Abstract
The glycoprotein (Gp) Ib/IX complex contains three transmembranous leucine-rich repeat polypeptides (GpIb, GpIbβ, and GpIX) that form the platelet von Willebrand factor (vWF) receptor. GpIb/IX functions to effect platelet adhesion, activation, and aggregation under conditions of high shear stress. GpIb/IX is expressed late in the ontogeny of megakaryocytes, the precursor cell that releases platelets when it reaches its terminal stage of differentiation. Because signal pathways can be reused at different stages of development by integration with different effector pathways and because cellular adhesion through other receptor families often modulates cell growth, the hypothesis that GpIb/IX regulates cell growth was investigated. The surface expression of recombinant GpIb decreases the proliferation of transduced CHO cells. GpIb causes growth arrest in the G1 phase of the cell cycle associated with the induction of the cyclin-dependent kinase inhibitor p21. G1 arrest induced by recombinant GpIb in heterologous cells requires signaling through the 14-3-3ζ binding domain of GpIb and is partially dependent on its engagement by the extracellular ligand vWF. Growth arrest induced by the expression of recombinant GpIb/IX is followed by apoptosis of the transduced cells. The endogenous expression of GpIb in human hematopoietic cells is associated with decreased proliferation. These results suggest that the expression of the GpIb/IX complex regulates megakaryocyte growth.
CELL GROWTH (proliferation and differentiation) involves reciprocal communications between a cell and its environment. Two extracellular substances that influence cell growth are soluble growth factors and insoluble matrix proteins. The essential growth response modulated by these environmental stimuli is mitosis. Growth factors and the extracellular matrix modulate entry into the mitosis phase of the cell cycle through signal pathways that are sometimes redundant, complementary, synergistic, or even antagonistic. Such precise modulation is required for normal development, and abnormal modulation results in the pathogenesis of many acquired diseases, such as cancer or atherosclerosis.1There is abundant evidence that the extracellular matrix modulates cell proliferation through the integrin family of cell surface proteins coupled by adapter proteins and protein kinases to the cellular restriction point transition apparatus. The restriction point occurs about 1 to 2 hours before S phase of the cell cycle. The restriction point is when a cell becomes programmed for autonomous entry into mitosis, independent of all mitogens and refractory to all antiproliferative stimuli. Cell surface integrins binding to extracellular matrix proteins activate the transcription of D cyclins, and newly synthesized cyclin D (particularly D1) activate the cyclin-dependent kinases (cdk) cdk 4 and cdk 6, which phosphorylate the retinoblastoma protein (Rb) and related protein (p107). The phosphorylation of Rb and p107 releases the transcription factor E2F, which activates a variety of growth-regulating genes, including cyclin A, irreversibly driving the cell into S phase followed by mitosis.2 A variety of cyclin-dependent kinase inhibitors (CDKIs) counterbalance the proliferative effects of the cyclin/cdk interactions. The most important of these may be p21, a 21,000-Dalton universal cdk inhibitor that is the effector of p53-mediated growth arrest.3 P53 also signals a pathway leading to apoptosis, although the route of this signal pathway bifurcation and the precise mechanism by which apoptosis-promoting target genes are activated remain obscure.4
The glycoprotein (Gp) Ib/IX complex contains three transmembranous leucine-rich repeat polypeptides (GpIbα, GpIbβ, and GpIX) that form the platelet von Willebrand factor (vWF) receptor.5 GpIb/IX functions to effect platelet adhesion, activation, and aggregation under conditions of high shear stress.6 GpIb/IX is expressed late in the ontogeny of megakaryocytes, the precursor cells that release platelets when they reach their terminal stage of differentiation. Although there are recent data relating to transcriptional effectors of megakaryocyte differentiation,7,8 growth factor control of megakaryocytopoiesis,9 and nuclear events associated with the final phases of megakaryocyte maturation leading to platelet production,10 11 there is little or no published information about adhesion receptor function in megakaryocytopoiesis. Because signal pathways may be functionally conserved in cells at different stages of development and because GpIb/IX binding to vWF triggers platelet activation, the hypothesis that GpIb/IX regulates megakaryocytopoiesis was developed. To begin to investigate this hypothesis, the effect of GpIb/IX on the growth of cultured cells was examined.
MATERIALS AND METHODS
Cell lines and culture conditions.
CHOβ/IX cells (CHO cells expressing GpIbβ and GpIX ) were a gift from Dr J.A. López and Dr J.F. Dong (Baylor College of Medicine and Veterans Affairs Medical Center, Houston, TX). The cells were grown in α-minimum essential medium (MEM; Life Technologies Inc, Grand Island, NY) containing 5% fetal bovine serum (FBS; Life Technologies Inc), 500 μg/mL G418 (Sigma, St Louis, MO), 80 μmol/L methotrexate (Sigma), and an antibiotic-antimycotic (Life Technologies Inc). The human hematopoietic DAMI cell line12 was purchased from the ATCC (Rockville, MD; ATCC.CRL-9792, batch no. F-10415). DAMI cells were grown in 10% FBS. All cells were maintained in an atmosphere of 5% CO2 and 95% humidity at 37°C.
Transfections of recombinant GpIbα.
The full-length human GpIbα cDNA (from −42 to 2,420 bp; obtained from Dr J.A. López) was cloned into pBluescript SK vector (Stratagene, La Jolla, CA) at its EcoRI insertion site and then subcloned into the mammalian expression vector pcDNA3.1/Zeo (Invitrogen Co, San Diego, CA) at BamHI and Xho I sites. A truncated GpIbα (amino acid residues Met1-Leu540) was generated by ligation of two fragments of GpIbα cDNA in the SK vector from BamHI toXba I and Xba I to Pst I sides and then cloned into pcDNA3.1/Zeo expression vector at BamHI and Xho I sites. A second truncated GpIbα (amino acid residues Met1-Gly594) was constructed by making a fragment of wild-type GpIbα in pBluescriptSK with an excision from its HindIII to its Sma I restriction site. This fragment was ligated to a HindIII plus Sma I-digested product of wild-type GpIbα cDNA after amplification by polymerase chain reaction (PCR) using primers spanning codons 547 through 595 (5′ACAGTGCCCCGGGCCTGGCTGCTC3′ and 5′CAGGTCCTGACCTCGAGCCTGACTCAG3′, respectively). A cDNA for GpIbα deleted of its actin-binding domain (Gln541-Leu589) was constructed by ligating three fragments of GpIbα cDNA in pBluescript SK. The first fragment was wild-type GpIbα with an excision from its HindIII to itsXba I restriction sites. The second was wild-type GpIbα with an excision from its Xba I to its Pst I restriction site. The third fragment was generated by PCR amplification of wild-type GpIbα using primers that span codon 587 (5′ TCAGCTCTGCTGCAGGGTCGTGGTCAG3′) with a sequence for the 3′ untranslated region of the cDNA (5′ATGCAGCATCTCGAGCTTTGTCTTGTC3′). After ligation, the mutant GpIbα species were cloned into pcDNA3.1/Zeo at HindIII and Xho I sites. In all cases, the integrity of the mutant cDNA was verified by sequence analysis.
The ecdysone-inducible GpIbα (αIn) gene expression plasmid was constructed by cloning GpIbα cDNA from the SK vector into the ecdysone-inducible mammalian expression vector pIND (Invitrogen) atBamHI and Xho I sites. CHOβ/IX cells (5 × 105) in 25-cm2 culture flasks were washed twice with phosphate-buffered saline (PBS) and maintained in 1.5 mL of MEM serum-free medium.12 A mixture of 15 μL lipofectAMINE (Life Technologies, Inc) with 5 μg of plasmid DNA (full-length GpIbα, truncated GpIbα, or control empty vector) was kept at room temperature for 15 minutes in 100 μL PBS before being added to each flask. The transfection mixtures were incubated at 37°C for 10 hours. After transfection, cells were selected in MEM supplemented with 10% FBS, 500 μg/mL Zeocin (Invitrogen), 500 μg/mL G418, and 80 μmol/L methotrexate. For inducible gene expression, CHOβIX cells were cotransfected with plasmids pVgRXR (Invitrogen) and pIND-α and then selected in the same medium as Zeo-α cells.
Flow cytometry and cell cloning.
The expression of the GpIb complex on the cell surface was analyzed by flow cytometry. Stable transfected CHO cells were washed with PBS, harvested with 1:5,000 EDTA (Life Technologies, Inc), and incubated with 1 μg/mL fluorescein isothiocyanate (FITC)-conjugated AN51 (DAKO, Carpinteria, CA), a monoclonal antibody to GpIbα, or SZ1, a monoclonal antibody against GpIX (provided by Dr J.A. López). For wild-type GpIbα and truncated GpIbα, samples were washed twice with PBS, resuspended in 0.5 mL of PBS, and directly analyzed for emission at 520 nm in a Becton Dickinson FACStar flow cytometer (Becton Dickinson, San Jose, CA) after stimulation with an argon ion laser at a wavelength of 488 nm. For GpIX, the samples were immunostained with a second antibody (a goat antimouse antibody conjugated with FITC), washed twice with PBS, and then analyzed by flow cytometry. For inducible GpIbα, transfected CHO-Inα/β/IX cells were grown in medium containing 1 μmol/L muristerone for 24 to 96 hours at 37°C before analysis.13 Muristerone has no direct effect on the growth of CHO cell. Cells with the highest expression level of GpIbα, truncated GpIbα and GpIX, were isolated with DYNABEADS M-450 rat antimouse IgG2a (Dynal, Inc, Lake Success, NY). These cells were incubated with 5 μg/mL AN51 or SZ1 for 1 hour at 4°C, washed twice with PBS, and then incubated with 4 × 107 beads. The mixture was incubated for 1 hour at 4°C with gentle rotation before the beads were collected by a magnetic separation. The beads were washed twice with PBS and the cells were detached by adding 2% EDTA at 37°C for 30 minutes. Cell lines with comparable expression of GpIbα were cloned by limiting dilution selection.
DAMI cells expressing GpIbα were first isolated by affinity purification with solid-phase vWF. From this pool, cells demonstrating increased surface expression were selected by repeated rounds of fluorescence-activated cell sorting with the anti-GpIbα antibody AN51.
Western blot analyses.
The expression of GpIbα, mutant GpIbα, and induced GpIbα and the cell cycle regulatory proteins p21 (WAF1/Cip1), cyclin D1, and cyclin E was analyzed by Western blot. Cell lysis buffer was added to washed cells (50 mmol/L Tris-HCl, pH 7.4; 1% Triton-X 100; 0.25% sodium deoxycholate; 150 mmol/L NaCl; 1 mmol/L EGTA; 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]; 1 μg/mL aprotonin, leupeptin, and pepstatin; 1 mmol/L Na3VO4; and 1 mmol/L NaF). The protein mass in each lysate sample was measured using a BCA Protein Assay Kit from Pierce (Rockford, IL). One microgram of total protein in lysis buffer was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF), and blotted with either WM23 (a mouse monoclonal antibody to GpIbα; a gift from Dr M.C. Berndt, Baker Medical Research Institute, Prahran, Victoria, Australia) or M-20, H-295, or C-19 (affinity-purified rabbit polyclonal anti-cyclin E, anti-cyclin D1, and anti-p21 antibodies, respectively; all from Santa Cruz Biotechnology, Inc, Santa Cruz, CA). Proteins were detected by the ECL system (Amersham, Arlington Heights, IL).
In vitro growth assay.
The growth rate of transfected CHO cells was measured by three different assays. First, cell numbers were measured in the continuous presence of 5% FBS. Cells were grown in 6-well plates containing 5% FBS, trypsinized, and counted with a hemacytometer at 0, 1, 2, 3, and 4 days after plating. Second, DNA synthesis was measured after the cells were growth arrested in serum-free medium for 24 hours. Five thousand cells per well were grown on 24-well plates containing 5% FBS at 37°C. 5-Bromo-2′-deoxyuridine (BrdU; Boehringer Mannheim Co, Indianapolis, IN) was added at 10 μmol/L after 16 hours of serum repletion. After 4 hours of incubation with BrdU, the cells were fixed and stained with an anti-BrdU antibody. BrdU incorporation was measured by the Cell Proliferation BrdU Colorimetric Kit (Boehringer Mannheim). Third, DNA content and ploidy were measured by propidium iodide staining. Growth-arrested cells were grown in 6-well plates containing 5% FBS for 20 hours at 37°C. Cells were fixed in 70% ethanol at 4°C for 30 minutes, washed twice with PBS, and incubated in PBS containing 200 μg/mL DNAase-free RNAase A (Boehringer Mannheim) for 30 minutes at 37°C. Propidium iodide (50 μg/mL; Boehringer Mannheim) was then added to the cell suspension and fluorescence was analyzed by flow cytometry. For CHO-Inα/β/IX cell growth measurements, experiments were performed in the continuous presence of 5% FBS. Control growth conditions were defined in the uninduced cells, and the effect of GpIbα on growth was determined in CHO-Inα/β/IX cells at times after the induction of GpIbα by adding 1 μmol/L muristerone to the medium plus FBS. To determine the effect of vWF on cell growth, incubation plates were coated with 20 μg/mL purified vWF (a gift of Nancy Turner, Rice University, Houston, TX). To eliminate any potential influence of bovine vWF in the FBS, CHO cells were maintained in MEM supplemented with CD CHO (serum-free growth factors designed for CHO cells; Life Technologies Inc, Gaithersburg, MD). Apoptosis was measured using flow cytometry to identify surface phosphatidylserine by its binding to phycoerythrin-conjugated annexin V (Pharmingen, San Diego, CA).
Data analysis.
All bar graphs present the mean values with the standard errors of the means. P values were calculated by the Student’st-test.
RESULTS
Cells expressing GpIbα are selected against during routine passage.
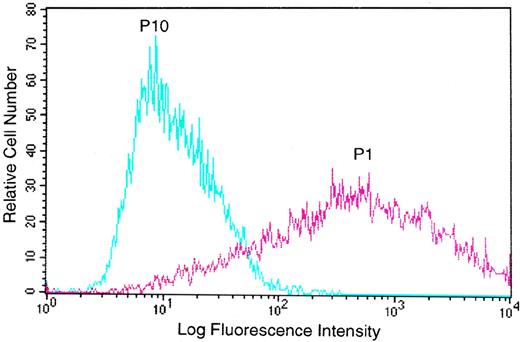
During the course of studies on the recombinant GpIb/IX complex, poor cell surface expression was observed in a variety of stably transfected cell lines. To investigate the basis for this observation, CHO cells transduced with the cDNAs for GpIbβ and GpIX were transfected with the cDNA for GpIbα inserted into pcDNA3.1 vector. Excellent expression of GpIbα was identified after selection with Zeocin, G418, and methotrexate and separation with immunomagnetic beads (Fig 1, passage 1). This decreased progressively with each cell passage to the point that, after the tenth passage, no expression was detectable (Fig 1, passage 10). Similar decreases over time in the expression of GpIbα were observed with other cell lines (Jurkat and L2H; data not shown).
Cells expressing GpIb are selected against during routine passage. Expression of GpIb (reported by flow cytometry fluorescence with FITC-conjugated anti-GpIb antibody AN51) was identified immediately after antibiotic and immunomagnetic bead selection (passage 1), but this decreased progressively with each cell passage to the point that, after the tenth passage, no expression was detectable (passage 10).
Cells expressing GpIb are selected against during routine passage. Expression of GpIb (reported by flow cytometry fluorescence with FITC-conjugated anti-GpIb antibody AN51) was identified immediately after antibiotic and immunomagnetic bead selection (passage 1), but this decreased progressively with each cell passage to the point that, after the tenth passage, no expression was detectable (passage 10).
Cell number is decreased after passage of CHO cells expressing the GpIbα/β/IX complex.
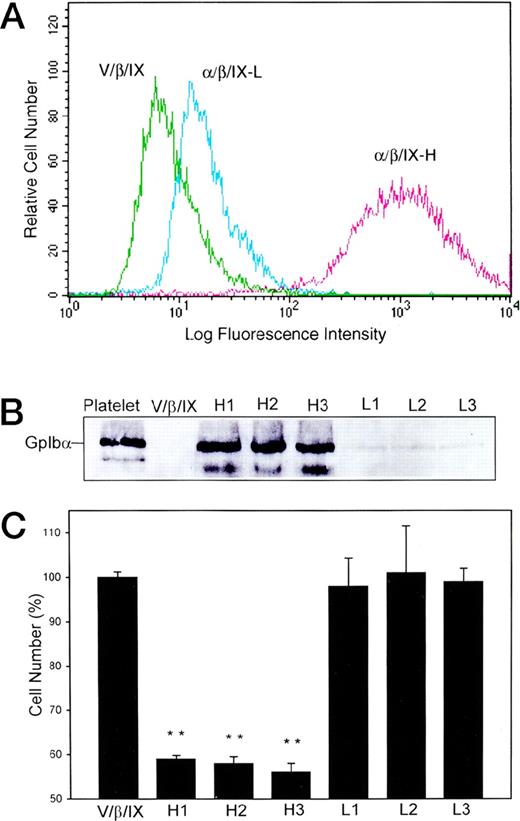
To determine if the decrease in cell surface expression of GpIbα is due to a decline in protein expression in cells proliferating normally or to a decrease in the proliferation of cells expressing GpIbα normally, CHOα/β/IX cells were selected as described above and isolated with immunomagnetic beads. Single cells were cloned by limiting dilution. The quantity of GpIbα expressed in each cloned cell line was checked by flow cytometry. Figure 2A shows that GpIbα expressed on the cell surface is increased from vector (V) to low (L) to high (H) expressing cell clones. Three cell lines with high expression of GpIbα and three cell lines with low expression of GpIbα were selected for analysis of immunoreactive GpIbα by immunoblotting whole cell lysates with the monoclonal antibody WM23 (Fig 2B). These cells were cultured on a vWF substrate and the cell number was counted 4 days after passage. Figure 2C demonstrates that the cell number is decreased in proportion to the quantity of GpIbα expressed by that cell line.
Cell number is decreased after passage of CHO cells expressing GpIb. Single cells were cloned by limiting dilution and the quantity of GpIb expressed in each cloned cell line was checked by flow cytometry as in Fig 1. (A) shows the stable expression of GpIb in vector (V/β/IX), low (/β/IX-L), and high (/β/IX-H) expressing cloned cell lines. Three cell lines with high expression of GpIb and three cell lines with low expression of GpIb, as defined by immunoblot analysis of total GpIb (B), were cultured on a vWF substrate and the cell number was counted 4 days after passage. (C) demonstrates that the cell number is decreased in proportion to the quantity of GpIb expressed by that cell line. (**P < .0001 in comparison with V/β/IX cells; n = 3.)
Cell number is decreased after passage of CHO cells expressing GpIb. Single cells were cloned by limiting dilution and the quantity of GpIb expressed in each cloned cell line was checked by flow cytometry as in Fig 1. (A) shows the stable expression of GpIb in vector (V/β/IX), low (/β/IX-L), and high (/β/IX-H) expressing cloned cell lines. Three cell lines with high expression of GpIb and three cell lines with low expression of GpIb, as defined by immunoblot analysis of total GpIb (B), were cultured on a vWF substrate and the cell number was counted 4 days after passage. (C) demonstrates that the cell number is decreased in proportion to the quantity of GpIb expressed by that cell line. (**P < .0001 in comparison with V/β/IX cells; n = 3.)
The inhibitory effect of the GpIbα/β/IX complex on cell growth is specifically dependent on the surface expression of GpIbα and is mediated by its cytoplasmic domain.
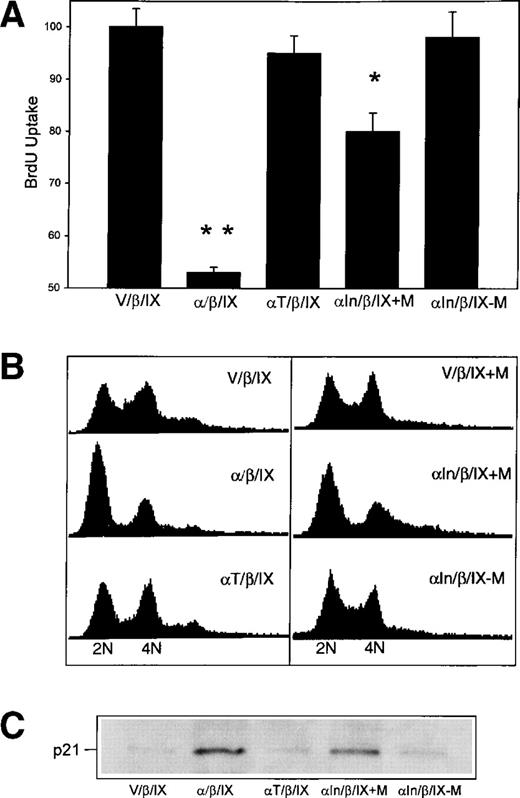
To corroborate the observed effects of stable expression of GpIbα on cell growth, cell number was measured on day 4 after the induced expression of GpIbα with muristerone (M) in CHO αIn/β/IX cells grown in serum on plates coated with vWF.12Figure 3 shows that, 4 days after the induction of GpIbα with muristerone (αIn/β/IX + M in upper part of Fig 3A), the αIn/β/IX + M CHO cells are reduced in number (lower part of Fig 3B). To explore further the specificity of GpIbα in mediating this effect, cell number was measured 4 days after replating in CHO cells transduced with GpIbα truncated at amino acid 540, which eliminates its actin binding protein and 14-3-3 adapter protein interactions.14,15 These interactions are theorized to mediate GpIbα signaling, although there is currently no direct evidence for this. Figure 3A shows that truncated GpIbα (αT/β/IX) is expressed on the surface of transduced cells at levels comparable to the wild-type GpIbα/β/IX shown in Fig 2. Figure 3B shows that the truncation of the cytoplasmic tail of GpIbα completely reverses the growth-inhibitory effect of the wild-type GpIbα/β/IX complex. To confirm that the growth-inhibitory effect of GpIbα is due to its surface expression and not to a nonspecific toxic effect of high-level cytoplasmic expression of a recombinant protein, CHO cells were transiently transfected with pcDNA3.1/Zeo without and with an insert for GpIbα. Cell number was measured after 3 days of growth in culture medium and 2 days after selection in the antibiotic Zeocin (500 μg/mL). Under these conditions, the majority of the translated protein is not surface expressed, but rather is degraded and secreted after processing in the golgi.5 The number of cells was the same 3 and 5 days after transfection with pcDNA3.1/Zeo regardless of whether it carried an insert for GpIbα (data not shown).
The inhibitory effect of GpIb on cell growth is specifically dependent on the expression of GpIb and is mediated by its cytoplasmic domain. Expression of GpIb with muristerone (M) was induced in CHO In/β/IX cells grown on vWF. Four days after the induction of GpIb with muristerone (upper part of [A]), the In/β/IX CHO cells are reduced in number (lower part of [B]). Cell number was also measured 4 days after replating in CHO cells transduced with a truncated GpIb (at aa 540, which eliminates its actin binding protein and 14-3-3 adapter protein interactions). The mutant GpIb is expressed on the cell surface at levels comparable to the wild-type (A), but this truncation eliminates the growth-inhibitory effect of wild-type GpIb. (**P < .0001 compared with V/β/IX; *P < .001 compared with V/β/IX; n = 3.)
The inhibitory effect of GpIb on cell growth is specifically dependent on the expression of GpIb and is mediated by its cytoplasmic domain. Expression of GpIb with muristerone (M) was induced in CHO In/β/IX cells grown on vWF. Four days after the induction of GpIb with muristerone (upper part of [A]), the In/β/IX CHO cells are reduced in number (lower part of [B]). Cell number was also measured 4 days after replating in CHO cells transduced with a truncated GpIb (at aa 540, which eliminates its actin binding protein and 14-3-3 adapter protein interactions). The mutant GpIb is expressed on the cell surface at levels comparable to the wild-type (A), but this truncation eliminates the growth-inhibitory effect of wild-type GpIb. (**P < .0001 compared with V/β/IX; *P < .001 compared with V/β/IX; n = 3.)
GpIbα-mediated growth arrest is partially dependent on extracellular vWF.
To determine the extent to which the GpIbα/β/IX complex regulates CHO cell growth in a ligand-dependent manner, cell number was measured in cells in which serum is replaced by serum-free growth factors. Ristocetin (1.5 mg/mL; which promotes vWF binding to GpIbα in static conditions) was added to the serum-free medium, and cells were grown in either the absence or presence of vWF coating the culture dish (20 μg/mL). Figure 4 shows that there was approximately 50% inhibition of the number of CHOα/β/IX cells 4 days after replating when they were grown on vWF. In the absence of vWF, a decrease in cell number by approximately 35% was observed. Using CHOαIn/β/IX cells treated continuously for 4 days with muristerone, there was an approximately 25% reduction in cell number when the cells were grown on vWF and an approximately 15% reduction in cell number when they were grown in plastic dishes without vWF. Ristocetin alone had no effect on cell growth.
GpIb-mediated growth arrest is partially dependent on extracellular vWF. The relative number of CHO/β/IX cells growing in serum-free medium 4 days after replating or serum-free CHO In/β/IX cells after 4 days of continuous treatments with muristerone is smallest when they are grown on vWF. Ristocetin (15 μg/mL) was added to every culture well. In the absence of vWF, a significant decrease in cell number is still observed in CHO cells expressing GpIb. (**P < .0001 compared with V/β/IX cells growing in serum-free medium; *P < .001 compared with V/β/IX cells growing in serum-free medium; n = 3.)
GpIb-mediated growth arrest is partially dependent on extracellular vWF. The relative number of CHO/β/IX cells growing in serum-free medium 4 days after replating or serum-free CHO In/β/IX cells after 4 days of continuous treatments with muristerone is smallest when they are grown on vWF. Ristocetin (15 μg/mL) was added to every culture well. In the absence of vWF, a significant decrease in cell number is still observed in CHO cells expressing GpIb. (**P < .0001 compared with V/β/IX cells growing in serum-free medium; *P < .001 compared with V/β/IX cells growing in serum-free medium; n = 3.)
The expression of the GpIbα/β/IX complex causes p21-dependent G1 growth arrest.
To begin to identify the mechanism by which the GpIb complex decreases cell proliferation, CHO α/β/IX cells were synchronized to enter the cell cycle by serum deprivation for 24 hours, followed by serum repletion for 20 hours, at which time BrdU incorporation and DNA contentwere determined. The same assays were performed in CHO αIn/β/IX cells growing in serum and treated with muristerone for 20 hours. Figure 5A shows that the expression of GpIbα inhibits BrdU incorporation when transduced cells are grown on vWF and serum depleted/repleted, or induced cells are treated with muristerone (M). Figure 5 also shows that cells expressing truncated GpIbα (at amino acid 540) have normal BrdU uptake after serum-repletion. Figure 5B shows that serum-repleted GpIbα-expressing cells are arrested in G1 (represented by the decreased width and amplitude of the second 4N DNA peak) and that this G1 arrest is eliminated in cells transduced with the truncated cDNA for GpIbα. Figure 5C shows that growth arrest is associated with increased immunoreactive p21, which is not observed in cells that are stable transfectants of the truncated GpIbα/β/IX complex. At the time that GpIbα-dependent G1 arrest is observed, there are no changes in the quantity of immunoreactive cyclins D and E (data not shown).
The expression of GpIb causes p21-dependent G1 growth arrest. CHO /β/IX cells growing on vWF were serum deprived for 24 hours and then serum repleted for 20 hours, at which time BrdU incorporation and DNA content were determined. Identical measurements were made in CHO In/β/IX treated with muristerone. (A) shows that serum repletion of stable transfectants or the induced expression of GpIb inhibits BrdU incorporation and that this is reversed by the truncation of GpIb at amino acid 540 of the cytoplasmic domain. (B) shows that these cells are arrested in G1 and that G1 arrest is eliminated in cells transduced with the truncated cDNA for GpIb. (C) shows associated levels of immunoreactive p21 (n = 2). (**P< .0001 compared with V/β/IX; *P < .001 compared with V/β/IX; n = 3.)
The expression of GpIb causes p21-dependent G1 growth arrest. CHO /β/IX cells growing on vWF were serum deprived for 24 hours and then serum repleted for 20 hours, at which time BrdU incorporation and DNA content were determined. Identical measurements were made in CHO In/β/IX treated with muristerone. (A) shows that serum repletion of stable transfectants or the induced expression of GpIb inhibits BrdU incorporation and that this is reversed by the truncation of GpIb at amino acid 540 of the cytoplasmic domain. (B) shows that these cells are arrested in G1 and that G1 arrest is eliminated in cells transduced with the truncated cDNA for GpIb. (C) shows associated levels of immunoreactive p21 (n = 2). (**P< .0001 compared with V/β/IX; *P < .001 compared with V/β/IX; n = 3.)
GpIbα’s growth-inhibitory effect maps to its 14-3-3 interaction domain.
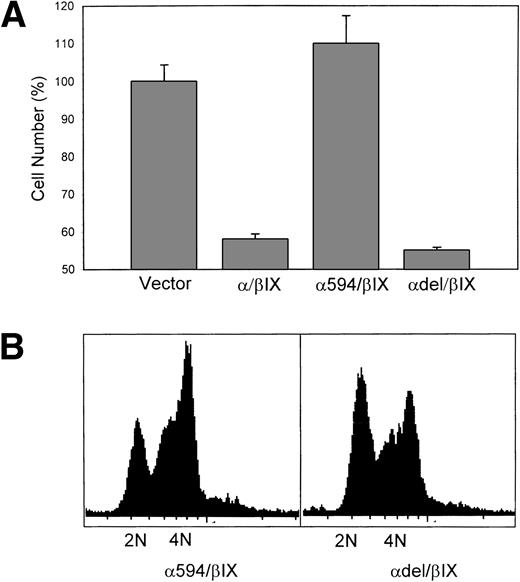
To begin to identify the specific intracellular switch that signals GpIbα-induced CHO cell growth arrest, two additional GpIbα mutations were expressed in CHOβ/IX cells. The first mutation eliminated the 16 C-terminal amino acids (610 through 595) of GpIbα, a region reported to represent the binding site for 14-3-3ζ.15 The second mutation created a deletion of amino acids 541 through 589, eliminating the actin binding domain,16 but preserving the 14-3-3 binding domain of GpIbα. Figure 6A shows that expression of mutant GpIbα with the C-terminal truncation (α594/β/IX) is associated with proliferation comparable to control (empty vector-transfected) CHO cells. In contrast, expression of mutant GpIbα with the actin-binding domain deletion (αdel/β/IX) results in decreased proliferation comparable to that observed in CHO cells transduced with wild-type GpIbα/β/IX. Figure 6B shows that the expression of αdel/β/IX, but not α594/β/IX, is associated with G1 growth arrest.
GpIb’s growth-inhibitory effect maps to its 14-3-3 interaction domain. In (A), cell number was measured 4 days after replating. Truncation of 16 C-terminal amino acids of GpIb (594/β/IX), a region reported to represent the binding site for 14-3-3ζ, restores cell number to control (vector) levels. A second mutation eliminating the actin binding domain, but preserving the terminal 14-3-3 binding domain of GpIb (del/β/IX), shows decreased cell number comparable to CHO/β/IX cells. In (B), propidium-labeled cells were analyzed for DNA content. CHO cells expressing 594/β/IX enter S-phase normally, but those expressing del/β/IX are arrested in G1 (n = 3).
GpIb’s growth-inhibitory effect maps to its 14-3-3 interaction domain. In (A), cell number was measured 4 days after replating. Truncation of 16 C-terminal amino acids of GpIb (594/β/IX), a region reported to represent the binding site for 14-3-3ζ, restores cell number to control (vector) levels. A second mutation eliminating the actin binding domain, but preserving the terminal 14-3-3 binding domain of GpIb (del/β/IX), shows decreased cell number comparable to CHO/β/IX cells. In (B), propidium-labeled cells were analyzed for DNA content. CHO cells expressing 594/β/IX enter S-phase normally, but those expressing del/β/IX are arrested in G1 (n = 3).
GpIbα-mediated growth arrest is followed by apoptosis.
Integrin adhesion receptors are involved in regulating proliferation and programmed cell death,17 and these two responses may be coupled together by p53, which activates both the cyclin-dependent kinase inhibitor p21 and the apoptosis-promoting genetic machinery.4 To examine for apoptosis in CHOα/β/IX cells whose growth is arrested in G1, annexin V binding was measured by flow cytometry 24 and 48 hours after serum repletion. At both time points, there was an increase in the number of GpIbα/β/IX-expressing CHO cells undergoing apoptosis. Figure 7 shows that the α594/β/IX truncation, but not the actin-binding domain deletion (αdel/β/IX), decreases the apoptosis of CHO cells 48 hours after serum-repletion to control levels (vector/β/IX).
GpIb-mediated growth arrest is followed by apoptosis. The Y-axis is the mean fluorescence of FITC-conjugated Annexin V (which binds to phosphatidylserine exposed during apoptosis) and the X-axis is a log scale of cell number. Flow cytometry for Annexin V-binding cells was performed 48 hours after serum repletion. The population of CHO cells expressing /β/IX shows increased annexin V binding in comparison to control cells (vector/β/IX). This is reversed by expressing a mutant complex with a truncation of its 14-3-3 interaction domain (594/β/IX). A mutant with a deleted actin binding protein domain (del/β/IX) continues to show an increased number of apoptotic cells 48 hours after serum-repletion (n = 2).
GpIb-mediated growth arrest is followed by apoptosis. The Y-axis is the mean fluorescence of FITC-conjugated Annexin V (which binds to phosphatidylserine exposed during apoptosis) and the X-axis is a log scale of cell number. Flow cytometry for Annexin V-binding cells was performed 48 hours after serum repletion. The population of CHO cells expressing /β/IX shows increased annexin V binding in comparison to control cells (vector/β/IX). This is reversed by expressing a mutant complex with a truncation of its 14-3-3 interaction domain (594/β/IX). A mutant with a deleted actin binding protein domain (del/β/IX) continues to show an increased number of apoptotic cells 48 hours after serum-repletion (n = 2).
The endogenous GpIbα/β/IX complex regulates the proliferation of human DAMI cells.
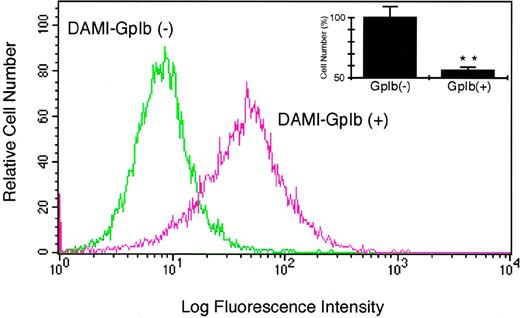
Under routine culture conditions, DAMI cells express very little detectable GpIbα/β/IX. However, immunofluorescent examinations do show rare DAMI cells that stain relatively brightly for GpIbα (data not shown). To develop a system for analyzing the potential significance of endogenous GpIbα/β/IX in megakaryocyte biology, DAMI cells expressing GpIbα were isolated. These cells were affinity-purified with solid-phase vWF and then selected by several rounds of fluorescence-activated cell sorting with the anti-GpIbα antibody AN51. The sorted cells stain almost 10 times brighter than unsorted cells (Fig 8A). The inset in Fig8A shows that the expression of GpIbα is associated with decreased proliferation of DAMI cells 4 days after replating.
Endogenous GpIb regulates the growth of hematopoietic cells. DAMI cells expressing GpIb were first isolated by affinity purification with solid-phase vWF and then selected by repeated rounds of fluorescence-activated cell sorting with the anti-GpIb antibody AN51. After selection, a population of cells designated DAMI GpIb (+) stains brighter with FITC-conjugated AN51. The inset shows that, when cells are counted 4 days after replating, DAMI GpIb (+) cell number is significantly decreased compared with DAMI GpIb (−) cells. (**P < .0001 compared with DAMI cells not expressing GpIb; n = 3.)
Endogenous GpIb regulates the growth of hematopoietic cells. DAMI cells expressing GpIb were first isolated by affinity purification with solid-phase vWF and then selected by repeated rounds of fluorescence-activated cell sorting with the anti-GpIb antibody AN51. After selection, a population of cells designated DAMI GpIb (+) stains brighter with FITC-conjugated AN51. The inset shows that, when cells are counted 4 days after replating, DAMI GpIb (+) cell number is significantly decreased compared with DAMI GpIb (−) cells. (**P < .0001 compared with DAMI cells not expressing GpIb; n = 3.)
DISCUSSION
GpIbα is a lineage-restricted receptor for vWF. It is expressed late in the development of megakaryocytes, and it is fairly densely packed (up to 30,000 molecules per cell) on the surface of platelets released from fully mature megakaryocytes.5 It is also expressed on the surface of some human vascular endothelial cells stimulated by inflammatory cytokines.18 The best-described function for this protein is mediating platelet adhesion to the subendothelial extracellular matrix. Platelet adhesion develops when the extracellular globular domain of GpIbα binds to vWF. vWF may be an insoluble constituent of the subendothelial matrix (thereby effecting direct adhesion of platelets) or it may be soluble in plasma (thereby effecting adhesion indirectly by bridging platelets to extracellular collagen).6,19 Platelet GpIbα also mediates homotypic platelet cohesion (aggregation) and is associated with platelet signaling, including stimulatory calcium and protein kinase responses.20-22
The GpIb/IX complex is expressed late in the ontogeny of megakaryocytes. For example, when CD34(+)/CD41(+) human bone marrow cells are first isolated, less than 20% demonstrate surface expression of GpIbα, but after 4 days of growth in a medium containing thrombopoietin, approximately 90% of the cells express GpIbα.23 This expression coincides with increasing polyploidization of these progenitor cells associated with p21-dependent cell cycle arrest.10 These cellular changes appear to precede terminal megakaryocyte differentiation, resulting in cellular apoptosis associated with platelet production.24,25 The genetic program that regulates the final phase of megakaryocyte maturation appears to be activated independent of exogenous growth factors.11
Experiments presented in this report demonstrate that recombinant GpIbα decreases the proliferation of CHO cells and that the decreased proliferation is associated with the induction of the cyclin-dependent kinase inhibitor p21 and G1 cell cycle arrest. G1 arrest induced by recombinant GpIbα in transduced CHO cells requires signaling through the 14-3-3ζ interaction domain of GpIbα and is partially dependent on extracellular vWF. G1 cell cycle arrest is followed by cellular apoptosis.
Taken together, the data suggest that the expression of GpIbα during the maturation of hematopoietic stem cells into megakaryocytes may be an important growth-regulating event. Megakaryocytopoiesis requires a convergence of multiple factors that function in a time- and dose-dependent manner to drive a multilineage program towards a single lineage commitment. The expression of GpIbα during the later stage of megakaryocytopoiesis may be one factor, of a combination of many, that regulates cell growth by slowing cell proliferation. Such a hypothesis is consistent with clinical observations of humans with congenital deficiencies of platelet GpIbα. Patients with this bleeding disorder (Bernard-Soulier syndrome) have thrombocytopenia, with the majority of circulating platelets enlarged and dysfunctional.26,27 Some recent investigations of the bone marrow from patients with Bernard-Soulier syndrome provide ambiguous data about the possible function of the GpIb/IX complex in regulating megakaryocytopoiesis. For example, Hourdillé et al28 and Nurden and Nurden29 demonstrate abnormal morphology of such megakaryocytes and suggest that this represents qualitatively abnormal maturation, whereas Tomer et al30 observed a normal number of megakaryocytes with normal morphology. Further studies should help to determine if human progenitor cells require GpIbα expression to complete normal maturation into megakaryocytes capable of producing platelets.
ACKNOWLEDGMENT
The authors thank Drs Michael Berndt, Bruce Ewenstein, JoséLópez, Andrew Schafer, and Sandy Shattil for helpful discussions.
Supported by the Research Service of the Department of Veterans Affairs and the National Heart, Lung and Blood Institute (HL 18584). This work was performed by M.H.K. during the tenure of an Established Investigator Award from the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael H. Kroll, MD, Section of Hematology-Oncology (111H), VA Medical Center, 2002 Holcombe Blvd, Houston, TX 77030; e-mail: mkroll@bcm.tmc.edu.

![Fig. 3. The inhibitory effect of GpIb on cell growth is specifically dependent on the expression of GpIb and is mediated by its cytoplasmic domain. Expression of GpIb with muristerone (M) was induced in CHO In/β/IX cells grown on vWF. Four days after the induction of GpIb with muristerone (upper part of [A]), the In/β/IX CHO cells are reduced in number (lower part of [B]). Cell number was also measured 4 days after replating in CHO cells transduced with a truncated GpIb (at aa 540, which eliminates its actin binding protein and 14-3-3 adapter protein interactions). The mutant GpIb is expressed on the cell surface at levels comparable to the wild-type (A), but this truncation eliminates the growth-inhibitory effect of wild-type GpIb. (**P < .0001 compared with V/β/IX; *P < .001 compared with V/β/IX; n = 3.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/12/10.1182_blood.v93.12.4256/4/m_blod412fen03z.jpeg?Expires=1763519664&Signature=rWfpn9liYB0Yj2969zD9kbxavxJDmA6gfDXuAnfqpt2lBzVmaRJgpty0eTLmWM6ZT1jF2flcfIj~6KOdUSLMs~GID7qlJOOYceDgQVdvy7hLYglcconKmgix-lsCdH97TGskfaT8hHamwrq4S5mFrP8dOD0FnDGD3UqFxjMLJk4N~dNSxHQ84Oe8DuNlIdkuNJMXkBQTNorZzacqTePFVPn98q1vQPZE02WKcnIzo0JjvEVvS11HlibIXJdIB0a3dDUgGshkjL8goG4t8Oh2y~-CZn7INNxsuTofEhVWq5iyRFIwi4y5FF19Q8dwsJoxzoL-5wJWoFxtynjopt9sHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal