Abstract
Unrelated bone marrow transplantation (BMT) is often complicated by fatal opportunistic infections. To evaluate features unique to immune reconstitution after unrelated BMT, the lymphoid phenotype, in vitro function, and life-threatening opportunistic infections after unrelated and related T-cell–depleted (TCD) BMT were analyzed longitudinally and compared. The effects of posttransplant donor leukocyte infusions to treat or prevent cytomegalovirus (CMV) or Epstein-Barr virus (EBV) infections on immune reconstitution were also analyzed. This study demonstrates that adult recipients of TCD unrelated BMTs experience prolonged and profound deficiencies of CD3+, CD4+, and CD8+ T-cell populations when compared with pediatric recipients of unrelated BMT and adults after related BMT (P < .01), that these adults have a significantly increased risk of life-threatening opportunistic infections, and that the rate of recovery of CD4 T cells correlates with the risk of developing these infections. Recovery of normal numbers of CD3+, CD8+, and CD4+ T-cell populations is similar in children after related or unrelated BMT. This study also demonstrates that adoptive immunotherapy with small numbers of unirradiated donor leukocytes can be associated with rapid restoration of CD3+, CD4+, and CD8+T-cell numbers, antigen-specific T-cell responses, and resolution of CMV- and EBV-associated disease after unrelated TCD BMT.
DISEASE-FREE SURVIVAL (DFS) after unrelated bone marrow transplantation (BMT) continues to improve from the 40% event-free survival in good risk patients initially reported by the National Marrow Donor Program (NMDP) in 1993,1 with single centers now reporting extended DFS rates of 60% or greater after such transplants when applied to patients with acute leukemia in early remission or chronic-phase chronic myeloid leukemia (CML).2-9 Despite these advances, severe graft-versus-host disease (GVHD), particularly in patients lacking an HLA-A, B, DRβ1-matched donor,2-5,10-12 and infection1,3,5-11,13-16 remain significant obstacles to improving results. In recently reported series, fatal opportunistic infections after successful engraftment occurred in 12% to 28%1,3,6-10,11,13-16 of unrelated transplant recipients compared with 4% to 15%7,15-20after HLA-matched sibling BMT. The higher incidence of opportunistic infections after unrelated BMT is likely due to, in part, the influence of micro and macro HLA-disparities between donor and host,2,3,5,10-12,21,22 the more intensive immunosuppressive regimens often used to prevent graft rejection or GVHD,2-10and the higher incidence of severe acute and extensive chronic GVHD.1-5 10-12
Although unrelated marrow grafts depleted of T cells are generally associated with a relatively low incidence of acute and chronic GVHD,6-9 they have still been associated with a higher number of fatal opportunistic infections, including Epstein-Barr virus-associated lymphoproliferative disorders (EBV-LPD), when compared with HLA-matched related T-cell–depleted (TCD) BMT, even when identical cytoreduction, graft rejection prophylaxis, and supportive care measures have been used.6-9,15,23,24 To better understand the biological basis of this enhanced risk of infection, we have conducted a comparative analysis of the reconstitution of the immune system after TCD transplants from matched related and unrelated donors and correlated these findings with risk of opportunistic infections in the posttransplant period. In the course of these studies, we, like others, have been examining the use of adoptive transfer of donor leukocytes or in vitro propagated viral specific T cells23-27 in an attempt to reduce the lethality of these infections. In this report, we describe the results of our analyses of the distinctive cellular and functional characteristics of the immune reconstitution observed after TCD unrelated BMT. We also describe the alterations in immune function induced by posttransplant adoptive immunotherapy.
PATIENTS AND METHODS
For this study, we reviewed 71 consecutive patients at this institution who received an unrelated BMT depleted of T cells by soybean agglutination followed by sheep red blood cell rosetting between January 17, 1992 and August 8, 1997. These transplants were administered for the treatment of a hematologic malignancy (n = 66), aplastic anemia (n = 3), paroxysmal nocturnal hemoglobinuria (n = 1), or familial erythrophagocytic lymphohistiocytosis (FEL; n = 1). Characteristics of these patients are summarized in Table 1. All patients received cytoreduction with hyperfractionated total body irradiation (1,500 cGy in 12 fractions), thiotepa (5 mg/kg/dose for 2 days), and cyclophosphamide (60 mg/kg/dose for 2 days). To prevent graft rejection, patients received immunosuppression with antithymocyte globulin (ATG; Upjohn, Kalamazoo, MI) and methylprednisolone either before (n = 36) or after (n = 35) the transplant, according to the protocol active at the time of their BMT. Pretransplant graft rejection prophylaxis consisted of 2 to 4 doses of ATG at 30 mg/kg/dose with 2 mg/kg of methylprednisolone. Posttransplant rejection prophylaxis included 5 doses of ATG at 15 mg/kg every other day, from day +5 to day +13. Both groups received 2 mg/kg/d of methylprednisolone daily from day +5 through day +13, with rapid subsequent taper, unless GVHD developed. Since 1993, all cytomegalovirus (CMV)-seropositive patients or those with CMV-seropositive donors have received prophylactic ganciclovir (5 mg/kg/d) initiated when the absolute neutrophil count was greater than 2,000 cells/μL. The ganciclovir dose was increased to 10 mg/kg/d if CMV viremia was documented or clinical infection ensued. This treatment was continued for 2 to 3 weeks. Thereafter, maintenance ganciclovir was resumed until evidence of a CMV proliferative response was documented in vitro. Patients who developed severe cytopenias during ganciclovir treatment have been treated with foscarnet.
Clinical Characteristics of Recipients of SBA−E− BMT
| . | Unrelated . | Related . | ||
|---|---|---|---|---|
| Adults (n = 32) . | Children (n = 30) . | Adults (n = 142) . | Children (n = 12) . | |
| Male/female | 14/18 | 18/12 | 77/65 | 9/3 |
| Age | ||||
| Median | 37.8 | 12.0 | 41.1 | 14.0 |
| Range | 19.7-58.9 | 2.13-18.1 | 18.0-69.2 | 4.15-19 |
| Diagnosis | ||||
| Acute leukemia | 4 (12.5) | 23 (76) | 60 (42) | 10 (83) |
| ALL | 3 | 14 | 11 | 6 |
| AML | 1 | 7 | 47 | 4 |
| Biphenotypic | 0 | 2 | 1 | |
| CML | 16 (50) | 3 (10) | 55 (38) | 0 (0) |
| NHL | 2 | 0 | 17 | 1 |
| Multiple myeloma | 0 | 0 | 5 | 0 |
| AA | 1 | 1 | 1 | 0 |
| MDS | 8 | 2 | 5 | 1 |
| Myelofibrosis | 1 | 0 | 0 | 0 |
| FEL | 0 | 1 | 0 | 1 |
| CMV status | ||||
| Patient/donor | ||||
| Neg/neg | 44% | 46% | 27% | 42% |
| Neg/pos | 9% | 13% | 15% | 0% |
| Pos/neg | 40% | 23% | 17% | 42% |
| Pos/pos | 6% | 17% | 41% | 17% |
| . | Unrelated . | Related . | ||
|---|---|---|---|---|
| Adults (n = 32) . | Children (n = 30) . | Adults (n = 142) . | Children (n = 12) . | |
| Male/female | 14/18 | 18/12 | 77/65 | 9/3 |
| Age | ||||
| Median | 37.8 | 12.0 | 41.1 | 14.0 |
| Range | 19.7-58.9 | 2.13-18.1 | 18.0-69.2 | 4.15-19 |
| Diagnosis | ||||
| Acute leukemia | 4 (12.5) | 23 (76) | 60 (42) | 10 (83) |
| ALL | 3 | 14 | 11 | 6 |
| AML | 1 | 7 | 47 | 4 |
| Biphenotypic | 0 | 2 | 1 | |
| CML | 16 (50) | 3 (10) | 55 (38) | 0 (0) |
| NHL | 2 | 0 | 17 | 1 |
| Multiple myeloma | 0 | 0 | 5 | 0 |
| AA | 1 | 1 | 1 | 0 |
| MDS | 8 | 2 | 5 | 1 |
| Myelofibrosis | 1 | 0 | 0 | 0 |
| FEL | 0 | 1 | 0 | 1 |
| CMV status | ||||
| Patient/donor | ||||
| Neg/neg | 44% | 46% | 27% | 42% |
| Neg/pos | 9% | 13% | 15% | 0% |
| Pos/neg | 40% | 23% | 17% | 42% |
| Pos/pos | 6% | 17% | 41% | 17% |
Of the 71 consecutive patients, 9 were not evaluable in this analysis due to primary graft failure in 5, death before engraftment in 3 (pulmonary hemorrhage day + 10, severe mucositis day + 15, or respiratory syncytial virus [RSV] pneumonia day +16), or death in 1 patient due to subacute bacterial endocarditis (day +29) after engraftment but before the first date of immunologic analysis. The lymphoid phenotype, function, and infectious complications of the remaining 62 patients form the basis of this report. The median age (range) of these 62 patients was 23.5 (2.1 to 58.8) years. Thirty (48%) of the evaluable patients were children, defined as individuals less than 19 years of age. Of the 32 adults, 15 were greater than 40 years of age at the time of BMT. There were no statistically significant differences in the proportions of CMV-seropositive donors or recipients among the pediatric and adult unrelated transplant groups (Table 1). Differences were observed in the proportion of pediatric and adult unrelated transplant recipients who were EBV-seropositive before transplant (70% v 97%). Whereas none of the adult recipient/donor pairs were both EBV-seronegative, 10% of the pediatric recipient/donor pairs were EBV-seronegative. Thirty of 31 donors for adult patients were EBV-seropositive, compared with 25 of 29 pediatric donors. One donor in each group was not tested for EBV serology.
Thirty-six of the 62 donor/recipient pairs included in the analysis (58%) were HLA-A, B, DRβ1 identical as defined by analysis of HLA class I alleles by isoelectric focusing28 and class II alleles by sequence-specific oligonucleotide probe hybridization (SSOP).29 Of the remaining 26 donor/recipient pairs, 10 were serologically mismatched for 1 HLA class I or class II alleles; the rest were serologically HLA-A, -B, -DR matched but mismatched for one (n = 14) or two alleles by iso-electric focusing or molecular analysis.
Twelve of the 62 patients received unirradiated donor leukocyte infusions (DLI) post-BMT to treat or prevent viral infections. All except one recipient of these leukocyte infusions (UPN 1380; DRβ1-mismatched) were HLA-A, B, DRβ1-matched with their donor (Table 2). Five patients received DLI for the treatment of an EBV-LPD that developed at a median (range) of 116 (103 to 190) days after transplant. These infusions consisted of peripheral blood mononuclear cells (PBMNC; n = 4) or PBMNC and bone marrow (n = 1) calculated to provide a median of 0.5 × 106 (range, 0.2 to 1.00 × 106) CD3+ cells/kg. Seven patients received 0.1 × 106 CD3+ cells/kg as PBMNC in an effort to prevent an EBV-LPD (n = 5) or CMV disease (n = 2) in the context of CMV viremia refractory to antiviral therapy. At the time of donor leukocyte infusion, 1 of the 12 patients was on systemic steroids for the treatment of upper gastrointestinal GVHD.
Characteristics of Recipients of DLI and Outcome of Treatment
| UPN/Age (yr) . | Reason for DLI . | No. of CD3 Cells/kg/d Infused . | Outcome . | GVHD (Y/N) . | Primary Malignancy . | Relapse of Primary Malignancy (Y/N) . |
|---|---|---|---|---|---|---|
| 1330/28.6 | EBV-LPD + 120 | 0.57 × 106/+120 | EBV-LPD eradicated | N | CML-CP | Y |
| 1332/48.8 | EBV-LPD, +181 | 0.20 × 106/+190 | EBV-LPD eradicated | Y | NHL | N |
| 1341/19.7 | EBV-LPD, +99 | 1.00 × 106/+103 | EBV-LPD eradicated | N | Ph+ ALL (CR3) | N |
| 1358/23.3 | EBV-LPD, +102 | 0.23 × 106/+104 | EBV-LPD eradicated | N | CML-CP | Y |
| 1596/14 | EBV-LPD, +113 CMV retinitis, +102 | 0.50 × 106/+116 | EBV-LPD eradicated | N | ALL-2nd relapse | Y |
| 1630 | CMV viremia | 0.10 × 106/+110 | Negative CMV shell vial/antigen | N | Aplastic Anemia | N |
| 1725 | CMV viremia/retinitis | 0.10 × 106/+110 | Negative CMV shell vial/antigen | N | Myelofibrosis | N |
| 1351 | Prophylaxis | 0.10 × 106/+157 | N | ALL-3rd CR | N | |
| 1380 | Prophylaxis | 0.10 × 106/+88 | N | CML-Blastic | N | |
| 1395 | Prophylaxis | 0.10 × 106/+49 | Y | CML-Blastic | N | |
| 1421 | Prophylaxis | 0.10 × 106/+76 | Y | Secondary AML | N | |
| 1377 | Prophylaxis | 0.10 × 106/+79 | Y | CML, accelerated | N |
| UPN/Age (yr) . | Reason for DLI . | No. of CD3 Cells/kg/d Infused . | Outcome . | GVHD (Y/N) . | Primary Malignancy . | Relapse of Primary Malignancy (Y/N) . |
|---|---|---|---|---|---|---|
| 1330/28.6 | EBV-LPD + 120 | 0.57 × 106/+120 | EBV-LPD eradicated | N | CML-CP | Y |
| 1332/48.8 | EBV-LPD, +181 | 0.20 × 106/+190 | EBV-LPD eradicated | Y | NHL | N |
| 1341/19.7 | EBV-LPD, +99 | 1.00 × 106/+103 | EBV-LPD eradicated | N | Ph+ ALL (CR3) | N |
| 1358/23.3 | EBV-LPD, +102 | 0.23 × 106/+104 | EBV-LPD eradicated | N | CML-CP | Y |
| 1596/14 | EBV-LPD, +113 CMV retinitis, +102 | 0.50 × 106/+116 | EBV-LPD eradicated | N | ALL-2nd relapse | Y |
| 1630 | CMV viremia | 0.10 × 106/+110 | Negative CMV shell vial/antigen | N | Aplastic Anemia | N |
| 1725 | CMV viremia/retinitis | 0.10 × 106/+110 | Negative CMV shell vial/antigen | N | Myelofibrosis | N |
| 1351 | Prophylaxis | 0.10 × 106/+157 | N | ALL-3rd CR | N | |
| 1380 | Prophylaxis | 0.10 × 106/+88 | N | CML-Blastic | N | |
| 1395 | Prophylaxis | 0.10 × 106/+49 | Y | CML-Blastic | N | |
| 1421 | Prophylaxis | 0.10 × 106/+76 | Y | Secondary AML | N | |
| 1377 | Prophylaxis | 0.10 × 106/+79 | Y | CML, accelerated | N |
To identify features unique to immune reconstitution after unrelated BMT, the lymphoid phenotype, in vitro T-cell function, specific antibody production, and opportunistic infections of these 62 patients were compared with that of 154 consecutive, contemporaneously transplanted adult (n = 142) or pediatric (n = 12) recipients of an HLA-matched related SBA−E− BMT receiving identical cytoreduction. Graft rejection prophylaxis with ATG and steroids was administered pretransplant to 77 adult recipients of an HLA-related BMT and posttransplant to 65. We have previously found that patients less than 15 years of age transplanted with an HLA-matched sibling graft are not a risk for graft rejection or graft failure.30 Because of this finding, only 1 pediatric recipient (age, 16 years) received ATG and steroids after his matched sibling TCD graft. The characteristic of the HLA-matched related transplant recipients are shown in Table 1.
Cell preparations.
Heparinized blood was collected monthly from patients for the first 3 months when possible. Thereafter, samples were obtained every 2 to 6 months until normalization of immune function and phenotype was observed. Patients were studied only once during each time period. PBMNC were isolated by Ficoll-Hypaque density gradient separation.
Immunofluorescence analysis.
Directly fluoresceinated (FITC) antibodies, including HLe-1 (CD45), common leukocyte antigen (positive control), MsIgG, Leu-4 (CD3, pan T-cell), Leu-16 (CD20, pan-B cell), Leu-20 (CD20, pan B-cell), Leu-M3 (CD14), Leu-M3 (CD13), and phycoerythrin (PE)-conjugated antibodies MsIgG, Leu-2 (CD8), Leu-3 (CD4), Leu-4 (CD3, pan-T cell), Leu-16, Leu-18 (CD45RA), and Leu-19 (CD56, natural killer [NK] cells) were purchased from Becton Dickinson (Mountain View, CA).
Immunofluorescence was performed on peripheral blood lymphocytes or on whole blood. The whole blood technique consisted of the addition of appropriate amounts of undiluted conjugated antibody to 50 μL of whole blood, followed by 30 minutes of incubation at room temperature. Red blood cells were then lysed with a 1/10 dilution of FACS lysis solution (Becton Dickinson), followed by two washes. Immunofluorescence samples were analyzed on a FACScan (Becton Dickinson). The lymphoid populations to be analyzed were gated on using log 90° and forward angle scatter characteristics. The leukocyte-specific monoclonal antibody HLe-1 (CD45) was used to gate out any residual red blood cells. Monocyte contamination was ruled out by lack of reactivity (<1%) of the gated lymphoid cells with the monocyte-specific marker CD14.
In vitro lymphocyte proliferation assays.
PBMC (5 × 104), resuspended in RPMI, supplemented with 10% pooled human serum, penicillin/streptomycin, and L-glutamine, were plated in round-bottom microtiter wells in a volume of 175 μL/well. The T-cell mitogens used were phytohemaglutinin-P (PHA-P; DIFCO, Detroit, MI) used at optimal final concentrations of 42.9 and 21.4 μg/mL, soluble anti-CD3 (OKT3; Ortho Biotech, Raritan, NJ) at 1 and 2 μg/mL, tetanus toxoid (Lederle, Wayne, NJ) at a final culture concentration of 1:50 and 1:200, and CMV CF antigen (Biowhittaker, Walkersville, MD) at a final dilution of 1:50 and 1:100. Cultures were pulse labeled with 0.03 to 0.04 μCi/well of 14C or 1.0 μCi/well3H-thymidine for the last 24 hours of the 72-hour (PHA, tetanus toxoid) or 120-hour (CMV antigen) culture period, harvested onto glass-fiber filter paper, and counted in a liquid scintillation counter. The absolute proliferative response was calculated as the median counts per minute (cpm) of triplicate wells minus the unstimulated medium control. The PHA responses in this study are presented as a percentage of the lower limit of normal, calculated as the median cpm of the patient divided by the lower 5th percentile PHA response of 85 normal controls in cpm, multiplied by 100. This percentage was chosen, rather than the absolute PHA response, to compare patients evaluated over the last 5 years, during which time the isotope used to label proliferating cells was changed from14C to 3H-labeled thymidine, leading to higher range of normal control values.
Statistical analysis.
The cumulative incidence function was used to estimate the probability of opportunistic infection. Comparisons of these rates across covariate groups were evaluated using the logrank statistic.31 Two sample comparison for continuous data were analyzed by the nonparametric Wilcoxon rank sum test.32
The marginal regression model for longitudinal data was used to determine the relationship between T-cell recovery post-BMT and the covariates age and donor type. The marginal regression coefficient of interest measures the average change in T-cell recovery within a given follow-up time posttransplant for a change in covariate group. The Wald statistic using robust standard errors and the independence working correlation model was used to formally test for association between covariate and T-cell recovery.33 In addition, the Cox proportional hazards model with a time-dependent covariate was used to determine the relationship between T-cell recovery and time to life-threatening opportunistic infection.34
RESULTS
The median nucleated cell dose per kilogram for the unrelated and related SBA−E− transplant recipients were 2.74 × 107/kg (range, 1.01 to 14.28 × 107/kg) and 2.2 × 107/kg (range, 0.1 to 14.1 × 107/kg), respectively (P < .01). For the 62 evaluable recipients of an unrelated SBA−E− BMT, the median time (range) to engraftment (neutrophil count >500 cells/μL) was 12 (8 to 24) days. There were no statistically significant differences in the time to reach an absolute lymphocyte count of 500 and 1,000 cells/μL after unrelated or related SBA−E− BMT. Of the unrelated transplant recipients who achieved a circulating lymphocyte count of 500 (n = 58) and 1,000 cells/μL (n = 37), the median time (range) to reach these values were 42 (9 to 152) and 51 (10 to 242) days, respectively. After related transplants, the median (range) days to reach an ALC of 500 and 1,000 cells/μL were 35 (16 to 193; n = 134) and 49 (23 to 338; n = 105).
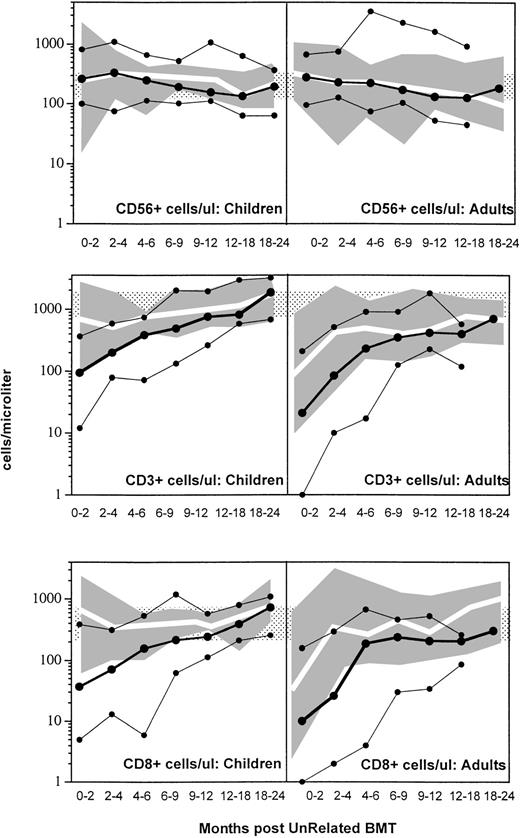
Figure 1 compares the recovery of CD56+ (upper), CD3+ (middle), and CD8+ (lower) lymphocytes in children and adults following related (shaded solid area) and unrelated SBA−E− BMT. Recovery of normal to increased numbers of circulating NK cells occurred in the majority of patients by 1 month posttransplant regardless of donor type or patient age. In contrast, CD3+ and CD8+ T-cell recovery was affected both by donor type and patient age. Adult recipients of an unrelated SBA−E− transplant experienced prolonged CD3+ and CD8+ T lymphopenia when compared with children after unrelated BMT and adults after related BMT (P < .01). Children who received an unrelated BMT had significantly decreased CD3+ and CD8+ T-cell numbers during the first 4 posttransplant months compared with those who received an HLA-matched sibling transplant (P < .01). After these early time points, CD3+ T-cell recovery was similar in both groups of children, despite ATG prophylaxis in the unrelated transplant group only (Fig 1, middle and lower panels).
Shown are the 10th-50th-90th% (solid lines, •) of CD56+ NK cells (upper), CD3 (middle), and CD8 (lower) cells per microliter in children (left) and adults (right) after SBA−E− unrelated BMT. The shaded gray area represents the 10th-90th% values of related SBA−E− transplant recipients; the white line represents the 50th% of SBA−E−related transplant recipients. Patients were studied only once during each interval. The stippled area represents the 10th-90th% of 65 normal controls.
Shown are the 10th-50th-90th% (solid lines, •) of CD56+ NK cells (upper), CD3 (middle), and CD8 (lower) cells per microliter in children (left) and adults (right) after SBA−E− unrelated BMT. The shaded gray area represents the 10th-90th% values of related SBA−E− transplant recipients; the white line represents the 50th% of SBA−E−related transplant recipients. Patients were studied only once during each interval. The stippled area represents the 10th-90th% of 65 normal controls.
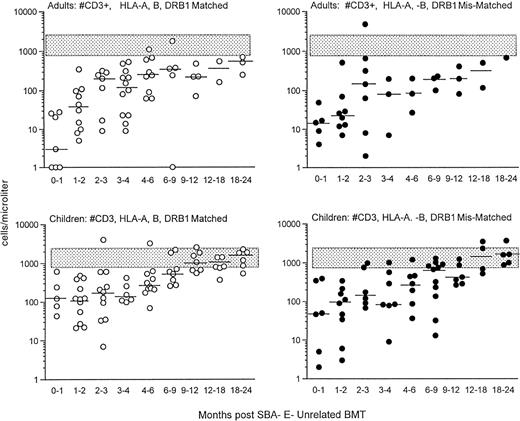
Before donor leukocyte infusions, 16 of 62 patients (27%) developed acute GVHD (19% of children v 31% of adults). Five patients developed grade I acute GVHD, 7 developed grade II GVHD, and 4 developed grade III-IV acute GVHD. Of the 16 patients who developed acute GVHD, graft rejection prophylaxis with ATG was administered pretransplant in 12 and posttransplant in 4. Four patients succumbed to complications of acute GVHD, all with grade III-IV. CD3+T-cell recovery was not significantly different in patients with or without GVHD (Fig 2). For children who received an HLA-matched versus HLA-mismatched unrelated BMT, no differences in recovery of CD3+ T-cell populations were observed (Fig 3, upper panel). Although the group size for adults was small, there was a trend toward more profound and prolonged CD3+ T lymphopenia in the HLA disparate adult transplant group (Fig 3, lower panel).
Shown is the recovery of CD3+ T cells in adults (upper) versus children (lower) after unrelated SBA−E− BMT. T-cell recovery is further divided between unrelated transplant recipient patients with (•) or without GVHD (○). The stippled rectangle denotes the 10th-90th% CD3 values of 65 normal adults. The horizontal bar represents the median of patients studied within each time period.
Shown is the recovery of CD3+ T cells in adults (upper) versus children (lower) after unrelated SBA−E− BMT. T-cell recovery is further divided between unrelated transplant recipient patients with (•) or without GVHD (○). The stippled rectangle denotes the 10th-90th% CD3 values of 65 normal adults. The horizontal bar represents the median of patients studied within each time period.
Shown is the recovery of T cells in adults (upper) and children (lower) who were transplanted from HLA A, B, DRβ1 matched (left; ○) versus mismatched (right; •) unrelated donors. The stippled rectangle denotes the 10th-90th% of 65 normal adults. The horizontal bar represents the median of patients studied within each time period.
Shown is the recovery of T cells in adults (upper) and children (lower) who were transplanted from HLA A, B, DRβ1 matched (left; ○) versus mismatched (right; •) unrelated donors. The stippled rectangle denotes the 10th-90th% of 65 normal adults. The horizontal bar represents the median of patients studied within each time period.
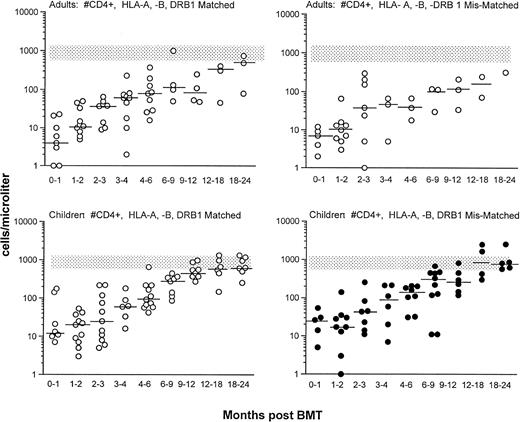
Significant differences were noted in the recovery of CD4+lymphocyte numbers not only between children and adult recipients of an SBA−E− unrelated BMT (P = .07), but also between adults given a related or unrelated transplant (P < .01). The majority of adults had CD4 cell counts of less than 100 cells/μL for at least 6 months posttransplant, with counts less than 200 cells/μL persisting for 12 to 18 months (Fig 4, upper panel). Although most children had low CD4 cell counts for the first 4 months after an unrelated BMT, the majority developed CD4 cell counts greater than 200 cells/μL by 6 months and normal numbers within 6 to 12 months post-BMT. Within the CD4 cell population, CD45Ra reconstitution (Fig 4, lower panel) was appreciably delayed in adult recipients of both related and unrelated SBA−E− BMT compared with pediatric recipients of an unrelated transplant (P < .01). After the initial 6 months posttransplant (P < .01), no differences were observed in the recovery of CD4 or CD4+ CD45RA populations between children who received an unrelated compared with a related SBA−E− BMT. In children and adults, recovery of CD4 cell counts was similar regardless of whether the unrelated donor was HLA-matched or mismatched (Fig 5).
Upper figure compares the recovery of CD4+T cells after unrelated BMT in adults (right) and children (left) with that of adult recipients of a related SBA−E− BMT receiving the same cytoreductive regimen and graft rejection prophylaxis. Unrelated transplant recipients are shown as circles, and related transplant recipients are shown by the shaded gray area (10th-90th%; 50th% shown by white line). The figure also compares the CD4+ cell counts of patients who did (•) or did not (○) develop a life-threatening opportunistic infection during that time interval after unrelated BMT. The stippled rectangle denotes 10% to 90% normal control values. (Lower panel) CD4+ CD45RA recovery in adults (right) and children (left) after unrelated SBA−E− BMT (circles), compared with patients after SBA−E− related BMT (shaded gray area). The horizontal bar represents the median of patients studied within each time period. The stippled rectangle denotes 10th-90th% of normal controls.
Upper figure compares the recovery of CD4+T cells after unrelated BMT in adults (right) and children (left) with that of adult recipients of a related SBA−E− BMT receiving the same cytoreductive regimen and graft rejection prophylaxis. Unrelated transplant recipients are shown as circles, and related transplant recipients are shown by the shaded gray area (10th-90th%; 50th% shown by white line). The figure also compares the CD4+ cell counts of patients who did (•) or did not (○) develop a life-threatening opportunistic infection during that time interval after unrelated BMT. The stippled rectangle denotes 10% to 90% normal control values. (Lower panel) CD4+ CD45RA recovery in adults (right) and children (left) after unrelated SBA−E− BMT (circles), compared with patients after SBA−E− related BMT (shaded gray area). The horizontal bar represents the median of patients studied within each time period. The stippled rectangle denotes 10th-90th% of normal controls.
Shown is the recovery of CD4+ cells per microliter cells in adults (upper) and children (lower) who were transplanted from HLA A, B, DRβ1 matched (○) versus mismatched (•) unrelated donors. The rectangle denotes the 10th-90th% CD4 cell counts of 65 normal adults. The horizontal bar represents the median of patients studied within each time period.
Shown is the recovery of CD4+ cells per microliter cells in adults (upper) and children (lower) who were transplanted from HLA A, B, DRβ1 matched (○) versus mismatched (•) unrelated donors. The rectangle denotes the 10th-90th% CD4 cell counts of 65 normal adults. The horizontal bar represents the median of patients studied within each time period.
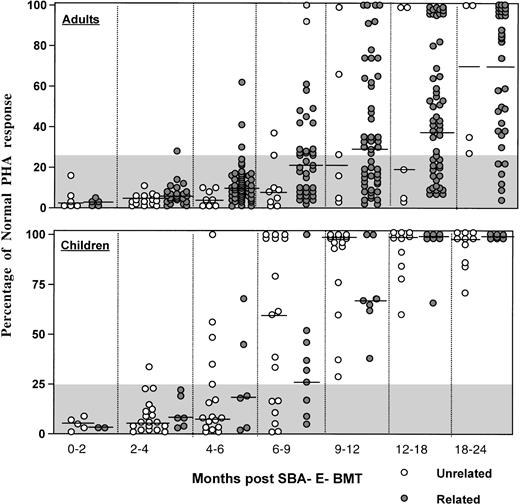
As shown in Fig 6, the majority of adult patients had persistently low PHA responses (<25% of normal) for the first 12 months posttransplant, regardless of donor type. In contrast, most children normalized their PHA response by 9 to 12 months after unrelated BMT. Antigen-specific responses were analyzed in 16 patients after vaccination with tetanus toxoid (n = 16) or after clinical reactivation of varicella virus in 4. Patients received 3 doses of tetanus toxoid at 2-month intervals starting at a median (range) of 16.7 (10.7 to 41.9) months post-BMT. Specific T-cell proliferative responses against tetanus antigen were present in all 16 patients vaccinated, with 15 of 16 developing protective tetanus antitoxin titers after the third vaccination. After clinical Herpes zoster infection, all 4 patients developed a proliferative response to varicella antigen that was negative before reactivation.
Percentage of normal PHA response of adults (upper) and children (lower) after unrelated SBA−E− BMT (○) compared with patients (◍) after SBA−E− related BMT. The gray shaded area denotes values less than 25% of normal. The horizontal bar represents the median of patients studied within each time period.
Percentage of normal PHA response of adults (upper) and children (lower) after unrelated SBA−E− BMT (○) compared with patients (◍) after SBA−E− related BMT. The gray shaded area denotes values less than 25% of normal. The horizontal bar represents the median of patients studied within each time period.
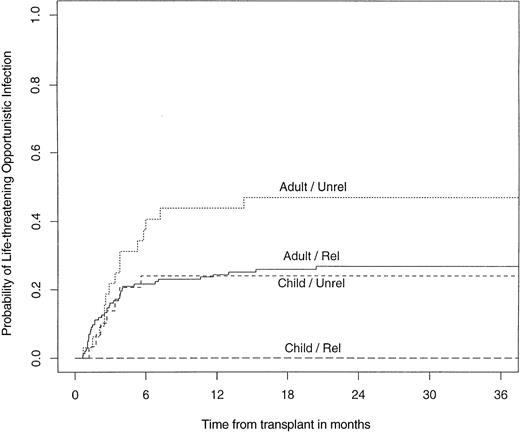
The infectious complications, including EBV-LPDs, that occurred after durable engraftment after unrelated SBA−E− BMT were analyzed. Thirty-four percent of unrelated transplant recipients (adults, 44%; children, 23%) developed a life-threatening opportunistic infection. When analyzed according to the degree of HLA disparity, opportunistic infections occurred in 25% of individuals who were HLA A, B, DRβ1 identical, compared with 42% who were HLA class I or class II mismatched. An opportunistic infection occurred in the setting of GVHD in 38% of cases. The probability of developing a life-threatening opportunistic infection was significantly higher in adults after an unrelated transplant compared with children after unrelated BMT (P = .02) or adults after related BMT (P = .02; Fig 7).
Probability of developing a life-threatening opportunistic infection after a related or unrelated SBA−E− BMT, children versus adults.
Probability of developing a life-threatening opportunistic infection after a related or unrelated SBA−E− BMT, children versus adults.
The most common causes of life-threatening opportunistic infection were EBV-LPD (n = 7), CMV alone (n = 5) or with HHV-6 (n = 1), adenovirus alone (n = 2) or with CMV (n = 1), and toxoplasmosis (n = 3). The median time (range) to develop an EBV-LPD after unrelated BMT was 3.4 (2.1 to 6.25) months. EBV-LPD were successfully eradicated in the 5 patients who received donor leukocytes and fatal in the 2 who did not. CMV interstitial pneumonia developed in 5 patients at a median (range) of 2.0 (1.8 to 6.0) months after unrelated BMT and was fatal in all patients. Two patients developed CMV retinitis at 3.5 and 9.0 months despite antiviral therapy. CMV retinitis did not resolve in either patient until donor leukocytes were administered in addition to continued antiviral therapy.
The potentially life-threatening opportunistic infections detailed in this series occurred exclusively in patients whose CD4 cell counts were less than 200 cells/μL (Fig 4) and were fatal in all patients except those receiving donor leukocytes. We examined the relationship between age, T-cell recovery, and risk of life-threatening opportunistic infections. The rate of CD4 recovery was associated with the risk of developing a life-threatening opportunistic infection after an unrelated SBA−E− BMT (P = .07). After adjusting for age, the relationship between CD4 reconstitution and the risk of a life-threatening infection was strengthened (P = .04). In addition, we examined the effect of age and risk of opportunistic infections, independent of CD4 recovery. This analysis demonstrated that an adult had a 3.1-fold increased risk of developing a life-threatening opportunistic infection relative to a child (P = .04; 95% confidence interval, 1.2 to 8.5). This demonstrates that other factors, in addition to CD4 recovery, contribute to the increased risk of these infections in adult unrelated transplant recipients.
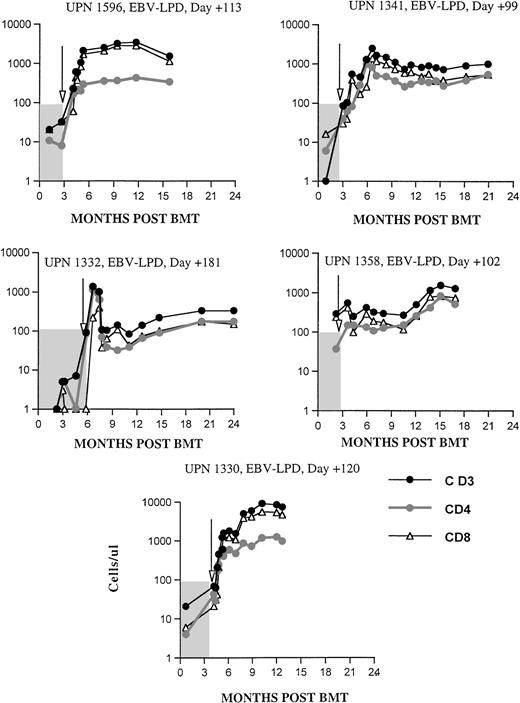
The effects of donor leukocytes administered at doses of 0.2 to 1.0 × 106 CD3+ T cells/kg (median, 0.5 × 106/kg) for the treatment of an EBV-LPD are shown in Fig 8. These resulted in the rapid normalization of T-cell numbers in 4 of 5 patients treated for an EBV-LPD, each of whom had CD3+ T-cell numbers of less than 100 cells/μL immediately before the infusion. Normal numbers of circulating CD8+ cells were found in each of these 5 patients within 4 weeks of the infusion and CD4 counts greater than 200 cells/μL were achieved in 4 of 5 patients. UPN 1332, the only patient with an EBV-LPD who developed de novo GVHD post-DLI that required systemic steroid therapy, normalized his T-cell numbers within 12 days of DLI infusion, during which time his multiorgan EBV-LPD was eradicated. Although this patient became markedly lymphopenic after initiation of intravenous methylprednisolone, he remains in continuous remission 61+ months post-BMT, off immunosuppressive therapy.
Recovery of CD3, CD4, and CD8 cells per microliter in patients (n = 5) treated with donor leukocytes for a documented EBV-LPD after unrelated SBA−E− BMT. Arrows denote time of DLI. The shaded area denotes values before DLI.
Recovery of CD3, CD4, and CD8 cells per microliter in patients (n = 5) treated with donor leukocytes for a documented EBV-LPD after unrelated SBA−E− BMT. Arrows denote time of DLI. The shaded area denotes values before DLI.
The effect of donor leukocytes at a dose of 0.1 × 106CD3+ T cells/kg administered in an effort to prevent EBV-associated lymphoproliferative disorders in 5 EBV-seropositive patients or to eradicate CMV viremia that persisted in 2 patients despite protracted antiviral therapy was less consistent. The increment of CD3+ and CD4+ T cells observed in these 7 patients was smaller and often transient due to the development of GVHD requiring immunosuppressive therapy (data not shown). All 3 patients receiving 0.1 × 106 donor CD3+ T cells/kg less than 80 days posttransplant developed de novo GVHD post-DLI, compared with none of the 4 individuals who received this cell dose 103 to 157 days post-BMT.
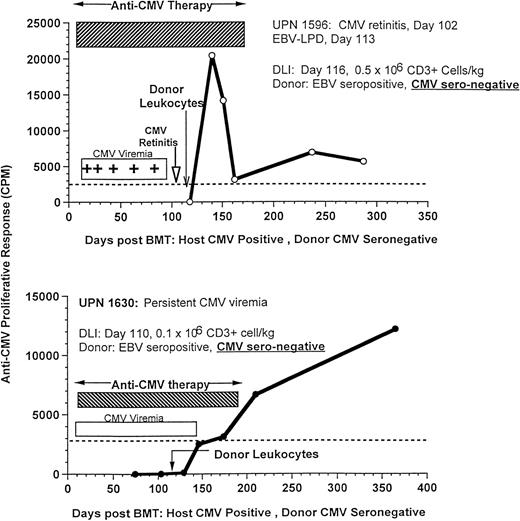
The effect of DLI on antigen-specific responses is shown in 3 CMV-seropositive patients (UPNs 1596, 1630, and 1725) who developed recurrent CMV viremia with (n = 2) or without retinitis (n = 1), despite prolonged antiviral therapy with ganciclovir, foscarnet, and immune globulin. After the infusion of only 0.1 × 106/kg T cells/kg (n = 2) or 0.5 × 106/kg T cells (n = 1) from their marrow donors, all 3 patients rapidly developed an anti-CMV proliferative response, cleared their CMV viremia, and remained CMV antigen negative, despite cessation of antiviral therapy (Fig 9). These responses were particularly striking, because the donors had CMV-specific titers of less than 1:10, which is in the range of donors used to provide CMV-negative blood products.
Recovery of CMV-specific T-cell proliferation after donor leukocytes from EBV-seropositive, CMV-seronegative donors for the treatment of an EBV-LPD in UPN 1596, who had concurrent CMV retinitis (upper, 500,000 CD3+ cells/kg), and UPN 1630, who received 100,000 CD3+ T cells/kg for the treatment of persistent CMV viremia despite antiviral therapy (lower).
Recovery of CMV-specific T-cell proliferation after donor leukocytes from EBV-seropositive, CMV-seronegative donors for the treatment of an EBV-LPD in UPN 1596, who had concurrent CMV retinitis (upper, 500,000 CD3+ cells/kg), and UPN 1630, who received 100,000 CD3+ T cells/kg for the treatment of persistent CMV viremia despite antiviral therapy (lower).
DISCUSSION
Although relapse rates are often lower after transplantation of marrow derived from unrelated donors when compared with HLA-matched siblings,1,2,9,35 DFS is often compromised by a higher incidence of fatal opportunistic infections1,3,6-10,11,13-16 and GVHD.1-5,10-12In the series of 462 recipients of bone marrow derived from unrelated donors facilitated by the NMDP,1 infection and interstitial pneumonia were the primary and secondary cause of death in greater than 30% of patients, compared with 14% from disease recurrence. In recent reports, leukemic relapse accounted for 8% to 25% of deaths compared with 38% to 75% from infection.2,9,10,11 15
Despite this increased risk of infection, only one published study has analyzed parameters of immune reconstitution after unrelated BMT36 and none has correlated them with infection rates. In this report, we have shown that SBA−E− TCD BMT derived from unrelated adult donors are associated with prolonged T-cell lymphopenia and extreme CD4 lymphopenia in adults but not children, that opportunistic infections have occurred only in patients with CD4 cell counts less than 200 cells/μL, and that adoptive immunotherapy with small numbers of unirradiated donor leukocytes can be associated with rapid restoration of CD3+, CD4+, and CD8+ T-cell numbers, antigen-specific T-cell responses, and resolution of CMV- and EBV-associated disease. Comparison of this data with a similar study performed on recipients of HLA-matched sibling BMT20 demonstrates a similar time to engraftment of donor NK cells (1 month) after related and unrelated SBA−E− BMT in both children and adults. No differences were observed in the time to develop normal numbers of circulating CD3+, CD4+, CD4+ CD45RA+, and CD8+ T lymphocytes in children transplanted with SBA−E− bone marrow derived from unrelated donors when compared with children transplanted from matched sibling donors. However, in contrast to adult recipients of a related SBA−E− BMT who developed normal numbers of CD3+ T cells by 2 to 4 months even when receiving ATG and steroids for graft rejection prophylaxis, adult recipients of unrelated BMT experienced prolonged T lymphopenia, with delayed reconstitution of CD8+ and CD4+populations, including CD45RA+ CD4+ T lymphocytes.
Several factors could be hypothesized to account for the more profound and prolonged immunologic deficiencies observed among recipients of transplants from unrelated donors. First, major or microvariant HLA disparities between donor and host might, on one hand, alter the capacity of the donor’s lymphoid progenitors to migrate to or mature within the host thymus, or on the other hand, stimulate clinical or subclinical GVHD, thereby further potentiating thymic injury.11,12,21,22 HLA class I microvariant disparities have been detected in up to 48% of unrelated donor-recipient pairs matched by serology for HLA class I alleles and molecularly matched for DRβ1.22 Furthermore, increasing evidence suggests that such HLA microvariant disparities may explain, in part, the increased risk of severe GVHD after unmodified matched unrelated marrow grafts when compared with transplants from similarly matched HLA-identical siblings.2,3,11 12 Although the incidence of grade II-IV GVHD after SBA−E− BMT from unrelated donors is low (17%), it is greater than the 4% incidence of acute grade II GVHD and absence of grade III-IV GVHD observed in adults transplanted with SBA−E− matched sibling marrow. In this context, it is possible that mild clinical or even subclinical forms of GVHD could contribute to the extended immunodeficiency observed after unrelated BMT. The significance of microvariant HLA disparities for the redevelopment of T-cell populations may also differ in children versus adults. Thus, in this series, one or two HLA class I or class II microvariant disparities did not appear to affect recovery of T-cell populations or their function in children, but did appear to predispose to more prolonged and profound deficits in adults.
A second factor that likely contributes to the delayed and incomplete immune reconstitution of T cells observed in adults is the involution of the thymus, which normally occurs in adulthood, beginning late in the second decade of life (reviewed in Sprent37). Analysis of immune reconstitution in our patients grouped according to age indicated that, after the first 4 to 6 months posttransplant, there were no significant differences between children who received an unrelated or related SBA−E− BMT despite administration of ATG and steroids to children receiving unrelated marrow grafts.20 Indeed, the kinetics of reconstitution resembles that recorded in children after autologous or unmodified matched sibling transplants.38 In contrast, adult recipients of SBA−E−marrow transplants have prolonged states of T-cell cytopenias that are significantly more protracted and severe in the unrelated transplant recipients. The differences in immune reconstitution observed in adults versus pediatric recipients are difficult to ascribe to differences in the lymphoid progenitors or mature lymphocytes administered in the grafts as there were no differences in the adult marrow donor pool used to transplant these two patient groups. This suggests that differences in the host environment, such as the thymus may underlie limitations in immune recovery in adults39 40 and further that genetic disparities between donor and host may affect not only the capacity of mature lymphoid cells to interact with host antigen-presenting cells or host cells infected by viruses, but also the migration of naive cells to the thymus as well as other sites of lymphoid differentiation.
Grade III to IV GVHD can be reduced to less than 10% in children after T10B9-depleted unrelated BMT6-9 and in all patients transplanted with SBA−E−TCD marrow, even when derived from HLA-mismatched donors. This contrasts with the 20% to 35% incidence of acute grade III-IV GVHD reported after unmodified marrow transplants derived from HLA-A, B DRβ1 identical unrelated donors, as defined by high resolution molecular typing2,3,12 and the 50% to 70% incidence of severe GVHD recorded when donors who are matched only by serology.3-5,11,12 Unfortunately, rigorous T-cell depletion to prevent GVHD often requires additional immunosuppression to prevent graft failure, which can increase the risk of opportunistic infections. After related SBA−E− BMT, the probability of developing pneumonia secondary to an opportunistic infection increased from 0.10 in patients not receiving ATG and steroids to 0.21 in adults who were.20 The risk of EBV-LPD has also increased from less than 1% to 8% for patients receiving ATG and steroid to prevent graft failure after a related SBA−E− BMT20 to 11% in recipients of unrelated SBA−E−grafts. Although the probability of developing an opportunistic pneumonia was similar in adult recipients of an unrelated and related SBA−E− BMT, the fatality rate for these pneumonias in the unrelated group was 100%, compared with 30% in similarly treated recipients of a related BMT.20
This group has previously demonstrated a rapid expansion of EBV-specific cytotoxic cells in patients receiving doses of donor leukocytes containing 0.5 to 1.0 × 106CD3+ T cells/kg for the treatment of EBV-LPD after HLA-matched related TCD BMT.41 As shown in this study, infusions of donor leukocytes transferring as few as 0.2 to 1.0 × 106 CD3+ T cells/kg recipient weight regularly induced normalization of CD3+, CD4+, and CD8+ T lymphocyte populations within 18 to 21 days in these unrelated transplant recipients. Striking increases in virus specific immune responses to EBV (Lucas et al41 and data not shown) and to CMV were also detected. These responses were sufficient to induce durable remissions in each of the 5 unrelated transplant recipients with monoclonal EBV-LPD and the 2 patients with CMV-induced retinitis refractory to antiviral therapy. In the latter cases, cessation of viremia was detected in association with recovery of T-cell proliferative responses to CMV antigens. These latter responses are striking in that the donors of the leukocytes themselves had such low titers of CMV-reactive antibody. Although it may be assumed that these donors had low levels of preprimed CMV-specific T cells in their circulation, the rapid recovery of CMV responses in the patients suggests that, in CMV infections as in EBV infections, the numbers of T cells required to induce effective immunity may be small. At this time our data are insufficient to determine whether the increment in T-cell numbers and viral specific responses observed after DLI reflect the presence and/or magnitude of antigenic stimuli (ie, viral load) or the dose of cells infused.
The obvious disadvantage of donor leukocyte infusions is the risk of inciting severe GVHD, particularly in recipients of matched unrelated or HLA-disparate unrelated grafts. In this series, 1 of 7 patients who received donor leukocytes providing 0.1 to 1.0 × 106CD3+ T cells/kg as treatment for an EBV-LPD or persistent CMV viremia developed de novo GVHD. In these 7 cases, the donor leukocytes were infused 103 to 190 days posttransplant. In contrast, 3 of 4 patients who received 0.1 × 106 CD3+donor T cells/kg to prevent EBV-LPD less than 90 days posttransplant developed de novo GVHD requiring steroid therapy. These studies confirm what we have previously observed in our analyses of the threshold doses of T cells required to induce GVHD at different times posttransplant.42 In HLA-matched sibling grafts marrow, T-cell doses exceeding 105 T cells/kg are associated with a significant incidence of GVHD when administered in the absence of GVHD prophylaxis.42 In contrast, when donor leukocytes containing 107 T cells/kg patient weight are given greater than 9 to 12 months posttransplant to treat relapsed CML post-SBA−E− BMT, only 2 of 17 patients developed grade II GVHD (MacKinnon et al,43Papadopoulos et al44), indicating that, late after transplant, higher doses of T cells may be tolerated without risk of GVHD. A similar reduction in GVHD risk late after transplant has also been detected in murine models.45 The mechanism responsible for these alterations of GVHD risk remain unclear, but may, in part, include the relatively lower sensitivity of host tissues to GVH-induced injury late after myeloablative radiation and chemotherapy, the development of donor T cells from the marrow graft capable of modulating or suppressing the function of alloreactive T cells transferred in the donor leukocyte infusion, or the replacement of host antigen-presenting cells, such as dendritic cells capable of stimulating alloreactive GVH-inducing donor T cells, with donor-derived cells. Although the threshold doses of donor T cells required to induce GVHD are different and often lower than those required for GVHD in HLA-matched recipients, the experience in this small series suggests that doses of 106 T cells/kg derived from a matched unrelated donor may be tolerated if administered more than 3 months posttransplant, whereas doses of 0.1 × 106/kg may induce significant GVHD if administered earlier in the posttransplant course. If these dose-response relationships are taken into consideration, cautious use of donor leukocytes may be indicated in the treatment of potentially lethal infections due to CMV, EBV, and potentially other viruses (eg, adenovirus), for which other antiviral therapies are either unavailable, ineffective, or, in specific cases, inadequate or intolerable.46
Clearly, other approaches for adoptive immunotherapy offer the potential of effectively transferring antiviral immunity without the risk of GVHD, specifically, the use of donor-derived cloned antiviral cytotoxic cells or T-cell lines, such as those used to prevent or treat CMV or EBV-related infections post-BMT.24-26 However, this approach is often logistically difficult, generally requiring 7 to 9 weeks for generation.24-26 Furthermore, although in vitro propagated cells may provide resistance to one specific viral pathogen, they have little capacity to foster general recovery of the immune system, because they generally include CD8+, but lack CD4+ T cells. Infusions of donor leukocytes genetically modified to express a marker for cell selection and a suicide gene (eg, HSV thymidine kinase) can circumvent these obstacles by providing a population of broadly reactive T cells requiring less in vitro generation time, cells that can be eliminated by treatment with ganciclovir in the event that clinically signficant GVHD ensues, as shown by Bordignon et al.27 The success or failure of this latter strategy depends on the sustained expression of transduced genes in the infused cells sufficient to permit the eradication of these cells at any time an untoward GVH reaction develops as well as the inability of the transduced cells to recruit other alloreactive cells that could sustain an acute or chronic GVHD reaction.
Supported by Grant No. PO1 CA23766 from the National Cancer Institute, National Institutes of Health; by the Vincent Astor Chair in Clinical Research (R.J.O.); and by the Butler Fund (T.N.S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to T.N. Small, MD, Department of Pediatrics, Bone Marrow Transplant Service, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: smallt@mskcc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal