mesenteric LN are known to drain the spleen and intestinal mucosa whereas peripheral LN drain essentially the skin. With regard to Mac-1 expression, our data illustrate that upon migration LC partially downregulated this molecule, although they still expressed Mac-1 at high levels, comparable to that expressed by CD8int FITC- DC found in the auricular LN of the FITC-treated mice (Fig 4), or by CD8int DC from control peripheral LN (Fig 1). Therefore, the expression of Mac-1 by CD8int DC from the peripheral LN most likely reflects the fact that at least a proportion of these cells derive from skin LC that express high levels of Mac-1.
On the other hand, as discussed above, the FITC+CD8int DC population obtained in the draining LN after skin sensitization may be result of the migration of both epidermal LC and dermal DC. The relative contribution of LC to the FITC+CD8int DC population would most likely be more important than that of dermal DC because our experiments of skin DC purification, performed in parallel with epidermal and dermal sheets, indicate that epidermal LC and dermal DC are present in the adult mouse ear skin at a 5:1 ratio. In support of this rationale, it has been recently reported that TNF-α or oxazolone-induced LC migration to the draining LN was blocked by antibodies against the α6 integrin subunit, which is expressed by epidermal LC but not by LN DC.23
Our data strongly suggest that at least a proportion of FITC+ CD8int LN DC derive from epidermal LC, and therefore that LC undergo the upregulation of CD8 and LFA-1 upon migration. Therefore, LC acquire a lymphoid DC-like phenotype upon migration to the LN, suggesting that they belong to the lymphoid CD8+ DC lineage. This hypothesis is supported by the fact that LC have been shown to migrate to the T-cell areas of the lymphoid organs on antigen stimulation,26 and that CD8+DC have been shown to be located in the T-cell areas of the spleen and LN.10 19
Concerning LC lineage, classically, LC have been considered to be of myeloid origin,1 although this hypothesis has not been formally shown. In fact, DC were also globally considered as myeloid precursor-derived, until it was shown in the mouse that the CD8+ DC category derived from lymphoid precursors.13 In this sense, the origin and precursors of the so-called myeloid DC, not expressing CD8 in the mouse, also remain undefined. Similarly, the immediate precursors of LC have not yet been identified. In the first instance, the observation that in mice homozygous for a dominant-negative mutation in the Ikaros gene (Ikaros DN −/− mice), T cells, B cells and splenic DC did not develop, whereas LC and myeloid cells were produced,27 suggested a correlation between LC and the myeloid lineage. However, the analysis of bone marrow chimeric mice reconstituted with Ikaros DN −/− precursor cells12 showed that Ikaros DN −/−mice might have an intrinsic defect in LC/DC differentiation, the defficiency in the generation of T and B cells being accompanied by a blockade in DC differentiation at an immature LC stage. Thus the data derived from Ikaros-deficient mice have not provided an explanation so far for the correlation between DC and LC lineages. Therefore further experiments are needed to conclusively determine the origin of epidermal LC.
In conclusion, our results support the view that lymphoid DC comprise thymus DC, CD8+ spleen DC, CD8high and CD8int Peyer’s patch and LN DC as well as LC, whereas CD8− DC from spleen and LN represent the so-called myeloid DC. Although, as mentioned before, functional differences between mouse CD8+ and CD8− splenic DC have been reported,7,8 a precise analysis of the APC capacity of the different DC subsets has to be achieved, and importantly, their respective precursors remain to be characterized. In this sense, although thymus DC have been shown to derive from thymic CD4low precursors,13 the progenitors of both CD8+ and CD8− peripheral DC continue to be largely unknown. The definition of DC precursors might be specially relevant to define the best source of DC to obtain in vitro the large number of DC required for antitumor therapeutic purposes. On the other hand, it is important to take into account that DC differentiated in vitro may differ in their phenotype and APC function depending on the culture conditions and on their origin.1 Therefore, the study of the precursors and function of the different DC subtypes is crucial to fully exploit the DC tumor immunotherapy potential, because it can provide the information required to define the most adequate experimental conditions to induce an optimal DC-mediated T-cell antitumor response.
ACKNOWLEDGMENT
The authors thank Anton Rolink for the anti-CD40 hybridoma FGK45 (Basel Institute for Immunology, Basel, Switzerland), and the Flow Cytometry facility of the Fundación Jiménez Dı́az for making it possible to perform FACS analysis after hours.
Supported by a grant from the DGICYT (PB95-0376), Ministerio de Educación y Ciencia, Spain (C.A.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal