Abstract
The standard enzyme-linked immunosorbent assay (ELISA) for anticardiolipin antibodies (ACA) detects a heterogenous group of antibodies against cardiolipin on its own, β2-glycoprotein I (β2GPI), and, potentially, other phospholipid-binding plasma proteins from bovine or human origin. In an attempt to identify new proteic targets of ACA, we selected 6 patients who possessed cofactor-dependent ACA but no antibody to human or bovine β2GPI detectable in the β2GPI-ELISA. Three of these samples proved to recognize β2GPI in combination with cardiolipin, but not β2GPI directly immobilized on γ-irradiated polystyrene or agarose beads. In the other cases, the component required for ACA binding was purified from adult bovine serum or plasma by means of ammonium sulfate precipitation and chromatography on Phenyl-Sepharose, diethyl aminoethyl (DEAE)-cellulose, heparin-Ultrogel, and Sephacryl S-300 columns. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis coupled to N-terminal amino acid microsequencing identified the cofactors of patients no. 4, 5, and 6 ACA as lipopolysaccharide binding protein (LBP), complement C4b-binding protein (C4BP), and the thrombin-antithrombin (AT) complex, respectively. Adsorption of each of these cofactor preparations with cardiolipin liposomes led to suppression of ACA reactivity, concomitant with the loss of bands from SDS gels corresponding to sequenced material. Bacterial lipopolysaccharide (which forms high-affinity complexes with LBP) specifically neutralized the cofactor activity of the LBP preparation in a concentration-dependent manner. Bovine serum and plasma, as well as the C4BP preparation, optimally supported the binding of a rabbit anti-C4BP antiserum to immobilized cardiolipin. The binding of a rabbit anti-AT antiserum to solid-phase cardiolipin was sustained by the thrombin-AT preparation and bovine serum, but neither by bovine plasma nor by native AT, thus reproducing the behavior of patient no. 6 ACA. Taking advantage of the restricted recognition by the latter ACA of a cofactor from bovine origin appearing upon clotting, we studied the generation of such activity in human plasma supplemented with bovine AT or bovine prothrombin before clotting. In these conditions, patient no. 6 antibody binding to cardiolipin required the addition of bovine AT, whereas addition of bovine prothrombin alone was ineffective. We therefore concluded that those ACA targeted bovine AT once it has been modified/cleaved by thrombin. These findings underline the wide heterogeneity of ACA and the links that may exist between various coagulation pathways, inflammation and the complement system.
ANTIPHOSPHOLIPID antibodies (aPL) consist of a heterogenous group of autoantibodies that have different specificities for phospholipids and phospholipid-binding plasma proteins, especially β2-glycoprotein I (β2GPI), and prothrombin for those antibodies detectable in standard anticardiolipin (ACA) and lupus anticoagulant (LA) assays.1 So as to improve the reliability of ACA detection by enzyme-linked immunosorbent assay (ELISA) using solid-phase cardiolipin, the addition of 10% animal (usually bovine) serum or plasma in the blocking buffer/patient sample diluent has long been recommended. More recently, the serum component required was found to be β2GPI,2,3 and its addition was reported to either enhance ACA binding (samples from autoimmune patients) or depress it through a competition mechanism (cases of infection-associated true ACA). The identification of the antigenic target of most ACA associated with the antiphospholipid syndrome (APS) also led to the development of a more straightforward and specific assay for anti-β2GPI antibodies that uses purified β2GPI in the absence of phospholipids, the β2GPI-ELISA.4
In addition to detecting antibodies to β2GPI and to cardiolipin proper, the standard ACA assay could potentially detect antibodies to other bovine or human serum proteins that bind to cardiolipin-coated wells independently of calcium.1 5 For further insight into the spectrum of ACA specificities, 6 patients were selected whose antibodies showed an absolute serum requirement in the ACA assay but were not detectable in the β2GPI-ELISA using the human and bovine proteins. Three of these antibodies proved to recognize the β2GPI-cardiolipin complex, involving human (patient no. 1) or bovine β2GPI (patients no. 2 and 3). In the 3 other cases (patients no. 4, 5, and 6), a series of experiments was conducted to characterize the component responsible for ACA cofactor activity leading to the identification of lipopolysaccharide binding protein (LBP), complement C4b-binding protein (C4BP), and thrombin-modified antithrombin (AT), respectively. These results demonstrate for the first time that a variety of phospholipid binding proteins in addition to β2GPI are involved in epitope formation of some ACA. Also, the finding of new ACA cofactors offers a broader view of the so-called aPL, establishing links between various coagulation pathways, inflammation, and the complement system.
MATERIALS AND METHODS
Patients.
Six female patients were selected on the basis of the presence of serum-dependent ACA in the absence of antibodies to human or bovine β2GPI detectable in the β2GPI-ELISA. Pertinent clinical and laboratory findings of these patients are summarized in Table 1. None fulfilled the classification criteria for definite antiphospholipid syndrome, either primary or secondary to systemic lupus erythematosus. Indeed, patient no. 1 (88 years of age) presented other well-established risk factors for ischemic stroke, including hypertension and prosthetic heart valve, in addition to the presence of ACA. Also, the two pregnancy losses (1 fetal loss and 1 neonatal death) in patient no. 3 (now 59 years of age) were not consecutive (2 uncomplicated pregnancies in between) and occurred long before the search of aPL.
Clinical and Laboratory Features of Patients
| Patients . | Diagnosis . | Autoantibodies (titer) . | LA . | ACA . | |||
|---|---|---|---|---|---|---|---|
| No. . | Age/Sex . | GPL . | MPL . | Cofactor . | |||
| 1 | 88/F | Stroke | No | No | 67 | <15 | Human β2GPI |
| 2 | 28/F | Thrombocytopenia | No | No | 85 | <15 | Bovine β2GPI |
| Migraine | |||||||
| 3 | 59/F | Two fetal losses | ANA (1:100) | No | 100 | <15 | Bovine β2GPI |
| 4 | 66/F | Thrombocytopenia | ANA (1:100) | No | 126 | 25 | LBP |
| Elevated liver enzymes | APCA (1:20) | ||||||
| 5 | 34/F | Seizures (hysteria?) | No | No | 54 | <15 | C4BP |
| 6 | 73/F | Polymyalgia rheumatica | APCA (1:20) | No | 140 | <15 | Modified AT |
| Patients . | Diagnosis . | Autoantibodies (titer) . | LA . | ACA . | |||
|---|---|---|---|---|---|---|---|
| No. . | Age/Sex . | GPL . | MPL . | Cofactor . | |||
| 1 | 88/F | Stroke | No | No | 67 | <15 | Human β2GPI |
| 2 | 28/F | Thrombocytopenia | No | No | 85 | <15 | Bovine β2GPI |
| Migraine | |||||||
| 3 | 59/F | Two fetal losses | ANA (1:100) | No | 100 | <15 | Bovine β2GPI |
| 4 | 66/F | Thrombocytopenia | ANA (1:100) | No | 126 | 25 | LBP |
| Elevated liver enzymes | APCA (1:20) | ||||||
| 5 | 34/F | Seizures (hysteria?) | No | No | 54 | <15 | C4BP |
| 6 | 73/F | Polymyalgia rheumatica | APCA (1:20) | No | 140 | <15 | Modified AT |
Abbreviations: ANA, antinuclear antibodies; APCA, antiparietal cell antibodies.
ELISAs for ACA and anti-β2GPI antibodies.
The standard ELISA for ACA was performed using cardiolipin- or methanol (sample blank)-coated PolySorp plates (Nunc, Roskilde, Denmark) and 10% adult bovine serum (ABS) or adult bovine plasma (ABP) in phosphate-buffered saline (PBS) for saturation and patient serum dilution (1:100), essentially as described.4 The cut-off points for positivity were set at 15 GPL and 15 MPL using eight standards (from the Antiphospholipid Standardization Laboratory, University of Louisville, Louisville, KY) and after testing 100 blood donors as controls (96th percentiles).
To investigate the role of cofactors in ACA binding and monitor their purification, the standard assay was modified by replacing bovine serum or plasma by 0.6% gelatin (from cold water fish skin; Sigma, St Louis, MO) as blocking agent and sample diluent. The patients’ antibody binding step was performed with PBS/gelatin alone or containing one of the following: bovine or human β2GPI (10 μg/mL) purified from plasma,4 crude ABS or ABP (10%, unless otherwise specified), and column fractions being analyzed for ACA cofactor activity. The lysine residues in β2GPI were modified by carbamylation, resulting in complete loss of binding to anionic phospholipids.6
The ELISAs for anti-β2GPI antibodies were performed using purified human and bovine β2GPI, γ-irradiated polystyrene plates (Maxisorp; Nunc), and PBS/0.1% Tween buffer, as previously described.4
Purification of the ACA cofactors.
The ACA cofactors were purified from ABP and ABS by ammonium sulfate precipitation (25% saturation), followed by hydrophobic chromatography of the supernatant on a Phenyl-Sepharose column equilibrated in PBS 25% saturated with ammonium sulfate, pH 7.2. The column was eluted by a linear gradient of decreasing ammonium sulfate concentration and simultaneously increasing ethylene glycol concentration (final concentrations, 0% and 60%, respectively). Fractions containing ACA cofactor activity were pooled, dialysed against 50 mmol/L Tris-HCl, pH 8, and then applied to a diethyl aminoethyl (DEAE)-cellulose column run in this buffer. Adsorbed material was eluted by a linear gradient from 0 to 0.3 mol/L NaCl in 50 mmol/L Tris-HCl, pH 8. Again, fractions were pooled according to ACA cofactor activity. The individual pools were adjusted to the desired ionic strength (0.125 mol/L NaCl) with distilled water or NaCl and applied to a heparin-Ultrogel column (IBF, Villeneuve-la-Garenne, France) washed in PBS, and bound cofactors were recovered with a linear gradient from 0.125 to 0.5 mol/L NaCl. After concentration on a PM-10 membrane, the material was further separated by chromatography over Sephacryl S-300 equilibrated with PBS, and the fractions were pooled as described before.
Protein concentrations were determined by using the BCA (Pierce, Rockford, IL) reagents and BSA as standard.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and amino acid sequencing.
SDS-PAGE was performed in 7.5% homogenous resolving gels and 3% stacking gels. Samples were boiled for 2 minutes in 2% (wt/vol) SDS containing 0.1 mol/L Tris/HCl, pH 6.8, 20% (vol/vol) glycerol, 0.2% (wt/vol) bromophenol blue. Reduction of samples was performed in the presence of 1% (wt/vol) dithiothreitol. Gels were stained with Coomassie blue R250.
The N-terminal amino acid sequence analysis of proteins separated by SDS-PAGE was performed on a Procise 492A sequencer (Applied Biosystems, Foster City, CA) according to the manufacturer’s recommendations.
Inhibition experiments.
Cardiolipin liposomes were prepared as a stock 2.5 mg/mL suspension by vortexing dried cardiolipin in PBS. Varying amounts of liposomes were incubated with crude ABS or ABP (1:5 dilution in PBS) or with the purified cofactors (5 to 50 μg/mL) for 30 minutes at 20°C. After centrifugation at 20,000g for 20 minutes, the supernatants were assayed for residual cofactor activity in the modified ACA ELISA.
Fluid-phase lipopolysaccharide (LPS), obtained from Escherichia coli K12 D31m4 (gift of S. Chesne, INSERM U238, Grenoble, France), was studied as an inhibitor of ACA binding by premixing serial dilutions with crude ABS or ABP or the cofactor under study (5 to 50 μg/mL) for 10 minutes before adding this mixture together with patient serum to cardiolipin-coated, gelatin-blocked wells.
Other methods.
IgG depletion of human serum and citrated plasma pools from healthy blood donors was performed by passage through a protein G-Sepharose (Pharmacia, Uppsala, Sweden) column.
The ability of crude ABS, ABP, or the appropriate purified cofactors to support the binding to cardiolipin of polyclonal rabbit antisera raised against human C4BP (gift of M.B. Villiers, INSERM U238, Grenoble, France) and human AT (Diagnostica Stago, Asnières, France) but cross-reacting with their bovine counterparts was studied by substituting each antibody for patient serum in the modified ACA ELISA. Bound antibodies were detected by the use of peroxidase-conjugated goat antirabbit Ig (Bio-Rad, Hercules, CA).
The dilute Russell viper venom time (dRVVT) of human citrated plasma spiked with bovine AT (Diagnostica Stago) and/or bovine prothrombin (Kordia, Leiden, The Netherlands) was performed using Diagnostica Stago reagents for Stypven-cephalin clotting time.
RESULTS
Binding of patient antibodies in the modified ACA assay.
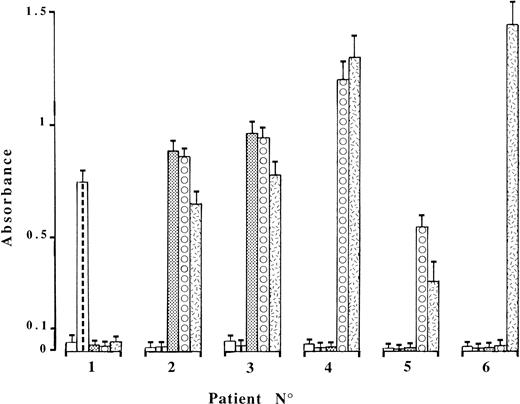
Six patients who possessed serum- and/or plasma-dependent ACA but no antibody to human or bovine β2GPI directly immobilized on γ-irradiated plates (β2GPI-ELISA) were selected for further study. Binding was little changed by replacing cardiolipin with phosphatidylserine or phosphatidylglycerol, whereas substitution by the neutral phospholipid, phosphatidylethanolamine, suppressed antibody binding from all six patient sera. In the modified ACA ELISA using gelatin buffer alone (Fig 1), no antibody binding was observed when serum from patients no. 2 through 6 was diluted 1:100. A higher serum dilution (1:600 or greater) was required for patient no. 1 so as to avoid the influence of the endogenous cofactor. Optimal ACA binding was ensured in this assay by adding 10 μg/mL purified β2GPI of human (patient no. 1) or bovine (patients no. 2 and 3) origin, and a concentration-dependent effect was observed at lower β2GPI concentrations. Increasing fluid-phase concentrations of β2GPI (human or bovine, as appropriate) up to 1.5 mg/mL had no inhibitory effect on patients no. 1, 2, and 3 antibody binding. If the lysine residues in β2GPI were first modified by carbamylation to suppress its phospholipid-binding capability, ACA binding was abolished (not shown). Furthermore, the antibodies from these 3 patients were not retained at all on affinity columns using β2GPI (human or bovine, as appropriate) covalently linked to agarose beads, contrasting with the usual behavior of anti-β2GPI antibodies.7 As a whole, these results indicate that solid-phase presentation of β2GPI on anionic phospholipids, but neither on γ-irradiated polystyrene and agarose beads nor in the fluid-phase, is required to induce epitope formation for ACA from patients no. 1, 2, and 3.
IgG binding in the modified ACA ELISA of the patient sera, diluted 1:600 (no. 1) or 1:100 (others). The antibody binding step was performed in the presence of gelatin buffer (□), 10 μg/mL purified β2GPI of human ( ) or bovine (▩) origin, or 10% bovine plasma (⧇) or serum (). The means ± SD of specific absorbance values from three independent experiments are shown.
) or bovine (▩) origin, or 10% bovine plasma (⧇) or serum (). The means ± SD of specific absorbance values from three independent experiments are shown.
IgG binding in the modified ACA ELISA of the patient sera, diluted 1:600 (no. 1) or 1:100 (others). The antibody binding step was performed in the presence of gelatin buffer (□), 10 μg/mL purified β2GPI of human ( ) or bovine (▩) origin, or 10% bovine plasma (⧇) or serum (). The means ± SD of specific absorbance values from three independent experiments are shown.
) or bovine (▩) origin, or 10% bovine plasma (⧇) or serum (). The means ± SD of specific absorbance values from three independent experiments are shown.
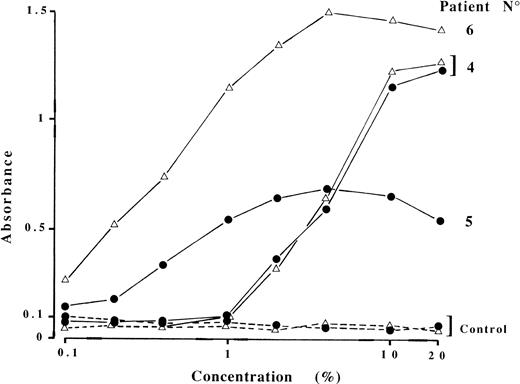
In the 3 other cases, a cofactor distinct from β2GPI was provided by ABS only (patient no. 6) or both ABS and ABP (patients no. 4 and 5; Fig 1). Antibodies from patient no. 4 were not species specific, because ABS or ABP could be replaced by IgG-free 10% human serum or plasma. By contrast, antibody recognition was restricted to bovine cofactors for patients no. 5 and 6. Figure 2 shows the absorbance in the modified ACA ELISA as a function of ABP or ABS concentrations. The requirement for relatively high (∼5%) ABP or ABS concentrations to sustain half-maximal binding of ACA from patient no. 4, in comparison with the two others, may indicate less abundant cofactor protein concentration. It also explains why the contribution to ACA binding made by endogenous cofactor was negligible at a 1:100 dilution of patient serum. Finally, the interaction between involved cofactors and cardiolipin appears to be independent of calcium ions, because the addition of EDTA or CaCl2 (5 mmol/L each) to the assay buffers did not change the results.
Bovine plasma or serum requirement for IgG ACA binding. Sera from patients no. 4, 5, and 6 and a negative control (dashed lines) were diluted 1:100 in PBS/gelatin containing different concentrations of bovine plasma (•) or serum (▵), and ACA reactivity was measured. Results are from a representative experiment.
Bovine plasma or serum requirement for IgG ACA binding. Sera from patients no. 4, 5, and 6 and a negative control (dashed lines) were diluted 1:100 in PBS/gelatin containing different concentrations of bovine plasma (•) or serum (▵), and ACA reactivity was measured. Results are from a representative experiment.
Purification and identification of ACA cofactors of patients no. 4, 5, and 6.
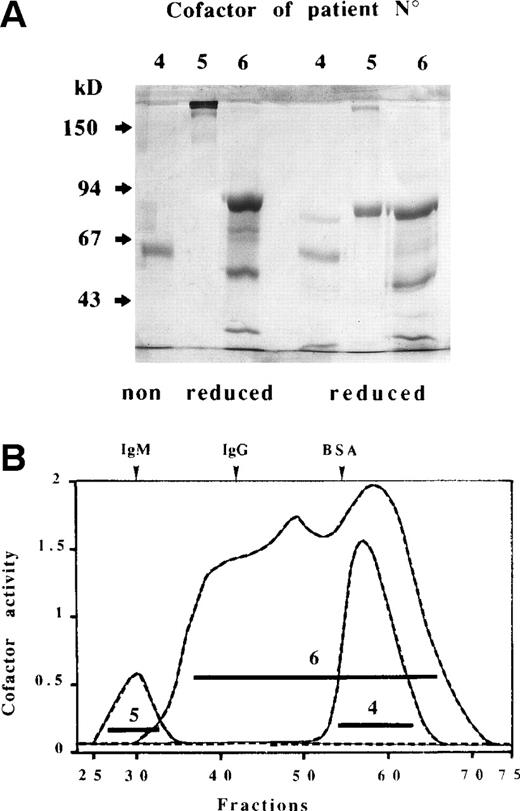
These cofactors were purified from ABS for patient no. 6 and from ABP for the 2 other patients using ammonium sulfate precipitation followed by four successive chromatographic steps. Both cofactors no. 4 and 5 were recovered in the eluant of the Phenyl-Sepharose column around 40% ethylene glycol, whereas no. 6 cofactor was detected in the fall-through fraction. The cofactors were totally retained on the next two columns (DEAE-cellulose and heparin-Ultrogel) and were eluted at different salt concentrations, eg, from the latter column 0.25, 0.3, and 0.45 mol/L NaCl for cofactors no. 4, 5, and 6, respectively. The concentrated heparin column eluents were applied to a Sephacryl S-300 gel-filtration column and the resultant fractions were submitted to electrophoretic separation on SDS-polyacrylamide gels (Fig 3A) and pooled according to cofactor activity (Fig 3B). The S-300 column yielded a coincident protein (A280) and activity peak that eluted at the estimated molecular weight (Mr) of 61 kD and close to the void volume for cofactor preparations no. 4 and 5, respectively. The latter high-Mr material (>200 kD on SDS-PAGE under nonreducing conditions) showed an approximate 80-kD band upon reduction. Seventeen N-terminal amino acids were identified from this 80-kD band and a sequence alignment search showed 100% correspondence with the N-terminus of the chain of bovine C4BP (Table 2). N-terminal microsequencing of the no. 4 cofactor preparation was determined on the 61-kD band from a nonreduced SDS gel and yielded two sequences. One of them closely matched the N-terminus of human LBP8 (bovine LBP was absent from current data base releases) with 8 identities among the 9 positions identified, whereas the second sequence, deduced, could not be assigned (Table 2). The S-300 elution profile displayed by no. 6 cofactor preparation was at first puzzling, in that cofactor activity emerged shortly after the void volume and then trailed to culminate around 60 kD. Furthermore, individual fractions spanning this broad activity peak exhibited essentially the same pattern on SDS gels. This pattern comprised, in addition to faint bands in the region of 60 to 71 kD, three prominent bands at 86, 54, and 36 kD under nonreducing conditions that migrated slightly faster after reduction at 80, 50 (closely spaced doublet), and 33 kD, respectively (Fig 3). It was established by N-terminal sequencing, successfully performed on the three dominant bands (reduced), that bovine AT and thrombin B chain comigrated in the higher 80-kD band, whereas free AT and thrombin B chain accounted for the 50- and 33-kD bands, respectively (Table 2). These findings have to be taken in context with the known mechanism of inhibition of serine proteinases (such as thrombin) by AT. This involves two steps, an initial weak association in a reversible Michaelis-type complex followed by the formation of a highly stable bond between the reactive center of AT and the active site of thrombin located on the B chain.9,10 It has also been demonstrated that the final products of thrombin inhibition during blood clotting are ternary complexes together with vitronectin, an adhesion glycoprotein of 65 to 75 kD in human plasma.11 12 In this respect, the faint bands observed around 60 to 71 kD on nonreduced and reduced SDS gels may testify to the presence of vitronectin in highMr thrombin-AT complexes from the S-300 column, the dissociation of which occurred in the presence of SDS.
SDS-PAGE analysis (A) of purified ACA cofactors of patients no. 4, 5, and 6 recovered after Sephacryl S-300 chromatography (B). The three effluents from this column were monitored for protein content and cofactor activity as described in Materials and Methods. Fractions were pooled as indicated by the horizontal bars. The elution profile of plasma proteins is depicted at top of (B). Electrophoresis was performed in 7.5% nonreducing and reducing polyacrylamide gels and then stained with Coomassie blue. Arrows indicate molecular weight markers.
SDS-PAGE analysis (A) of purified ACA cofactors of patients no. 4, 5, and 6 recovered after Sephacryl S-300 chromatography (B). The three effluents from this column were monitored for protein content and cofactor activity as described in Materials and Methods. Fractions were pooled as indicated by the horizontal bars. The elution profile of plasma proteins is depicted at top of (B). Electrophoresis was performed in 7.5% nonreducing and reducing polyacrylamide gels and then stained with Coomassie blue. Arrows indicate molecular weight markers.
N-Terminal Amino Acid Sequence Analysis of the Purified ACA Cofactors
| Molecular Weight (initial yield) . | Sequence Determined* (position−) . | Sequence Expected (protein identity and species) . | Accession (SWISS-PROT) . |
|---|---|---|---|
| Patient no. 4 | |||
| 61 kD (5 pmol) | 26-ANPGLV[VS]RI | ANPGLVARI (human LBP) | P18428 |
| 61 kD (5 pmol) | SALYSS[VS]DP | Unassigned | |
| Patient no. 5 | |||
| 80 kD (7 pmol) | 49-SCGIPPYLDFAFPINEL | SCGIPPYLDFAFPINEL (bovine C4BP α chain) | Q28065 |
| Patient no. 6 | |||
| 80 kD (10 pmol) | 1-HRSPVED | HRSPVED (bovine antithrombin) | P41361 |
| 80 kD (10 pmol) | 367-IVEGQDA† | IVEGQDA (bovine thrombin B chain) | P00735 |
| 50 kD (5 pmol) | 1-HRSPVED | HRSPVED (bovine antithrombin) | P41361 |
| 33 kD (7 pmol) | 367-IVEGQDA† | IVEGQDA (bovine thrombin B chain) | P00735 |
| Molecular Weight (initial yield) . | Sequence Determined* (position−) . | Sequence Expected (protein identity and species) . | Accession (SWISS-PROT) . |
|---|---|---|---|
| Patient no. 4 | |||
| 61 kD (5 pmol) | 26-ANPGLV[VS]RI | ANPGLVARI (human LBP) | P18428 |
| 61 kD (5 pmol) | SALYSS[VS]DP | Unassigned | |
| Patient no. 5 | |||
| 80 kD (7 pmol) | 49-SCGIPPYLDFAFPINEL | SCGIPPYLDFAFPINEL (bovine C4BP α chain) | Q28065 |
| Patient no. 6 | |||
| 80 kD (10 pmol) | 1-HRSPVED | HRSPVED (bovine antithrombin) | P41361 |
| 80 kD (10 pmol) | 367-IVEGQDA† | IVEGQDA (bovine thrombin B chain) | P00735 |
| 50 kD (5 pmol) | 1-HRSPVED | HRSPVED (bovine antithrombin) | P41361 |
| 33 kD (7 pmol) | 367-IVEGQDA† | IVEGQDA (bovine thrombin B chain) | P00735 |
Position including the putative leader sequence.
Numbering based on the prothrombin sequence.
Target recognition of ACA of patients no. 4, 5, and 6.
Further evidence for the involvement of those identified cofactors in patient antibody binding to cardiolipin was obtained by inhibition experiments and by using polyclonal antibodies (when available) against the above-noted cofactor proteins as probes in the modified ACA ELISA. It appears from Fig 4A that absorption of purified cofactors of patients no. 4, 5, and 6 with cardiolipin liposomes led to suppression of ACA reactivity. Concomitantly, the loss of bands from SDS gels corresponding to sequenced material was noticed. Because LPS forms high-affinity complexes with LBP in acute-phase serum and in solution when purified LBP and LPS are mixed,13purified LPS was preincubated with cofactor preparations to exclude the possibility that the unidentified contaminant of LBP was actually responsible for cofactor activity of patient no. 4 ACA. LPS specifically neutralized the cofactor activity of the LBP preparation with dose-response effect (Fig 4B). For both experimental designs (using cardiolipin liposomes and LPS as inhibitors), similar results were obtained when using ABS or ABP as a source of cofactors (not shown). Patient no. 3 antibodies, specific for bovine β2GPI complexed with cardiolipin, served as control in these experiments. The purified no. 5 cofactor (bovine C4BP), ABS, and ABP supported equally well the binding of a rabbit anti-C4BP antiserum to immobilized cardiolipin (not shown). The behavior of a rabbit anti-AT antiserum resembled that shown in Fig 1 for patient no. 6 ACA, as judged by the absorbances (mean ± SD) in the modified ACA ELISA with this reagent together with buffer (0.138 ± 0.034), ABP (0.161 ± 0.067), ABS (1.603 ± 0.102), no. 6 cofactor preparation (1.854 ± 0.176), and commercially prepared bovine AT (0.149 ± 0.076).
Inhibition of patient antibody binding to immobilized cardiolipin by preincubation of the corresponding purified cofactor (10 μg/mL) with increasing concentrations of cardiolipin containing liposomes (A) or fluid-phase LPS (B). Results are from a representative experiment. Patients no. 3 (□), 4 (○), 5 (•), and 6 (▵).
Inhibition of patient antibody binding to immobilized cardiolipin by preincubation of the corresponding purified cofactor (10 μg/mL) with increasing concentrations of cardiolipin containing liposomes (A) or fluid-phase LPS (B). Results are from a representative experiment. Patients no. 3 (□), 4 (○), 5 (•), and 6 (▵).
We also investigated which component of the thrombin-AT complex was targeted by antibodies of patient no. 6. To this end, we took advantage of the restricted recognition by those ACA of a cofactor from bovine origin that is generated upon clotting. A coagulation assay (dRVVT) of human plasma spiked with physiological concentrations of bovine AT and/or bovine prothrombin was thus performed, and the resultant serum was assayed for cofactor activity. In these conditions, patient no. 6 ACA bound only when supplementing human plasma with bovine AT or bovine AT plus bovine prothrombin, but not bovine prothrombin alone (mean absorbance after addition of the following: AT, 0.820; AT plus prothrombin, 1.358; prothrombin, 0.104; none, 0.113). We concluded that ACA from this patient recognized bovine AT, but only when it had been modified by thrombin.
Finally, the purified ACA cofactors were used as coating antigens for the development of direct ELISAs, ie, in the absence of cardiolipin (Table 3). Whatever the combination of plate and assay buffer chosen, the serum from patients no. 4 and 5 did not react with their respective cofactor in such an ELISA system. To the contrary, specific binding was observed between patient no. 6 ACA and the corresponding purified preparation and was optimal when using irradiated polystyrene plates and Tween-containing buffer as the blocking agent and sample diluent. The commercial preparation of bovine AT did not serve as an antigen for patient no. 6 antibodies, unless it was preincubated with purified thrombin before coating (not shown).
Reactivity of Sera From Patients No. 4, 5, and 6 Towards Their Respective Purified Cofactors, Directly Immobilized on ELISA Plates Under Various Experimental Conditions
| Plate/Buffer3-150 . | Patient No. . | Control3-151 . | ||
|---|---|---|---|---|
| 4 . | 5 . | 6 . | ||
| MaxiSorp/ Tween | 0.202 ± 0.070 | 0.178 ± 0.056 | 2.482 ± 0.168 | 0.158 ± 0.088 |
| MaxiSorp/ Gelatin | 0.256 ± 0.092 | 0.176 ± 0.042 | 1.911 ± 0.124 | 0.184 ± 0.073 |
| PolySorp/ Tween | 0.153 ± 0.042 | 0.183 ± 0.089 | 0.986 ± 0.097 | 0.190 ± 0.071 |
| PolySorp/ Gelatin | 0.215 ± 0.036 | 0.204 ± 0.075 | 1.693 ± 0.157 | 0.212 ± 0.093 |
| Plate/Buffer3-150 . | Patient No. . | Control3-151 . | ||
|---|---|---|---|---|
| 4 . | 5 . | 6 . | ||
| MaxiSorp/ Tween | 0.202 ± 0.070 | 0.178 ± 0.056 | 2.482 ± 0.168 | 0.158 ± 0.088 |
| MaxiSorp/ Gelatin | 0.256 ± 0.092 | 0.176 ± 0.042 | 1.911 ± 0.124 | 0.184 ± 0.073 |
| PolySorp/ Tween | 0.153 ± 0.042 | 0.183 ± 0.089 | 0.986 ± 0.097 | 0.190 ± 0.071 |
| PolySorp/ Gelatin | 0.215 ± 0.036 | 0.204 ± 0.075 | 1.693 ± 0.157 | 0.212 ± 0.093 |
Specific absorbance values (after sample blank subtraction) are shown.
PBS buffer containing 0.1% Tween or 0.6% gelatin to block plates and dilute samples.
Control denotes a pool of normal human sera reacted onto no. 6 cofactor; its reactivity towards the other purified preparations was essentially the same.
DISCUSSION
Since the widespread recognition of the APS, aPL continue to be scrutinized, with their very definition and significance being questioned. An important step has been the recognition of β2GPI as the antigenic target of most ACA associated with a thrombotic diathesis.2,3 In the present study, we have focused on a small series of patients with diverse clinical conditions whose antibodies exhibited a rare pattern, characterized by an absolute plasma and/or serum requirement in the standard ACA assay but no reactivity to human or bovine β2GPI directly immobilized on γ-irradiated plates. Despite such negative results in the β2GPI-ELISA, 3 of 6 patient antibodies actually targeted β2GPI but only when presented on a cardiolipin-coated surface. Most anti-β2GPI antibodies are able to react with β2GPI when the protein is immobilized on certain surfaces represented by anionic phospholipids, γ-irradiated polystyrene, or even agarose beads.1,5,7 Two hypotheses have been proposed to account for those reactivities: (1) antibody recognition of neo-epitopes exposed when β2GPI interacts with a suitable surface; and (2) alternatively, low intrinsic affinity of the antibodies thus requiring high density or clustering of β2GPI to allow their bivalent attachment.1The latter possibility is strongly supported by the recent demonstration, using various experimental approaches,14-16that more β2GPI binds to phospholipid surfaces in the presence of bivalent (but not monovalent) anti-β2GPI antibodies, the affinity of which increases upon dimerization of β2GPI.17 Another reason why patients no. 1, 2, and 3 antibody binding requires both components of the β2GPI-cardiolipin complex may stem from the report by Hörkkö et al18 that the neo-epitopes for some aPL are covalent adducts formed between breakdown products of oxidized phospholipid and associated proteins such as β2GPI. However, the hypothesis that our patient sera would be directed at such oxidation-dependent structures appears unlikely, because it is difficult to reconcile with the restricted species specificity of their antibodies (Fig 1). It is likely that there is much more heterogeneity among anti-β2GPI antibodies, in particular with respect to species and epitope specificities, than previously thought.7 An observation similar to ours has been made in the case of most antiphosphatidylethanolamine antibodies shown to react with high or low molecular weight kininogens or kininogen-binding proteins (eg, factor XI or prekallikrein) provided in combination with this neutral phospholipid.19 The investigators conclude that binding of kininogens to phosphatidylethanolamine induces novel antigenic epitopes.
By means of purification procedures, monitored by cofactor activity measurement in a modified ACA ELISA and coupled to N-terminal amino acid sequencing, it was possible to identify three hitherto unrecognized proteic targets of ACA, namely LBP, C4BP, and thrombin-modified AT. The three of them behaved as heparin-binding proteins in the purification process, in keeping with their known affinity for various lipids or membranes. It should be emphasized that the detection of cofactor activity depends on two successive or concurrent phenomena, a protein-cardiolipin interaction and antibody recognition of the available epitopes. A way to ascertain that putative ACA cofactors do bind to cardiolipin under the very conditions of the ACA assay (ie, from serum or plasma) is to use polyclonal antisera raised against the proteins being studied as probes because of the number and diversity of epitopes recognized by those highly specific reagents. In this respect, the thrombin-AT preparation (patient no. 6 ACA cofactor) and bovine serum, but neither bovine plasma nor native AT, supported the binding of a rabbit anti-AT antiserum to immobilized cardiolipin, with such a reactivity pattern being identical to the one of patient no. 6 ACA. This finding, together with evidence for the role of bovine AT in mixing experiments with human plasma before clotting, led us to conclude that those patient ACA do indeed target bovine AT once it has been modified/cleaved by thrombin.
AT is a single-chain glycoprotein of 58 kD that belongs to the serpin (serine proteinase inhibitors) superfamily and inhibits most proteinases of the coagulation cascade (including thrombin) by forming denaturant-stable, equimolar complexes with the enzymes.9The massive change in AT conformation that occurs upon complex formation with thrombin would offer the opportunity to acquire cardiolipin-binding properties and/or generate neoepitopes for ACA. It also probably accounts for the greatly reduced heparin affinity compared with native AT, as judged by the salt concentrations for elution from matrix-linked heparin: 1 mol/L NaCl for free AT and 0.15 mol/L NaCl for the thrombin-AT complex, with the value increasing to 0.45 mol/L NaCl upon ternary complex formation with vitronectin.11,20 Interestingly, patient no. 6 ACA cofactor activity eluted from the heparin-Ultrogel column around the latter ionic strength, which, together with the S-300 column activity profile, may indicate the presence of such ternary complexes in the final preparation and their participation in cofactor activity. It is also conceivable that interactions to the heparin affinity column and/or to solid-phase cardiolipin were actually mediated by the thrombin moiety of the thrombin-AT complex, because thrombin contains two positive poles to the east and northwest of the active site referred to as the anion-binding exosite and the heparin-binding site, respectively.21
C4BP, formerly called proline-rich protein,22 functions as an inhibitor of the classical pathway of the complement cascade and also regulates the protein C anticoagulant pathway through protein S binding.23 The major form of C4BP is composed of seven identical α chains (∼70 kD in human and 80 kD in the mouse) and one β chain (45 kD) linked by disulphide bridges.23 The latter chain, being highly glycosylated, has been noticed to stain poorly with Coomassie blue,23 probably explaining the absence of visible band in the 45-kD region when patient no. 5 ACA cofactor preparation was run on reduced SDS gels. Because circulating C4BP is known to be involved in calcium-dependent and high-affinity interactions with protein S (84 kD) and serum amyloid P component (5 noncovalently bound 25-kD subunits),24 their copurification in the C4BP preparation would have been expected. However, this did not prove to be the case possibly for the following reasons: (1) the weaker affinity for the bovine C4BP-protein S interaction than for the human complex,25 (2) the capability of heparin to disrupt the C4BP-serum amyloid P component interaction,24 and, most importantly, (3) the absence of calcium during the purification process. The presence of C4BP in triglyceride-rich lipoproteins (ie, chylomicrons and VLDL)26 is consistent with the property (shared with β2GPI) of binding to Intralipid,22 a lecithin-stabilized artificial triglyceride emulsion that behaves metabolically in a very similar way to chylomicrons. Both α and β chains of C4BP have been shown to contain several structural elements, referred to as short consensus or complement repeats (SCR), which are also found in approximately half of all complement proteins, including factor H, as well as in β2GPI.23 It is interesting to note the recent finding by Kertesz et al27 that complement factor H, which has a high degree of homology with β2GPI, binds to cardiolipin-coated plates under conditions used in the standard ACA assay. In addition, a high frequency of antibodies to several human complement regulatory proteins, including factor H and C4BP, has been reported in antiphospholipid syndrome patients using direct ELISAs.28 The possibility that these antibodies actually behave as cofactor-dependent ACA has not been investigated.
Finally, the antibodies of the last patient (no. 4) were shown to recognize LBP in combination with cardiolipin, and their binding was competed by fluid-phase purified LPS (or endotoxin, a glycolipid present in the outer membrane of all gram-negative bacteria) (Fig. 4B). LBP is a trace plasma glycoprotein of 60 kD that binds with high affinity to the lipid A moiety of rough and smooth forms of LPS13 and plays an important intermediary role in host-endotoxin interaction. LBP functions as an opsonin by bridging LPS-coated particles to the CD14 receptor of macrophages, thereby promoting tumor necrosis factor-α (TNF-α) secretion.29The interaction between LBP and a number of negatively charged polymeric substances, including DNA, RNA, heparin, and phospholipids, has been reported to be poor to nonobservable, stressing the high degree of specificity for the lipid A portion of LPS.13More recently, a novel function for LBP and soluble CD14 has been defined in transport of mammalian phospholipids both with purified recombinant proteins and in whole plasma.30 Of note, a valine residue at position 7 of the mature protein was identified in bovine LBP (versus alanine in human LBP; Table 2) as well as in a structurally related LPS binding protein found in granulocytes, human bactericidal/permeability-increasing protein.8
The exact causes of the development of antibodies against these newly identified ACA cofactors are unclear. However, both C4BP and LBP are known to behave as acute-phase reactants, the levels of which increase during various diseases, and an inflammation process might be involved in the generation of these antibodies. In addition, the quantitation of thrombin-AT complexes or modified AT in plasma is now available for the diagnosis of preclinical states of coagulation activation, and high values have been found in such conditions, eg, venous thromboembolism or inherited thrombophilia.31 The present study has not addressed two important issues: (1) the proportion and predictive value in various clinical settings of ACA corresponding to antibody binding to epitopes derived from the above-mentioned proteins, and (2) the putative biological effects of such antibodies on the functions of targeted proteins, as exemplified by antibodies to prothrombin and to inhibitors of coagulation, protein C, protein S, and annexin V.32-34 The isolated presence of ACA specificities of the kind described here appears quite uncommon, being detected through the systematic screening in the ACA- and β2GPI-ELISAs of thousands of consecutive serum samples sent to our laboratory for aPL testing. Obviously, patients could have more than one type of ACA, in which case it may prove difficult to determine the relative contribution made to binding in the standard ACA assay by the different antibody subsets for the following reasons: (1) the possible role of the endogenous cofactors from patient sera, (2) a competition phenomenon between various phospholipid-binding proteins for access to solid-phase cardiolipin, and, finally, (3) the influence of specific antibodies on the equilibrium between bound and fluid-phase cofactors. Although the involvement of these new ACA cofactors is for the moment limited to a few patient samples, the reported experimental approach is increasing our insight into the numerous ACA specificities. The real significance of those ACA is still elusive and clinical complications, such as thrombosis, seem uncommon, at least in our small series of patients.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Josiane Arvieux, MD, Laboratory of Immunology, Brest University Medical School Hospital, BP 824, F 29609 Brest Cedex, France.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal