Abstract
Alterations in the vascular system and the onset of angioproliferative lesions such as Kaposi’s sarcoma (KS) are common traits of human immunodeficiency virus-1 (HIV-1)–infected patients. To investigate possible factors involved in acquired immunodeficiency syndrome (AIDS)-associated vasculopathy and vascular malfunction, expression of vascular endothelial cell growth factor-A (VEGF-A) was analyzed in HUT 78 T lymphocytes upon infection with HIV-1. VEGF-A was found to be increased in supernatants from infected cells as compared with uninfected cells. In addition, VEGF-A mRNA expression and protein secretion were significantly increased in HUT 78 cells incubated with conditioned medium (CM) derived from HIV-1 chronically infected HUT 78 cells (HIV-TCM) as compared with CM from uninfected cells (TCM). Increase of VEGF-A production in T cells was promoted by inflammatory cytokines (IC) present in HIV-TCM, including tumor necrosis factor (TNF), interferon γ (IFNγ), interleukin-1β (IL-1β), and IL-6. These IC that have been shown to be increased in sera of HIV-1–infected patients and to be increased by HIV-1 infection or cell activation in these individuals as well as HIV-TCM also increased VEGF-A expression in primary T lymphocytes. Consistent with this, VEGF-A concentrations were found to be higher in sera of HIV-1–infected patients with (mean, 357.1 ± 197.9 pg/mL) and without KS (mean, 256.7 ± 137.5 pg/mL) as compared with uninfected individuals (mean, 188.6 ± 91.7 pg/mL). These data suggest that increased secretion of VEGF-A by T lymphocytes of HIV-1–infected individuals may induce vascular leakage and stimulate proliferation of vascular endothelial cells, which are hallmarks of AIDS-associated vasculopathy and especially of KS development.
VASCULAR DISORDERS are commonly found in human immunodeficiency virus-1 (HIV-1)–infected patients. These include the formation of cotton-wool spots in the eye due to vasculitis-induced ischemic injury,1,2 serum protein leakage across the blood-brain barrier,3,4 and enhanced transendothelial migration of HIV-1–infected monocytes in the brain that contributes to HIV-1–associated encephalitis.5,6 In addition, we have recently demonstrated the presence of severe morphologic alterations of the aortic endothelium in HIV-1–infected patients that were associated with endothelial cell activation and increased adhesion of mononuclear cells.7
However, the most profound mark of the acquired immunodeficiency syndrome (AIDS)-associated vasculopathy is Kaposi’s sarcoma (KS). KS is the most frequent tumor of HIV-1–infected individuals, particularly homosexual men.8,9 Tumors appear multifocally and are characterized by endothelial cell activation and proliferation as well as by prominent inflammatory cell infiltrates, particularly in early stages.8 9 In progressed stages, the so-called KS spindle cells, which are regarded as the tumor cells of KS, dominate the histological picture.
Previous data have suggested that elevated levels of inflammatory cytokines (IC), such as tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), IL-6, and interferon γ (IFNγ), may contribute to AIDS-KS pathogenesis (for review, see Ensoli and Stürzl9). These IC are released by activated or HIV-1–infected immune cells10 and have been found to be increased in sera11-15 or cell culture supernatants of blood cells derived from HIV-1–infected individuals16,17or in tumor tissues of KS patients.18,19 IC interrupt HIV-1 latency by activating viral gene expression and replication10,16,20 and, simultaneously, affect the normal properties of the vascular endothelium21,22 leading to endothelial cell activation10,23 and production of angiogenic factors.24-26 HIV-1 proteins such as gp120 and Tat can, in turn, activate production of TNFα, IL-1β, and IL-6.27,28 Long-term exposure of the endothelium to IC and other activating factors such as the HIV-1 Tat protein10 29-31 may, therefore, contribute to HIV-associated vasculopathy. However, the final mediators of the increased proliferation and permeability of vessels that is found in HIV-1–infected patients have not been identified as yet.
Vascular endothelial cell growth factor-A (VEGF-A) is a secreted dimeric protein that induces vascular permeability and endothelial cell proliferation,32-35 which are key events in the formation of KS lesions.9 Alternative splicing of VEGF-A mRNA gives rise to at least 4 VEGF-A isoforms of 121, 165, 189, and 206 amino acid residues. The two shorter forms are efficiently secreted, whereas the two longer forms remain mostly cell-associated.36-38Moreover, placental cells and various carcinoma cells of the female reproductive tract express a VEGF transcript that encodes an additional secreted isoform of 145 amino acids.39 New members of the VEGF family named VEGF-B, VEGF-C, and VEGF-D have also been identified.40-42
In this study, we focused on VEGF-A, which is a potent inducer of angiogenesis in vivo during normal physiological processes43 and embryonic vascular development44 and has also been shown to be involved in tumor angiogenesis.45-47 Recently, we showed that both VEGF-A mRNA and protein are highly expressed in the spindle cells of AIDS-KS lesions and that VEGF-A, in concert with basic fibroblast growth factor (bFGF), cooperate to induce angiogenesis and vascular permeability found in KS.24 26
In the present study, we show that VEGF-A expression is increased in an HIV-1 chronically infected T-lymphocyte cell line and that IC released from these cells upon infection or activation induce VEGF-A expression in uninfected T cells by a paracrine action. A role of VEGF-A in vivo is supported by the detection of VEGF-A protein in cell culture supernatants of primary T lymphocytes and of increased concentrations of VEGF-A in sera of AIDS-KS patients as compared with uninfected individuals. These data suggest that VEGF-A may contribute to the generalized vascular activation, vascular proliferation, and permeability observed in HIV-1–infected patients.
MATERIALS AND METHODS
Patients
AIDS-KS serum samples were taken from 39 HIV-1–positive male patients with KS diagnosis (36 Germans, 1 Italian, 1 Yugoslavian, and 1 Turk). The patients, ranging in age from 31 to 67 years (mean, 45 years), were treated with combinations of nucleosid-reverse transcriptase-inhibitors (AZT, ddI, and ddC). Sera of the HIV-1–positive/KS-negative group were taken from 37 HIV-1–positive male patients (32 Germans, 2 Irish, 1 Turk, 1 Italian, and 1 Sudanese) without KS. The patients, ranging in age from 20 to 71 years (mean, 44 years), were undergoing similar therapy as the AIDS-KS patients. Control sera were collected from 21 healthy, German male individuals ranging in age from 24 to 74 years (mean, 53 years) without known neoplasms and recent trauma or surgery, to avoid possibly elevated VEGF-A serum concentrations resulting from, eg, wound healing. Informed consent to take the blood samples was obtained from all of the individuals enrolled in the study.
Cell Cultures
Primary T lymphocytes.
Peripheral blood mononuclear cells (PBMC) were isolated from 5 healthy donors by Ficoll-Paque (Amersham Pharmacia, Freiburg, Germany) density centrifugation as described previously48 and enriched in T lymphocytes by magnetic beads using the Pan T cell Isolation Kit (Vario-MAC; Miltenyl Biotech, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Purification of CD3+ T lymphocytes was greater than 62% of the total PBMC in all 5 samples as monitored by fluorescence-activated cell sorting (FACS) analysis (FACS Calibur; Becton Dickinson, Heidelberg, Germany) using CD3 phycoerythrin and CD4 fluorescein isothiocyanate (FITC) staining. Cells were cultured in RPMI 1640, supplemented with 20% fetal calf serum (FCS), IL-2 (20 U/mL), L-glutamine (2 mmol/L), penicillin (100 U/mL), and streptomycin (100 μg/mL; GIBCO BRL, Gaithersburg, MD) at 37°C and 5% CO2.
HUT 78 T lymphocytes.
The lymphocytic T-cell line HUT 7849 50 was cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), IL-2 (10 U/mL), L-glutamine (2 mmol/L), penicillin (100 U/mL), and streptomycin (100 μg/mL; GIBCO BRL) at 37°C and 5% CO2. This culture medium does not contain any VEGF-A protein as determined by enzyme-linked immunosorbent assay (ELISA). Cell cultures were routinely tested for the absence of mycoplasma.
Cytokines and Tat Protein
Recombinant IL-2, TNFα, and IL-6 were purchased from Boehringer Mannheim (Mannheim, Germany). Recombinant IFNγ and IL-1β were purchased from Collaborative Biomedical Products (Bedford, MA). The recombinant Tat protein used in this study was expressed inEscherichia coli and purified to homogeneity as described previously.31 Handling and storage of Tat was performed as described by Barillari et al.10 Lyophilized Tat protein was resuspended and diluted in degassed buffer (phosphate-buffered saline–bovine serum albumin [PBS-BSA] 0.1%). This buffer was used as a negative control.
Transfection of HUT 78 Cells With HIV-1 Proviral DNA
Chronically infected HUT 78 cells were initially obtained upon transfection of the cells with the plasmid pHXB-2 (HTLV IIIB)51 by electroporation using a Bio-Rad Gene Pulser (Bio-Rad Laboratories, Inc, Hercules, CA). In a first-round cell-free culture supernatants of electroporated cells were used to infect new HUT 78 cells. To maintain HIV-1–infected cells, HUT 78 cells were freshly infected every month with cell-free culture supernatants obtained from the HUT 78 cells that were infected the month before. Virus production in infected cells was monitored by a p24-antigen capture assay (Abbott Laboratories, Abbott Park, IL).
Preparation of Conditioned Media (CM)
CM were prepared from 3 × 107 uninfected (TCM) or HIV-1 chronically infected (HIV-TCM) HUT 78 T lymphocytes, respectively. Cells were grown for 4 days in fresh medium, and cell culture supernatants were then collected and used for the experiments. For the preparation of TPA-TCM, HUT 78 cells (3 × 107) were incubated in culture medium containing 50 ng/mL phorbol 12-myristate 13-acetate (TPA; Sigma Chemical Co, St Louis, MO) for 3 hours. Cells were then harvested, washed twice with PBS, and incubated in RPMI 1640 containing 1% FBS for 18 hours. Cell culture supernatants were then harvested and used for the experiments. Synthetic HIV-TCM (SH-TCM) was prepared using culture medium supplemented with four recombinant cytokines at the following concentrations: 250 pg/mL TNFα, 1 U/mL IFNγ, 875 pg/mL IL-1β, and 200 U/mL IL-6.
Cell Stimulation
HUT 78 cells were grown to a cell density of 1 × 106cells/mL. The medium was changed and either TCM, HIV-TCM, TPA-TCM, SH-TCM, or cytokines diluted in RPMI 1640/10% FBS in the respective concentrations were added. Cells were then incubated for 1, 3, and 5 days. At each time point, cells and cell culture supernatants were harvested and further processed for analysis of VEGF-A RNA or protein content.
Northern Blot Analysis
Cells were harvested and washed twice with PBS. Total RNA was isolated by using the RNeasy kit (Qiagen, Hilden, Germany) according to the the manufacturer’s instructions. Twenty micrograms of total RNA was separated by electrophoresis in a 1% agarose/6% formaldehyde gel in the presence of ethidium bromide, transferred onto Hybond N+-nylon membrane (Amersham Life Science, Buckinghamshire, UK), and cross-linked by UV irradiation (264 nm for 2 minutes). A 450-bp cDNA fragment of the VEGF-A coding region that is present in all known splice variants of VEGF-A was radioactively labeled with [α-32P]-dCTP by using the High Prime oligonucleotide kit (Boehringer Mannheim) and used as a probe. Hybridization was performed as described previously.24 Band intensities on autoradiographic films were quantitatively determined using an Elscript 400 transilluminator (Hirschmann GmbH, Unterhaching, Germany).
VEGF-A ELISA
ELISA was performed with a commercially available ELISA kit (Quantikine; R&D Systems, Wiesbaden-Nordenstadt, Germany) for the detection of VEGF165 in cell culture supernatants (diluted 1:100) and patient sera (undiluted) according to the manufacturer’s instructions. All analyses and calibrations were performed in duplicate and each plate included recombinant human VEGF165standards. The color of the chromogenic reactions was evaluated spectrophotometrically at 450 nm using an ELISA reader (Dynatech Laboratories, Chantilly, VA), with a correction filter at 570 nm. As a control to determine quantitative reliability of detection in sera, known amounts of recombinant human VEGF165 were added to serum samples of healthy persons. The concentrations measured corresponded to the predicted values. Repeated blind analyses of identical serum samples showed identical VEGF-A concentrations.
Western Blot Analysis
HUT 78 cells were grown in 75-cm2 flasks (Greiner, Frickenhausen, Germany) with 25% of TCM in RPMI 1640/10% FBS from uninfected or chronically infected HUT 78 cells. After 5 days of incubation, the cell culture supernatants were collected and concentrated by 20-fold using a Centriprep-3 device (Amicon Corp, Beverly, MA) according to the manufacturer’s instructions. To avoid degradation of proteins, phenylmethyl sulfonylfluoride (PMSF; 2 mmol/L) and benzamidine (10 mmol/L; Sigma) were added to the supernatants. Concentrated samples prepared from equivalent numbers of cells (5 × 107) were boiled for 5 minutes in 1× loading buffer (50 mmol/L Tris-HCl, 100 mmol/L dithiothreitol [DTT], 2% sodium dodecyl sulfate [SDS], 0.1% bromphenol blue, and 10% glycerol) and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide) in the presence of 10% β-mercaptoethanol (Sigma). Electrophoretically separated proteins were then transferred onto a nitrocellulose membrane, and immunostaining with a polyclonal antiserum raised against VEGF165 was performed as described previously.24 As a control of specificity, recombinant human VEGF165 (R&D Systems) was used.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
RT-PCR was performed as described previously,36 with minor modifications. Shortly, 4 μg of total RNA was reverse transcribed by using 800 U Moloney murine leukemia virus (Mo-MLV) reverse transcriptase (GIBCO BRL) and 0.4 nmol oligo-dT15(Boehringer Mannheim) in a total volume of 50 μL. One microliter of the respective cDNA was amplified by using the VEGF-A–specific primers Ex5 and ET6 (0.25 μmol/L each). Primer Ex5 (5′-CCA AAG AAA GAT AGA GCA AGA CAA GAA-3′) binds in sense orientation within exon 5 of the VEGF-A gene and primer ET6 (5′-TCG ATC GTT CTG TAT CAG TCT-3′) binds in antisense orientation, as described by Ballaun et al.36 The PCR reaction mixture (50 μL) contained 20 mmol/L (NH4)2SO4, 75 mmol/L Tris-HCl, pH 9.0, 0.01% (vol/vol) Tween-20, 1.5 mmol/L MgCl2, 200 μmol/L dNTPs, and 1 U Red Hot DNA polymerase (Advanced Biotechnologies, Leatherhead, UK). After 30 cycles of amplification (94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute each cycle), the reaction products were separated by electrophoresis in an 1.5% agarose gel containing ethidium bromide (0.5 μg/mL).
Statistical Analysis
Statistical analysis was performed using the Student’s t-test (two-tailed for equal variances assumed) and the correlation analysis used Pearson Correlation (two-tailed). A P value less than .05 was considered to be significant.
RESULTS
VEGF-A Synthesis Is Increased in T Lymphocytes by HIV-1 Infection
To investigate the role of VEGF-A in AIDS-associated vasculopathy, cell culture supernatants from HIV-1–infected and uninfected HUT 78 T lymphocytes were tested by ELISA for VEGF-A content. VEGF-A concentrations were found to be 1.8-fold increased in cell culture supernatants of HIV-1–infected HUT 78 cells (Fig 1, column B) as compared with control cells (Fig 1, column A).
VEGF-A secretion in HIV-1–infected and uninfected HUT 78 cells. Cell culture supernatants of uninfected (A) and HIV-1 chronically infected HUT 78 cells (B) were analyzed in duplicate for the presence of VEGF-A by ELISA. VEGF-A concentration was 1.8-fold higher in infected cells as compared with uninfected cells.
VEGF-A secretion in HIV-1–infected and uninfected HUT 78 cells. Cell culture supernatants of uninfected (A) and HIV-1 chronically infected HUT 78 cells (B) were analyzed in duplicate for the presence of VEGF-A by ELISA. VEGF-A concentration was 1.8-fold higher in infected cells as compared with uninfected cells.
To investigate the mechanisms of VEGF-A increase, HUT 78 cells were incubated with either TCM or HIV-TCM for 1, 3, or 5 days, respectively, and expression of VEGF-A mRNA was examined by Northern blot hybridization (Fig 2A and B, left). Quantitative evaluation of band intensities showed no differences in VEGF-A expression after 1 day of incubation (Fig 2C, left, open bars). However, at 3 and 5 days, the level of VEGF-A mRNA was increased by 1.6-fold to twofold in HUT 78 cells incubated with HIV-TCM as compared with TCM (Fig 2C, left, shaded and solid bars). As compared with HUT 78 cells incubated in standard medium after 5 days of incubation, VEGF-A mRNA was already increased in TCM-treated HUT 78 cells and clearly further increased by HIV-TCM (Fig 2A, B, and C, right). The increase of VEGF-A expression in the presence of HIV-TCM as compared with TCM was highly reproducible, as demonstrated by two independent experiments (compare Fig 2, right and left). This was confirmed by determining the levels of secreted VEGF-A protein by ELISA (Fig 3) and by Western blot analysis (Fig 4). The VEGF-A protein concentrations established at day 5 in the cell culture supernatants of HIV-TCM–treated cells (Fig 3, solid columns, and Table 1) are capable of inducing vascular cell growth (data not shown), indicating that they are biologically relevant.
HIV-TCM increases VEGF-A expression in HUT 78 cells. (A) Northern blot analysis of VEGF-A mRNA expression in HUT 78 cells. Cells were incubated in normal cell culture medium (control) with TCM or HIV-TCM. In two separate experiments, total RNA was isolated after 1, 3, and 5 days of incubation (left) or after 5 days of incubation (right), subjected to electrophoresis (20 μg per lane), and blotted onto a nylon membrane. Hybridization was performed with a radioactively labeled human VEGF-A specific cDNA probe (specific activity, ≥8 × 108 cpm/μg DNA) at 42°C for 16 hours. VEGF-A mRNA expression in HUT 78 cells increased by twofold after 3 and 5 days of incubation with HIV-TCM as compared with TCM and threefold as compared with control cells incubated in normal medium. (B) Ethidium bromide staining of blotted RNA indicated that equal amounts of RNA were used in each lane. (C) Densitometric evaluation of band intensities from the Northern blot shown in (A). RNA derived from cells incubated with TCM or with HIV-TCM were blotted and hybridized on the same filter. VEGF-A expression was similar after 1 day of incubation with HIV-TCM or TCM (□). Increased VEGF-A expression was observed after 3 days (▩) and 5 days (▪) of incubation with HIV-TCM as compared with TCM and standard medium.
HIV-TCM increases VEGF-A expression in HUT 78 cells. (A) Northern blot analysis of VEGF-A mRNA expression in HUT 78 cells. Cells were incubated in normal cell culture medium (control) with TCM or HIV-TCM. In two separate experiments, total RNA was isolated after 1, 3, and 5 days of incubation (left) or after 5 days of incubation (right), subjected to electrophoresis (20 μg per lane), and blotted onto a nylon membrane. Hybridization was performed with a radioactively labeled human VEGF-A specific cDNA probe (specific activity, ≥8 × 108 cpm/μg DNA) at 42°C for 16 hours. VEGF-A mRNA expression in HUT 78 cells increased by twofold after 3 and 5 days of incubation with HIV-TCM as compared with TCM and threefold as compared with control cells incubated in normal medium. (B) Ethidium bromide staining of blotted RNA indicated that equal amounts of RNA were used in each lane. (C) Densitometric evaluation of band intensities from the Northern blot shown in (A). RNA derived from cells incubated with TCM or with HIV-TCM were blotted and hybridized on the same filter. VEGF-A expression was similar after 1 day of incubation with HIV-TCM or TCM (□). Increased VEGF-A expression was observed after 3 days (▩) and 5 days (▪) of incubation with HIV-TCM as compared with TCM and standard medium.
HIV-TCM increases VEGF-A secretion in HUT 78 cells. HUT 78 cells (1 × 106) were incubated in medium containing 25% of TCM or HIV-TCM. Cell culture supernatants were harvested after 1, 3, and 5 days of incubation and analyzed (in duplicate) for the presence of VEGF-A by ELISA. The data were subtracted of the VEGF-A levels present in TCM and HIV-TCM. In comparison to TCM-treated cells, VEGF-A concentrations were 1.8- to 2.0-fold higher in HUT 78 cells incubated with HIV-TCM after 1 day (□), 3 days (▩), and 5 days (▪) of incubation, respectively.
HIV-TCM increases VEGF-A secretion in HUT 78 cells. HUT 78 cells (1 × 106) were incubated in medium containing 25% of TCM or HIV-TCM. Cell culture supernatants were harvested after 1, 3, and 5 days of incubation and analyzed (in duplicate) for the presence of VEGF-A by ELISA. The data were subtracted of the VEGF-A levels present in TCM and HIV-TCM. In comparison to TCM-treated cells, VEGF-A concentrations were 1.8- to 2.0-fold higher in HUT 78 cells incubated with HIV-TCM after 1 day (□), 3 days (▩), and 5 days (▪) of incubation, respectively.
(A) Western blot analysis of VEGF-A in culture supernatants of HUT 78 cells incubated with HIV-TCM or TCM. HUT 78 cells were incubated in medium containing 25% of TCM or HIV-TCM. After 5 days of incubation, the cell culture supernatants were collected, concentrated, and subjected to SDS-PAGE under reducing conditions. Electroblotting and immunostaining were performed as described in Materials and Methods. A total of 10 ng of human recombinant VEGF165 was used as a control on the same membrane (rV). Two prominent bands corresponding to unglycosylated and glycosylated VEGF165 protein (24 and 26 kD, two upper bands) and a weaker band corresponding to glycosylated VEGF121 (20 kD, lower band) were detected in the cell culture supernatants. (B) Densitometric evaluation of band intensities from the Western blot shown in (A) indicates a 1.8-fold higher concentration of VEGF-A in HUT 78 cells after incubation with HIV-TCM as compared with incubation with TCM.
(A) Western blot analysis of VEGF-A in culture supernatants of HUT 78 cells incubated with HIV-TCM or TCM. HUT 78 cells were incubated in medium containing 25% of TCM or HIV-TCM. After 5 days of incubation, the cell culture supernatants were collected, concentrated, and subjected to SDS-PAGE under reducing conditions. Electroblotting and immunostaining were performed as described in Materials and Methods. A total of 10 ng of human recombinant VEGF165 was used as a control on the same membrane (rV). Two prominent bands corresponding to unglycosylated and glycosylated VEGF165 protein (24 and 26 kD, two upper bands) and a weaker band corresponding to glycosylated VEGF121 (20 kD, lower band) were detected in the cell culture supernatants. (B) Densitometric evaluation of band intensities from the Western blot shown in (A) indicates a 1.8-fold higher concentration of VEGF-A in HUT 78 cells after incubation with HIV-TCM as compared with incubation with TCM.
VEGF-A Concentrations in Supernatants of HUT 78 Cells Under Different Conditions of Stimulation
| Stimuli . | VEGF-A (ng/mL) . | ||
|---|---|---|---|
| Day 1 . | Day 3 . | Day 5 . | |
| TCM (25%)* | 3.1 ± 0.3 | 16.4 ± 1.5 | 28.7 ± 0.9 |
| HIV-TCM (25%)* | 5.8 ± 0.7 | 32.2 ± 3.4 | 53.4 ± 2.1 |
| TPA-TCM (25%)* | 8.5 ± 1.6 | 47.6 ± 1.2 | 65.6 ± 0.6 |
| TNFα (250 pg/mL) | 2.1 ± 0.1 | 21.5 ± 0.3 | 52.7 ± 2.6 |
| IFNγ (1 U/mL) | 2.1 ± 0.1 | 16.0 ± 0.4 | 45.0 ± 0.6 |
| IL-1β (875 pg/mL) | 2.3 ± 0.1 | 18.3 ± 0.1 | 51.3 ± 2.0 |
| IL-6 (200 U/mL) | 2.1 ± 0.2 | 20.2 ± 0.9 | 38.7 ± 0.2 |
| SH-TCM | 8.3 ± 1.2 | 54.3 ± 0.8 | 84.1 ± 2.4 |
| Stimuli . | VEGF-A (ng/mL) . | ||
|---|---|---|---|
| Day 1 . | Day 3 . | Day 5 . | |
| TCM (25%)* | 3.1 ± 0.3 | 16.4 ± 1.5 | 28.7 ± 0.9 |
| HIV-TCM (25%)* | 5.8 ± 0.7 | 32.2 ± 3.4 | 53.4 ± 2.1 |
| TPA-TCM (25%)* | 8.5 ± 1.6 | 47.6 ± 1.2 | 65.6 ± 0.6 |
| TNFα (250 pg/mL) | 2.1 ± 0.1 | 21.5 ± 0.3 | 52.7 ± 2.6 |
| IFNγ (1 U/mL) | 2.1 ± 0.1 | 16.0 ± 0.4 | 45.0 ± 0.6 |
| IL-1β (875 pg/mL) | 2.3 ± 0.1 | 18.3 ± 0.1 | 51.3 ± 2.0 |
| IL-6 (200 U/mL) | 2.1 ± 0.2 | 20.2 ± 0.9 | 38.7 ± 0.2 |
| SH-TCM | 8.3 ± 1.2 | 54.3 ± 0.8 | 84.1 ± 2.4 |
HUT 78 (1 × 106) cells were incubated for 1, 3, and 5 days with 25% of CM obtained from uninfected (TCM), HIV-1–infected (HIV-TCM), or TPA-treated (TPA-TCM) HUT 78 cells or with single cytokines or the combination of all the different cytokines in the respective concentrations (SH-TCM). VEGF-A concentrations were determined in duplicate by ELISA. The mean values and standard deviations of two independent experiments are shown.
Corrected VEGF-A concentrations are presented. The concentration of VEGF-A present in TCM, HIV-TCM, and TPA-TCM used for induction in the respective experiments has been subtracted.
Correlation of the results obtained by Northern blot hybridization and ELISA after 3 and 5 days of incubation suggested that the increase of VEGF-A concentration in the presence of HIV-TCM was predominantly due to a transcriptional activation of VEGF-A gene expression (compare Figs2 and 3, shaded and solid columns). Translational and secretory mechanisms may also be activated by HIV-TCM, as suggested by the slight increase of VEGF-A concentrations in supernatants of HIV-TCM–treated cells after 1 day of incubation, which was not detected at the mRNA expression analysis (compare Figs 2 and 3, open columns).
Western blot analysis showed three bands with molecular weights of 26, 24, and 20 kD (Fig 4A, HIV-TCM), representing the glycosylated and unglycosylated 165 aa VEGF-A isoform52 (Fig 4A, 26 and 24 kD, upper bands) and a glycosylated 121 aa isoform53 (Fig4A, 20 kD, lower band), respectively. Comparison of band intensities suggested that VEGF165 is the prevalently secreted VEGF-A isoform that is induced by HIV-TCM in T cells.
VEGF165 Is the Predominant VEGF-A mRNA Splice Variant in HIV-1–Infected HUT 78 Cells
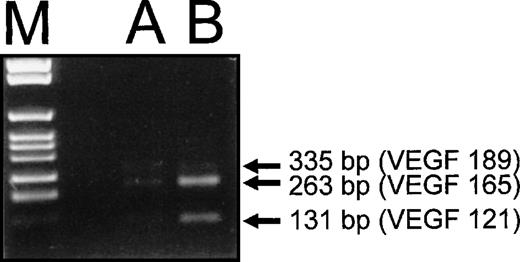
To verify whether the VEGF165 was the prevalent VEGF-A isoform induced by HIV-1 infection, RT-PCR analysis of VEGF-A mRNA splice variants was performed with HIV-1–infected HUT 78 cells (Fig 5). These data supported the Western blot results. Three different PCR products corresponding to the mRNA splice variants encoding VEGF121, VEGF165, and VEGF189 were detected in HUT 78 cells infected with HIV-1 (Fig 5, lane B). The most prominent band was the variant encoding VEGF165 (length of amplification product, 263 bp), whereas the band representing the VEGF121 variant (131 bp) was present at lower amounts. Only minimal amounts of the amplification product corresponding to VEGF189 (335 bp) were detectable (Fig 5, lane B).
RT-PCR analysis of VEGF-A mRNA splice variants synthesized by HIV-1–infected HUT 78 cells. RT-PCR was performed with specific primers, which allow discrimination of the different splice variants of VEGF-A mRNA by the length of the polymerization products. As a control, PCR products obtained with cDNA of a human epithelial cell line (A 431) known to produce VEGF-A are shown (A). The two splice variants coding for the secreted forms of VEGF-A, VEGF121and VEGF165 (131- and 263-bp amplification products) and the cell-associated VEGF189 (335 bp) were found to be synthesized in HUT 78 cells infected with HIV-1 (B). The PCR product corresponding to the mRNA splice variant encoding VEGF165was the most prominent, the one corresponding to VEGF121could be detected to a lesser extent, and the one corresponding to VEGF189 was only present in minimal amounts.
RT-PCR analysis of VEGF-A mRNA splice variants synthesized by HIV-1–infected HUT 78 cells. RT-PCR was performed with specific primers, which allow discrimination of the different splice variants of VEGF-A mRNA by the length of the polymerization products. As a control, PCR products obtained with cDNA of a human epithelial cell line (A 431) known to produce VEGF-A are shown (A). The two splice variants coding for the secreted forms of VEGF-A, VEGF121and VEGF165 (131- and 263-bp amplification products) and the cell-associated VEGF189 (335 bp) were found to be synthesized in HUT 78 cells infected with HIV-1 (B). The PCR product corresponding to the mRNA splice variant encoding VEGF165was the most prominent, the one corresponding to VEGF121could be detected to a lesser extent, and the one corresponding to VEGF189 was only present in minimal amounts.
IC, But Not HIV-1 Tat Protein, Induce VEGF-A Expression in T Lymphocytes
In chronically infected HUT 78 cells, HIV-1 p24 protein could be detected in more than 10% of the cells by immunocytochemical staining, whereas in HUT 78 cells incubated for 5 days with HIV-TCM, no or very few positive cells (<0.5%) were detected (data not shown). The low number of productively infected cells suggested that the induction of VEGF-A synthesis by HIV-TCM was not caused by autocrine mechanisms in de novo-infected cells, but, more likely, by paracrinely acting factors such as cytokines and/or HIV-1 proteins present in HIV-TCM. A preferential role of cytokines was supported by the observation that CM from TPA-treated-HUT 78 cells caused an increase of VEGF-A expression similar to that observed with HIV-TCM after 3 and 5 days of incubation (Table 1).
Single cytokines (TNFα, IL-1β, IL-6, and IFNγ) that are known to be elevated in sera of HIV-1–infected patients11-15 as well as in the cell culture supernatants of activated T lymphocytes10,17,23,25 were therefore used for cell stimulation. The concentrations of cytokines used were 25% of the concentrations that have been reported to be present in the cell culture supernatants of activated T cells (250 pg/mL TNFα, 1 U/mL IFNγ, 875 pg/mL IL-1β, and 200 U/mL IL-6).25 Analysis of VEGF-A secretion by ELISA showed that all of these cytokines increase VEGF-A expression in HUT 78 cells after 3 and 5 days of stimulation (Table 1). The most prominent effects were obtained with TNFα and IL-1β (1.8-fold increase as compared with TCM stimulation), whereas the effects of IFNγ (1.6-fold increase) and IL-6 (1.3-fold increase) were less pronounced under these conditions (Table 1). Application of a combination of these cytokines at the same concentrations (synthetic HIV-TCM, SH-TCM) increased VEGF-A mRNA expression (Fig 6) and secretion (Table 1) in HUT 78 cells threefold after 5 days of incubation, indicating additive mechanisms of induction. In contrast, the incubation of HUT 78 cells with various concentrations of the HIV-1 Tat protein (from 0.01 to 5 μg/mL) for 1, 3, and 5 days did not increase VEGF-A secretion as compared with treatment with buffer alone as determined by ELISA (data not shown). However, increased concentrations of VEGF-A in cell culture supernatants of HUT 78 cells were observed after 3 or 5 days of stimulation with very high concentrations of Tat (10 μg/mL; data not shown). Such high concentrations of Tat are unlikely to be present in vivo or produced by cultured cells.54 55 Therefore, these data suggest that the observed increase of VEGF-A expression by HIV-TCM may be preferentially mediated by cytokines released from HIV-1–infected HUT 78 cells.
SH-TCM increases VEGF-A expression in HUT 78 cells. (A) Northern blot analysis of VEGF-A mRNA expression in HUT 78 cells. Cells were incubated with buffer (control) or SH-TCM. Total RNA was isolated after 5 days of incubation, subjected to electrophoresis (20 μg per lane), and blotted onto a nylon membrane. Hybridization was performed with a radioactively labeled human VEGF-A–specific probe at 42°C for 16 hours. VEGF-A mRNA expression in HUT 78 cells increased by threefold after incubation with SH-TCM as compared with buffer. (B) Ethidium bromide staining of blotted RNA indicated that equal amounts of RNA were used in each lane. (C) Densitometric evaluation of band intensities from the Northern blot shown in (A).
SH-TCM increases VEGF-A expression in HUT 78 cells. (A) Northern blot analysis of VEGF-A mRNA expression in HUT 78 cells. Cells were incubated with buffer (control) or SH-TCM. Total RNA was isolated after 5 days of incubation, subjected to electrophoresis (20 μg per lane), and blotted onto a nylon membrane. Hybridization was performed with a radioactively labeled human VEGF-A–specific probe at 42°C for 16 hours. VEGF-A mRNA expression in HUT 78 cells increased by threefold after incubation with SH-TCM as compared with buffer. (B) Ethidium bromide staining of blotted RNA indicated that equal amounts of RNA were used in each lane. (C) Densitometric evaluation of band intensities from the Northern blot shown in (A).
Primary T Lymphocytes Secrete VEGF-A after Stimulation With IC or HIV-TCM
To investigate whether the mechanism of the VEGF-A induction may be operative in vivo, primary T lymphocytes were stimulated for 5 days with different cytokines, alone or in combination (SH-TCM), TCM, or HIV-TCM. SH-TCM and HIV-TCM clearly increased VEGF-A secretion in 3 of 5 persons as compared with incubation with buffer or TCM (Table 2). In addition, a slight increase of the VEGF-A secretion by primary T lymphocytes was induced by each of the single cytokines (Table 2). These data show that primary T cells are able to produce VEGF-A, supporting previous findings48 56 and indicating that cytokines released in increased amounts by HIV-1–infected cells may induce VEGF-A expression in uninfected T cells.
VEGF-A Concentrations in Supernatants of Primary T Cells Under Different Conditions of Stimulation
| Stimuli . | VEGF-A (pg/mL) . | ||
|---|---|---|---|
| Person 1 . | Person 2 . | Person 3 . | |
| Buffer | 11.4 ± 2.9 | 15.3 ± 0.8 | 21.9 ± 1.6 |
| TCM (25%)* | 15.6 ± 3.8 | 32.3 ± 3.8 | 30.5 ± 2.1 |
| HIV-TCM (25%)* | 34.6 ± 8.3 | 45.7 ± 8.3 | 48.6 ± 8.4 |
| TNFα (250 pg/mL) | 14.4 ± 1.3 | 20.1 ± 0.8 | 24.9 ± 5.8 |
| IFNγ (1 U/mL) | 13.5 ± 2.5 | 21.9 ± 0.8 | 24.0 ± 1.3 |
| IL-1β (875 pg/mL) | 18.3 ± 1.6 | 27.2 ± 1.7 | 27.9 ± 0.8 |
| IL-6 (200 U/mL) | 14.7 ± 1.7 | 22.2 ± 5.0 | 24.0 ± 0.4 |
| SH-TCM | 70.1 ± 12.9 | 51.8 ± 12.9 | 56.7 ± 1.7 |
| Stimuli . | VEGF-A (pg/mL) . | ||
|---|---|---|---|
| Person 1 . | Person 2 . | Person 3 . | |
| Buffer | 11.4 ± 2.9 | 15.3 ± 0.8 | 21.9 ± 1.6 |
| TCM (25%)* | 15.6 ± 3.8 | 32.3 ± 3.8 | 30.5 ± 2.1 |
| HIV-TCM (25%)* | 34.6 ± 8.3 | 45.7 ± 8.3 | 48.6 ± 8.4 |
| TNFα (250 pg/mL) | 14.4 ± 1.3 | 20.1 ± 0.8 | 24.9 ± 5.8 |
| IFNγ (1 U/mL) | 13.5 ± 2.5 | 21.9 ± 0.8 | 24.0 ± 1.3 |
| IL-1β (875 pg/mL) | 18.3 ± 1.6 | 27.2 ± 1.7 | 27.9 ± 0.8 |
| IL-6 (200 U/mL) | 14.7 ± 1.7 | 22.2 ± 5.0 | 24.0 ± 0.4 |
| SH-TCM | 70.1 ± 12.9 | 51.8 ± 12.9 | 56.7 ± 1.7 |
VEGF-A concentrations in supernatants produced from 5 × 105 primary T cells after 5 days of stimulation were determined in duplicate by ELISA.
Corrected VEGF-A concentrations are presented. The concentration of VEGF-A present in TCM and HIV-TCM used for induction in the respective experiments has been subtracted.
VEGF-A Concentrations Are Elevated in Sera of AIDS-KS Patients
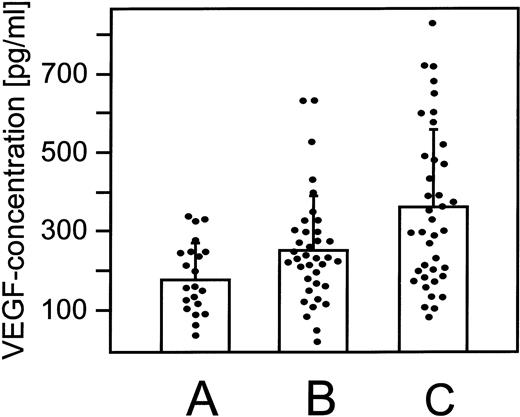
To determine whether increased expression and secretion of VEGF-A may occur in HIV-1–infected patients, VEGF-A serum concentrations were determined in HIV-1–infected patients without KS (n = 37) or with KS (n = 39) and in uninfected healthy individuals (n = 21). In the HIV-1–negative control individuals, VEGF-A serum concentrations ranged from 35.5 to 347.2 pg/mL, with a mean of 188.6 ± 91.7 pg/mL (Fig 7A). In contrast, elevated VEGF-A serum levels were detected in the HIV-1–positive/KS-negative group ranging from 26.2 to 634.8 pg/mL, with a mean of 256.7 ± 137.5 pg/mL (Fig 7B). Much higher VEGF-A serum levels were found in the AIDS-KS group, ranging from 87.5 to 835.4 pg/mL, with a mean of 357.1 ± 197.9 pg/mL (Fig 7C). The means of the VEGF-A concentrations of the different groups show a nearly linear increase. Specifically, it was found to be low in the control group, intermediate in the group of HIV-1–infected patients without KS, and highest in the AIDS-KS group (Fig 7). In the AIDS-KS group, 20 patients (51%) showed serum VEGF-A concentrations exceeding 300 pg/mL, whereas in the HIV-1–positive/KS-negative group only 9 patients (24%) and in the control group only 3 persons (14%) showed concentrations greater than 300 pg/mL. The Student’s t-test for equality of means showed highly significant differences between the means of the AIDS-KS and the control group (P = .001; Fig 7C and A) and between the AIDS-KS and the HIV-1–positive/KS-negative group (P = .018; Fig 7C and B). Difference in VEGF-A serum concentrations between the HIV-1–positive/KS-negative group compared with the control group was at borderline of significancy (P = .048; Fig 7B and A). No correlation between the VEGF-A concentration and the CD4+T-cell counts (Pearson correlation, 0.64), opportunistic infections, or therapy could be observed. The correlation between VEGF-A serum concentrations and KS development was further supported by the clinical follow-up of 2 patients that showed a 2.4-fold and 4.3-fold increase of VEGF-A serum concentrations after KS diagnosis as compared with the KS free stage of HIV-1 infection.
VEGF-A protein concentrations are increased in the serum of HIV-1–positive/KS-negative and AIDS-KS patients. VEGF-A protein concentrations were determined by ELISA in sera of 21 healthy control individuals without a known neoplasm, recent trauma, or surgery (A); in sera of 37 HIV-1–infected patients without KS (B); and in sera of 39 AIDS-KS patients (C). The VEGF-A serum concentration in group A ranged from 35.5 to 347.2 pg/mL, with a mean of 188.6 ± 91.7 pg/mL. Increased VEGF-A serum concentrations were determined in patients of group B, ranging from 26.2 to 634.8 pg/mL, with a mean of 256.7 ± 137.5 pg/mL. The highest VEGF-A serum concentrations were detected in patients of group C, ranging from 87.5 to 835.4 pg/mL, with a mean of 357.1 ± 197.9 pg/mL. The differences in VEGF-A concentrations between groups A and C (P = .001) and between groups B and C (P = .018) were statistically significant as determined by Student’s t-test analysis. The differences between the VEGF-A concentrations of groups A and B were at borderline statistical significance (P = .048).
VEGF-A protein concentrations are increased in the serum of HIV-1–positive/KS-negative and AIDS-KS patients. VEGF-A protein concentrations were determined by ELISA in sera of 21 healthy control individuals without a known neoplasm, recent trauma, or surgery (A); in sera of 37 HIV-1–infected patients without KS (B); and in sera of 39 AIDS-KS patients (C). The VEGF-A serum concentration in group A ranged from 35.5 to 347.2 pg/mL, with a mean of 188.6 ± 91.7 pg/mL. Increased VEGF-A serum concentrations were determined in patients of group B, ranging from 26.2 to 634.8 pg/mL, with a mean of 256.7 ± 137.5 pg/mL. The highest VEGF-A serum concentrations were detected in patients of group C, ranging from 87.5 to 835.4 pg/mL, with a mean of 357.1 ± 197.9 pg/mL. The differences in VEGF-A concentrations between groups A and C (P = .001) and between groups B and C (P = .018) were statistically significant as determined by Student’s t-test analysis. The differences between the VEGF-A concentrations of groups A and B were at borderline statistical significance (P = .048).
DISCUSSION
Vasculopathy is a common trait of HIV-1–infected patients, as indicated by the formation of cotton-wool spots in the eye, blood-brain barrier defects, and the occurrence of KS. VEGF-A, also known as vascular permeability factor, stimulates proliferation of endothelial cells in vitro and angiogenesis in vivo and induces capillary permeability.57 These properties suggested to investigate the presence of VEGF-A in T cells infected with HIV-1.
We demonstrate here that HIV-1 infection stimulates VEGF-A production in T cells, likely by paracrine mechanisms involving production of IC that mediate VEGF-A induction. In fact, VEGF-A expression and secretion in HUT 78 cells was significantly higher in the presence of HIV-TCM as compared with TCM, and the cytokines released by HIV-1–infected HUT 78 cells can mediate this effect. It is well documented that T lymphocytes secrete VEGF-A under various conditions.48,56 In the present study, VEGF-A expression and secretion was detected in HIV-TCM–treated and TCM-treated HUT 78 cells at mRNA and protein levels (Figs 2 through 5). The results obtained with the different assays closely correlated. In addition, increased VEGF-A secretion was found with primary T lymphocytes after stimulation with combinations of cytokines or HIV-TCM. These data proved that the mechanism of the VEGF-A induction, which has been investigated by the HUT 78 model system, reflects the in vivo situation. HUT 78 cells are in fact a useful and well-characterized model system for investigation of the impact of HIV-1 infection on VEGF-A expression in T lymphocytes, in contrast with a previous finding in which VEGF-A expression was undetectable in these cells by ELISA.58
Induction of VEGF-A expression may be paracrinely by HIV-1 proteins and/or cytokines secreted from infected cells. However, the HIV-1 Tat protein increased VEGF-A secretion in HUT 78 cells only in very high concentrations, which are not produced by infected cells in culture and are unlikely to be operative in vivo.54,55 In this regard, it is of note that IC such as IL-1β, IFNγ, TNFα, and IL-6 are released at increased concentrations by HIV-1–infected T lymphocytes.17,18,27,28 Furthermore, increased concentrations of the same IC have been detected in the sera of HIV-1–infected patients and in KS lesions or PBMC from patients with AIDS-KS.11-16 In this study, we showed that each of these IC induce VEGF-A expression in HUT 78 cells to levels similar to those observed with HIV-TCM. In fact, cell culture supernatants obtained from uninfected HUT 78 cells that were activated by TPA treatment caused a similar increase of VEGF-A expression as HIV-TCM. Thus, HIV-TCM–mediated increase of VEGF-A expression is predominantly mediated by IC, whereas paracrine activities by the HIV-1 Tat protein may have only limited effects.
Development of KS is the most evident mark of AIDS-associated vasculopathy. We detected highly significant (Student’st-test, P = .001) increased VEGF-A serum concentrations in AIDS-KS patients (mean, 357.1 ± 197.9 pg/mL; n = 39) as compared with the noninfected control group (mean, 188.6 ± 91.7 pg/mL; n = 21). The HIV-1–positive patients without KS showed intermediate VEGF-A serum concentrations (mean, 256.7 ± 137.5 pg/mL), but the difference with the control group was at the borderline of significancy (Student’s t-test, P = .048), whereas the difference of the AIDS-KS group with the group of HIV-1–positive patients without KS was significant (Student’s t-test,P = .018). Furthermore, determination of VEGF-A protein concentrations in sera taken before and after KS diagnosis of the same patients showed elevated VEGF-A concentrations upon development of KS. These data support the finding of the increased VEGF-A serum concentrations of AIDS-KS patients and the intermediate state of the HIV-1–positive patients without KS as compared with controls. This suggests that VEGF-A may contribute to the occurrence of AIDS-associated vasculopathy, especially to KS in vivo. Also, the spindle cells of KS primary lesions highly express and secrete VEGF165,24,26 but this may be locally trapped, because prominent binding of VEGF-A to its cognate receptors on blood vessel endothelial cells present in the lesions has been demonstrated.24 Therefore, in comparison to the VEGF-A secreted by circulating T cells, the impact of KS spindle cell-derived VEGF-A on serum concentrations may be small.
The impact of VEGF-A secreted from T cells on blood vessel endothelial cells may be further amplified by the fact that, in HIV-1–infected patients, a chronically increased adhesion of mononuclear cells to the endothelium was recently demonstrated.7 The same IC that are present at elevated levels in sera of AIDS-KS patients and are shown here to activate VEGF-A expression in T lymphocytes are also activating mononuclear cell adhesion to endothelial cells.59 IC-mediated adhesion to the endothelium of T lymphocytes with enhanced VEGF-A expression may establish disseminated foci of increased VEGF-A concentrations. VEGF-A is a potent inducer of endothelial cell proliferation and vascular permeability and by these activities may regulate the key features of AIDS-associated vasculopathy.
ACKNOWLEDGMENT
The authors thank Peter Hans Hofschneider (Max Planck Institute for Biochemistry, Martinsried, Germany) and Volker Erfle (GSF National Research Center for Environment and Health, Neuherberg, Germany) for generous support and helpful discussions. Furthermore, we acknowledge the kind support of Peter Engl (Munich, Germany), who provided sera from uninfected control persons; Filomena Nappi (Istituto Superiore di Sanità, Rome, Italy) for the HIV-1 p24 ELISA; and Oliver Loch (Policlinic of the Ludwig Maximilians University, Munich, Germany) for the statistical analysis.
Supported by grants of the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 464), the European Concerted Action “Pathogenesis of AIDS-KS,” and the BioFuture program sponsored by the German Ministry of Education and Research (BMBF) to M.S. and by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Progretto Sangue, and the IX AIDS project from the Italian Ministry of Health to B.E.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to M. Stürzl, PhD, GSF-National Research Center for Environment and Health, Institute of Molecular Virology, Ingolstädter Landstraβe 1, 85764 Neuherberg, Germany; e-mail: stuerzl@gsf.de.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal