Abstract
We have investigated the basis of antithrombin deficiency in an asymptomatic individual (and family) with borderline levels (≈70% antigen and activity) of antithrombin. Direct sequencing of amplified DNA showed a mutation in codon 135, AAC to ACC, predicting a heterozygous Asn135Thr substitution. This substitution alters the predicted consensus sequence for glycosylation, Asn-X-Ser, adjacent to the heparin interaction site of antithrombin. The antithrombin isolated from plasma of the proband by heparin-Sepharose chromatography contained amounts of β antithrombin (the very high affinity fraction) greatly increased (≈20% to 30% of total) above the trace levels found in normals. Expression of the residue 135 variant in both a cell-free system and COS-7 cells confirmed altered glycosylation arising as a consequence of the mutation. Wild-type and variant protein were translated and exported from COS-7 cells with apparently equal efficiency, in contrast to the reduced level of variant observed in plasma of the affected individual. This case represents a novel cause of antithrombin deficiency, removal of glycosylation concensus sequence, and highlights the potentially important role of β antithrombin in regulating coagulation.
INHERITED ANTITHROMBIN deficiency results in a predisposition to venous thromboembolism. Appreciable progress has been made in defining the molecular basis of deficiency, which mostly occurs in individuals heterozygous for point mutations in the antithrombin gene.1,2 As with many other deficiency states, antithrombin deficiency has been divided into two major types. Type I deficiency is characterized by a reduction in the antigen level usually to ≈50% of normal, a reduction paralleled by a decrease in functional activity. Type II deficiency is characterized usually by (near) normal levels of antithrombin antigen, but reduction, usually to 50%, in activity. Type II deficiency can be further subclassified and a number of schemes have been used.3-7 That adopted in recent reports of the antithrombin database of mutations applies a classification involving the functional domains.2 Thus, type II deficiency may be caused by mutations that alter the function of the reactive site, the heparin binding site, or that have multiple (or pleiotropic) effects on function. This latter group includes cases that present with reduced levels of antithrombin antigen, between 50% to 100%, with a disproportionate reduction in functional levels, usually ≈50%.
In most cases of deficiency, simple assays of function and activity are sufficient for diagnosis. Complication may arise when the levels of either assay are borderline to the normal range. Often this can be resolved by investigation of the kindred, by consideration of the clinical circumstances, or by genetic studies; although the latter is usually impractical unless the region of the antithrombin gene involved can be targeted for simple polymerase chain reaction (PCR) analysis.
We sought to clarify the reason for borderline levels of antithrombin in a panel of patients with phenotypic deficiency, which has formed the material used for a systematic investigation of the genetic basis of deficiency (the results of this work are summarized along with the vast majority of known mutations in Lane et al2). Once the genetic defects in most cases with clear cut phenotypic deficiency had been determined, those cases with borderline phenotypic values were then investigated. One of these cases, the subject of this report, was found to have a mutation that alters the glycosylation consensus sequence located adjacent to the proposed heparin binding site on antithrombin. The consequence of this mutation was an alteration in the proportion of antithrombin distributed into the two plasma subfractions of antithrombin, α antithrombin (with high-affinity for heparin), and β antithrombin (with very high-affinity for heparin).
MATERIALS AND METHODS
Kindred.
The proband, an Italian who at the age of 25 years had not experienced any thrombosis, was investigated at the request of her gynecologist. She was taking the oral contraceptive pill. A clinical history and plasma samples were also taken from her sister, mother, and father: there was no indication of venous thrombosis in the family, but the father had suffered a myocardial infarction at the age of 58 years (see Table 1).
Functional and immunoassays.
Antithrombin activity was determined both as antifactor IIa and antifactor Xa inhibitory activities in the presence of heparin and the chromogenic substrate S-2238 or S-2765 (Chromogenix, Molndal, Sweden). The immunologic determination of antithrombin levels was determined with a commercial radial immunodiffusion assay (Behring, Marburg, Germany). Interassay coefficient of variation for the functional and immunoassays was less than 5%.
Preparation of antithrombin.
Plasma antithrombin was extracted using the dextran sulphate precipitation method8 followed by heparin-Sepharose chromatography on a Pharmacia FPLC system (Amersham Pharmacia Biotech, St Albans, Herts, UK) in 0.05 mol/L Tris buffer pH 7.4. Antithrombin was either eluted with a single step with 0.4 mol/L to 3 mol/L NaCl or with a linear gradient from 0.4 to 3 mol/L NaCl, collecting 10 mL volumes of eluant.
Isoelectric focussing.
Isoelectric focussing was performed in a 0.75-mm thin polyacrylamide gel (8.5 × 7.5 cm) containing a final concentration of 0.7% Pharmacia Ampholine pH 4.0 to 6.5. The gels after fixation were stained with the Bio-Rad Silver Staining kit (Bio-Rad Laboratories, Herts, UK).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
SDS-PAGE was performed using a BioRad MiniProtean II system. Forty nanograms of total antithrombin and the elution fractions were separated. The gels were electroblotted using the Bio-Rad Mini Trans-Blot cell onto Hybond ECL Nitrocellulose membrane (Amersham Pharmacia Biotech). Antithrombin was located on the membrane using a peroxidase-conjugated rabbit antihuman antithrombin antibody (Dako Ltd, High Wycombe, UK) followed by ECL autoradiography (Amersham International).
Genetic analysis.
All six exons of the antithrombin gene were amplified by polymerase chain reaction (PCR) and sequenced using an automated sequencer (ABI PRISM/373 Stretch XL DNA Sequencer; Perkin Elmer Biosystems, Foster City, CA). Primers were as described using one biotinylated primer for each pair and single strand sequencing on magnetic beads.9
Mutagenesis of AT cDNA.
Wild-type AT cDNA was cloned into the vector pCR II (Invitrogen, Adelaide, Australia). The construct contained the complete AT coding sequence, but none of the 5′ or 3′ untranslated region. Two variant cDNAs were generated by site-directed mutagenesis (ExSite, Stratagene, Christchurch, New Zealand) using inverse PCR. Mutagenic primers were Asn135Thr forward 5′-ACCAAATCCTCCAAGTTAGTATC-3′ (nt 501-523, numbering of AT cDNA with the A of the initiation Met being nt 1), Ser137Ala forward 5′-AACAAAGCCTCCAAGTTAGTATC-3′ (nt 501-523), with a common reverse orientation primer 5′-GGCTTTTCGATAGAGTCGGCAG-3′ (nt 500-479). After ligation with T4 DNA ligase, the variant cDNA vector constructs were transformed in Epicurian Coli XL1-Blue supercompetent cells (Stratagene). DNA from the resulting colonies was sequenced to check that the mutation was present and no further changes had been introduced.
Cell-free expression of AT constructs.
RNA from wild-type and mutant AT cDNA was generated using the SP6 promoter of the pCR II vector. Plasmid DNA was linearized withNot1 (New England Biolab, Christchurch, New Zealand) and incubated at 37°C for 30 minutes in a 50-μL reaction containing 10 μL 5xSP6 buffer, 5 μL 0.1 mol/L dithiothreitol (DTT), 1 μL RNAguard (Pharmacia, Auckland, New Zealand), 10 μL guanosine triphosphate (GTP) mix (10 mmol/L adenosine triphosphate [ATP], cytidine triphosphate [CTP], uridine triphosphate [UTP], and 0.5 mmol/L GTP), 4 μL linear DNA (0.5 μg/μL), 2.5 μL CAP (New England Biolabs) and 1.5 μL SP6 polymerase (Pharmacia). After this, 5 μL of 10 mmol/L GTP was added with the reaction proceeding for a further 30 minutes. After phenol chloroform extraction, RNA was precipitated with 3 mol/L sodium acetate and 100% ethanol. Variant and wild-type antithrombins were produced using nuclease-treated rabbit reticulocyte lysate (Promega, Auckland, New Zealand) in a volume of 26 μL containing 17.5 μL lysate, 0.5 μL amino acids minus methionine, 5 μL 35S-methionine (>1,400 Ci/mmol, ICN, Auckland, New Zealand) and 3 μL RNA, in the presence (1.8 μL) or absence of canine pancreatic microsomal membranes (Promega, Dade Diagnostics, Aukland, New Zealand) for 1 hour at 30°C. Some products were incubated with endoglycosidase H (New England Biolabs), 500 U at 37°C for 60 minutes, to remove any Asn-linked carbohydrate. The products were electrophoresed in 10% acrylamide-bis (37.5:1) protein gels for 1 hour at 100 V, followed by fixation and autoradiography for 48 hours.
Mammalian cell expression of AT constructs.
A 1.4-kb EcoRI fragment containing the wild-type or mutant AT cDNA was isolated from pCR II and cloned into the EcoRI site of the mammalian expression vector pcDNA3 (Invitrogen). After transformation into DH5α competent cells, clones with the correct insert orientation were selected by screening with restriction enzyme digests. COS 7 cells at approximately 50% confluence were transfected with 1 μg of the pcDNA3 constructs using Tfx-20 (Promega) as described by the manufacturer. A reporter gene vector, pSEAP2-Control (Clontech, Sydney, Australia), was cotransfected with the AT constructs to act as a control of transfection efficiency. Assays for the reporter gene product, alkaline phospatase secreted into culture supernatant, were performed using a chemiluminescent assay (Great EscAPe, Clontech, San Diego, CA) according to the manufacturer’s instructions. Cells were then grown at 37°C in 10% CO2/air in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Auckland, New Zealand) with 2 mmol/L glutamine and 10% fetal bovine serum (FBS, Gibco BRL). Approximately 48 hours after transfection, culture supernatant was discarded and the cells were washed in DMEM lacking FBS and methionine. After resuspension of the cell pellet in the same media, newly synthesized cellular proteins were radiolabelled by the addition of 50 μCi35S-methionine for 3 hours. Culture supernatants were immunoprecipitated with rabbit polyclonal antisera raised against human AT (Dako) using formalin fixed Staphylococcus aureus(Gibco BRL). Electrophoresis was performed as described above, except in 12.5% acrylamide-bis (37.5:1) gels.
RESULTS
The proband, her father, and sister (but not her mother) all have antithrombin levels determined by two different functional assays and antigen assay that are lower than that expected for normal individuals (Table 1). This suggested an inherited abnormality leading to reduced levels. Interestingly, while the reduction in antigen level to almost 70% might have suggested the presence of a genetic mutation resulting in a pleiotropic effect, the functional activity was not reduced to 50%. The parallel reduction in plasma antithrombin antigen and activity in the proband, father, and sister was consistent with either production of antithrombin from one allele at a reduced rate or increased clearance of the protein, but no impairment of reactive site function. Furthermore, no slow-moving peak indicative of an antithrombin population with reduced heparin affinity was observed on crossed immunoelectrophoresis in the presence of heparin. These observations collectively suggest that this case is not similar to those with pleiotropic effects.
Phenotypic Investigation of the Affected Family
| Relationship . | Age (yrs) . | AT Activity (%) . | AT Antigen (%) . | Thrombosis . | |
|---|---|---|---|---|---|
| Anti-IIa . | Anti-Xa . | ||||
| Proband | 27 | 67 (6) | 66 (5) | 68 (3) | None |
| Mother | 50 | 94 | ND | 85 | None |
| Father | 60 | 72 (1) | 68 (6) | 67 (1) | MI, 58 yrs |
| Sister | 26 | 73 (1) | 63 (8) | 67 (2) | None |
| Normal range | 80-120 | 80-120 | 80-120 | ||
| Relationship . | Age (yrs) . | AT Activity (%) . | AT Antigen (%) . | Thrombosis . | |
|---|---|---|---|---|---|
| Anti-IIa . | Anti-Xa . | ||||
| Proband | 27 | 67 (6) | 66 (5) | 68 (3) | None |
| Mother | 50 | 94 | ND | 85 | None |
| Father | 60 | 72 (1) | 68 (6) | 67 (1) | MI, 58 yrs |
| Sister | 26 | 73 (1) | 63 (8) | 67 (2) | None |
| Normal range | 80-120 | 80-120 | 80-120 | ||
Mean (SD) of levels measured on three occasions over a 4-year period, except in the case of the mother where a single sample only was available.
Abbreviations: MI, myocardial infarction; ND, not done.
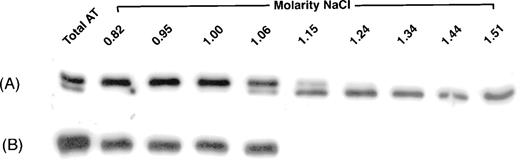
To investigate further the phenotype, plasma from the proband was applied to and eluted from heparin-Sepharose. Initially, elution was performed with a step gradient to recover all antithrombin in plasma. The antithrombin isolated from normal plasma migrated in SDS-PAGE and immunoblotting as two bands, the major band of α antithrombin and a barely visible β antithrombin band. In contrast, plasma of the patient contained a much greater apparent β antithrombin fraction than normally seen, as well as α antithrombin (see Fig 1 “Total AT”). Elution with a salt gradient was able to resolve the two bands in the proband plasma. Under these conditions, the antithrombin from the normal plasma eluted as expected between a NaCl concentration of 0.82 and 1.06 mol/L. The apparent β antithrombin band from the proband eluted later, between 1.06 and 1.51 mol/L NaCl (Fig 1).
Elution of antithrombin from heparin-Sepharose. Antithrombin from proband (A) and normal (B) plasmas was isolated either by a single-step (“Total AT”) or a gradient increase in NaCl concentration. Fractions were collected and analyzed by immunoblotting, as described in Materials and Methods. For fractions collected by gradient elution, the molar concentration of NaCl required for their elution is indicated.
Elution of antithrombin from heparin-Sepharose. Antithrombin from proband (A) and normal (B) plasmas was isolated either by a single-step (“Total AT”) or a gradient increase in NaCl concentration. Fractions were collected and analyzed by immunoblotting, as described in Materials and Methods. For fractions collected by gradient elution, the molar concentration of NaCl required for their elution is indicated.
On isoelectric focussing and silver staining of extracted normal antithrombin, at least nine components could be observed. The antithrombin extracted from the proband showed multiple bands in the same pH range, but with a different concentration distribution (results not shown).
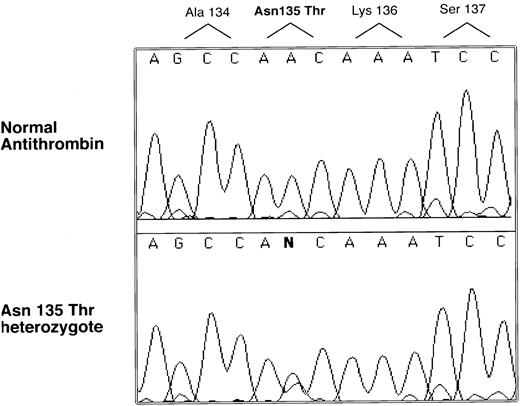
Genomic DNA was isolated from the proband, and the entire coding region was sequenced. A single nucleotide abnormality was identified in the codon for 135 Asn, AAC to ACC, which predicts an amino acid substitution Asn135Thr (see Fig 2). Inheritance of the mutation was demonstrated by PCR and sequencing in the case of the sister and the father (a sample could not be obtained from the mother). This nucleotide substitution was not found in more than 100 normal alleles, indicating it is not a common polymorphism.
Selected nucleotide sequence of proband and normal PCR-amplified DNA. All coding regions of the antithrombin gene were amplified and sequenced with an automated sequencer (see Materials and Methods). Only one region was found with a sequence difference between normal and patient products, and this difference is illustrated along with its predicted amino acid change.
Selected nucleotide sequence of proband and normal PCR-amplified DNA. All coding regions of the antithrombin gene were amplified and sequenced with an automated sequencer (see Materials and Methods). Only one region was found with a sequence difference between normal and patient products, and this difference is illustrated along with its predicted amino acid change.
The predicted amino acid substitution affects the consensus Asn-linked glycosylation sequence, Asn-X-Ser, beginning at position 135, and was predicted to block glycosylation at this site. The variant protein would be expected to have an almost identical molecular weight (MW) and properties to β antithrombin. This is entirely compatible with the results presented in Fig 1. One property that could not be explained is the reduced amount of variant in the plasma. Normal plasma contains ≈50% antithrombin from each of two normal alleles. In affected members of this family, the variant allele is assumed to have resulted in a reduced amount of plasma antithrombin, ≈20% rather than 50%, giving a total of ≈70% antithrombin (see Table 1). This estimate was compatible with one obtained by determining the relative peak areas on elution from heparin-Sepharose: the proportion of β antithrombin was then ≈20% to 30% of total antithrombin in the proband and her sister. The effects on glycosylation of the substitution and the reason for the reduced plasma concentration of antithrombin in the affected members of the family was examined by analysis of protein expressed in vitro.
Site-directed mutagenesis was used to generate variant AT cDNA encoding the Asn135Thr amino acid substitution identified in the proband. In addition, to check whether any effects of the substitution were related to effects on glycosylation of antithrombin at Asn135 or were residue-specific (that is, related to Asn135 rather than glycosylation), an additional variant cDNA was created encoding the substitution Ser137Ala. This substitution alters the Asn-linked glycosylation consensus sequence of Asn-X-Ser and was predicted to inhibit glycosylation at Asn135.
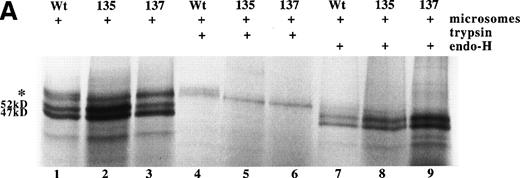
Using rabbit reticulocyte lysate as a cell-free expression system, RNA derived from wild-type and the two variant AT cDNAs were translated to protein with approximately equal efficiency (see Fig 3A). For all three proteins, a product of approximately 52 kD was observed, consistent with translation of the full-length antithrombin including signal peptide. In addition, all three translations produce a 47-kD form, which results from internal initiation of translation of the AT cDNA from Met17 (A.C.F. and R.J.O., unpublished results). In the presence of pancreatic microsomes, posttranslational processing of the wild-type Asn135Thr and Ser137Ala antithrombins was observed as larger MW products. Incubation of the translation products with endoglycosidase H left intact the 52-kD and 47-kD forms, but removed the larger MW bands, indicating the latter were the result of Asn-linked glycosylation of the protein. Careful inspection of the bands representing glycosylated protein showed the presence of a high MW band in wild-type antithrombin, which is missing for the variants (see Fig 3A, lanes 1 through 3). This is more clearly demonstrated after trypsin treatment, lanes 4 through 6, which removes nonglycosylated protein that has not been translocated into the microsomes. This shows that while the Asn135Thr and Ser137Ala variants are translated and can be glycosylated, complete glycosylation of the mutant proteins is prevented.
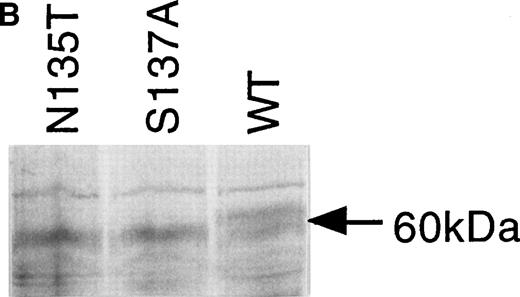
Expression of wild-type, As135Thr, and Ser137Ala antithrombins. (A) Antithrombin variants were expressed using rabbit reticulocyte lysate and coupled to posttranslational modification by the addition of microsomal membranes (+). Products above 52 kD in the coupled reactions represent N-linked glycosylation products. The uppermost of the doublet glycosylation isoform (*) produced from the wild-type protein (Wt) is absent in two variants with substitutions affecting the N-linked consensus glycosylation site at residue 135 (N135T and S137A, labelled 135 and 137, respectively); see lanes 1 through 3, but more clearly in lanes 4 through 6, where trypsin has been added (+) after protein has been incubated with microsomes, and protein not translocated into the protective environment of the microsomes has been degraded. Lanes 7 through 9 are control incubations of wild-type and two variant antithrombins with endoglycosidase H (+) and indicate that this enzyme strips the carbohydrate side chains from the high MW forms. The band labelled 47 kD results from internal initiation of translation at Met17. (B) Antithrombin was expressed in COS-7 cells. Supernatant of COS-7 cells transfected with antithrombin constructs was immunoprecipitated using polyclonal antithrombin antibody. Glycosylated antithrombin was found in the supernatants with a size of approximately 60 kD. At least two glycosylation isoforms were observed for wild-type (WT) antithrombin (just above and below the 60-kD arrow), but the larger form was not observed in the N135T or S137A variants. Several nonspecific bands are apparent in each lane and are also seen in supernatants from mock-transfected COS cells (not shown).
Expression of wild-type, As135Thr, and Ser137Ala antithrombins. (A) Antithrombin variants were expressed using rabbit reticulocyte lysate and coupled to posttranslational modification by the addition of microsomal membranes (+). Products above 52 kD in the coupled reactions represent N-linked glycosylation products. The uppermost of the doublet glycosylation isoform (*) produced from the wild-type protein (Wt) is absent in two variants with substitutions affecting the N-linked consensus glycosylation site at residue 135 (N135T and S137A, labelled 135 and 137, respectively); see lanes 1 through 3, but more clearly in lanes 4 through 6, where trypsin has been added (+) after protein has been incubated with microsomes, and protein not translocated into the protective environment of the microsomes has been degraded. Lanes 7 through 9 are control incubations of wild-type and two variant antithrombins with endoglycosidase H (+) and indicate that this enzyme strips the carbohydrate side chains from the high MW forms. The band labelled 47 kD results from internal initiation of translation at Met17. (B) Antithrombin was expressed in COS-7 cells. Supernatant of COS-7 cells transfected with antithrombin constructs was immunoprecipitated using polyclonal antithrombin antibody. Glycosylated antithrombin was found in the supernatants with a size of approximately 60 kD. At least two glycosylation isoforms were observed for wild-type (WT) antithrombin (just above and below the 60-kD arrow), but the larger form was not observed in the N135T or S137A variants. Several nonspecific bands are apparent in each lane and are also seen in supernatants from mock-transfected COS cells (not shown).
The supernatants of cultured COS-7 cells transfected with wild-type and the two variant antithrombin constructs were compared to determine the expression efficiency of the proteins. Protein products of approximately 60 kD were immunoprecipitated from the culture supernatants of all three transfectants using a polyclonal antibody to antithrombin. Figure 3B shows that in addition to the 60-kD species, there are several additional weak bands in each lane (a single band above and a triplet below the 60-kD species). Additional experiments (not shown) demonstrated that these bands were also present in supernatants from mock-transfected COS cells, suggesting that they are proteins, which are nonspecifically precipitated by the antibody. In further control experiments, we have shown with expressed wild-type antithrombin that the 60-kD form is glycosylated, as incubation of the cells with tunicamycin, which inhibits glycosylation, results in an antithrombin of about 50 kD. The glycosylated wild-type antithrombin synthesized by the mammalian cells was present in the electrophoretic separations of immunoprecipitated products as a doublet band, in contrast to the Asn135Thr and Ser137Ala variant proteins, which were each clearly expressed as a single band (Fig 3B). It is likely that the lower band of the doublet observed for wild-type AT represents β AT. Inspection of the products suggested wild-type antithrombin and the two variant proteins were secreted from cells with apparently similar efficiency, and comparison of the reporter gene product assays from the supernatants confirmed an equal efficiency of cell transfection by the constructs.
DISCUSSION
In this study, we have identified in an asymptomatic individual and her family a mutation in the antithrombin gene in codon 135, AAC to ACC, which results in an amino acid substitution, 135Asn to Thr. This alters the carbohydrate consensus sequence beginning at Asn135 and thereby prevents normal glycosylation at this site. Using two distinct in vitro transfection and expression systems, we have shown that glycosylation is specifically inhibited by this amino acid substitution. The consequence of the mutation is an increased concentration of the fraction of antithrombin in plasma that binds with very high-affinity to heparin, β antithrombin. Although the concentration of this fraction is greatly increased, it does not approach ≈50% (the concentration resulting from a single normal allele) and there is therefore a moderate deficiency, ≈70%, of total antithrombin in the plasma of the kindred.
In normal plasma, most (≈95%) antithrombin is glycosylated at Asn135 (α antithrombin) and a minor fraction is unglycosylated (β antithrombin).10,11 It has been shown that this normal variable glycosylation arises from the presence of Ser in the consensus Asn-X-Ser sequence at this site.12,13 Presence of the Ser residue reduces the efficiency of core glycosylation at position 135. A consequence of the lack of glycosylation in β antithrombin is increased affinity of this minor fraction for heparin and related glycosaminoglycans, such as heparan sulphate. Heparin is known to bind to antithrombin in a two-step reaction, initially with low-affinity, but then with high-affinity.14 High-affinity binding is believed to occur due to a conformational change involving also the reactive site loop. Recent rapid-kinetic studies of α and β antithrombins have suggested that lower affinity binding of the former to heparin is not due to interference of the initial (weak) binding step by the oligosaccharide side chain at position 135, but to decreased conformation flexibility resulting from incorporation of the oligosaccharide side chain.15 This decreases the rate constant for the conformational change in the second binding step and increases that for its reversal.
Estimates of the concentration of β antithrombin in normal plasma vary between 5% to 10%, but there is no precise means of determining its concentration and this might be an overestimate for human plasma. Electrophoretic analysis is at best semiquantitative, and the low relative concentration makes calculation of its recovery from precipitation and chromatography procedures difficult. Our data suggest that presence or absence of glycosylation at Asn135 will not significantly affect the export of antithrombin from hepatocytes. The finding that the concentration of the variant Asn135Thr in plasma is less than that of normal antithrombin in the present family (compared with ≈20% to 30% for the variant with ≈50% expressed by a normal single allele), therefore suggests that β antithrombin has a larger distribution volume, or higher clearance rate from the vasculature, or possibly it competes with α antithrombin for heparan sulphate within and outside of the circulation. This suggestion is compatible with results of turnover studies of antithrombin fractions (differing in heparin affinity) performed in the rabbit.16 Consequently, the level of β antithrombin in normal plasma may be determined by both efficiency of glycosylation and by events that occur at the vascular interface after export of the antithrombin into the circulation.
The physiological and pathological roles of β antithrombin have yet to be fully defined. Nevertheless, there is accummulating evidence that heparan sulphate-β antithrombin interaction could be important. First, there is much evidence that the normal endothelium synthesizes heparan sulphate, which is anticoagulantly active.17 This heparan sulphate contains some of the residues identified as being critical in the essential pentasaccharide of heparin, necessary for high-affinity binding to antithrombin. Second, there is a rapid reacting form of antithrombin, observable in a rat perfusion heart model system, which interacts with factor Xa at a rate indicative of heparin (-like) enhancement.18 Third, in models of vessel injury, β antithrombin associates more readily than α antithrombin with the vessel wall and preferentially inhibits thrombin coagulant activity.19 20 From consideration of these experimental studies, together with the kinetic data, it can be concluded tentatively that the anticoagulant function of antithrombin at the endothelial surface may be primarily determined by its β antithrombin content.
The considerations above on the potential role of the heparin-β antithrombin interaction bear on the current case of antithrombin deficiency. Antithrombin is lowered in the affected family to 70% of the normal level, inviting the suggestion that this could be associated with an increased risk of venous thromboembolism. That no venous thromboembolism has occurred in the family members studied (although we note the myocardial infarction in the father), could be due to a lack of concurrent or transient risk factors of sufficient strength. However, it could also be due to the protective effect of the relatively increased concentration of β antithrombin. Judging from the immunoblotting experiments illustrated herein, we speculate that the large increase in concentration of this fraction in this kindred may be sufficient to increase anticoagulant activity to compensate for the reduction in total antithrombin and to provide protection against venous thromboembolic events. Additional studies on the pathophysiology of β antithrombin will be required to substantiate or refute this speculation.
Supported by the Special Trustees of Charing Cross Hospital, the Charing Cross and Westminster Medical School Research Committee, by a small grant from the British Society of Haemostasis and Thrombosis and the Health Research Council of New Zealand.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to D.A. Lane, PhD, Department of Haematology, Imperial College School of Medicine, Charing Cross Hospital Campus, Hammersmith, London W6 8RP, UK; e-mail:d.lane@ic.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal