KSHV/HHV8 IS A NEWLY described oncogenic virus implicated in several hematological malignancies.1-4KSHV is consistently found in all the clinical forms of Kaposi’s sarcoma (KS),1,3,4 in primary effusion lymphomas (PEL),5 and in multicentric Castleman’s disease.3,4 Kaposi’s sarcoma, the major malignancy associated with acquired immunodeficiency syndrome (AIDS),3,6 is characterized by intense angiogenesis, proliferation of spindle-like cells, and inflammation.6,7Compelling evidence points to KSHV as the infectious etiologic agent of Kaposi’s sarcoma: (1) Infection precedes KS development and overlaps with KS risks3,4; (2) KSHV infects KS spindle and endothelial cells in the lesion3; and (3) KSHV infects and transforms cells thought to be premalignant for KS.8 Yet, as for most human oncogenic herpesviruses, it is still unknown why KSHV-mediated malignancy is mostly limited to certain scenarios.3,4,6 According to serological data, approximately 5% of the general population may be infected with KSHV; however, the incidence of KS is less than 1 in 100,000 in the general population.3,6 The incidence of KS increases almost 20,000-fold in patients with AIDS,6 suggesting that immune-dysregulation and HIV are important cofactors for KS- and KSHV-mediated pathogenesis.3,4,6 The high incidence of KS in AIDS was the focus of pioneering research conducted by Gallo6 and Ensoli et al7 that pointed to a role in KS pathogenesis for inflammatory cytokines that are increased in AIDS. However, the link between the inflammatory and the infectious mechanisms for KS pathogenesis remained unclear.
Two reports in the current issue of BLOOD9,10provide new insights into the KS-KSHV puzzle. Aoki et al9show that one of the KSHV genes, the viral interleukin-6–like gene11 (vIL-6) has potential for angiogenesis and for hematopoiesis. This observation adds to the molecular machinery of KSHV that might contribute to the pathogenesis of KS. Nevertheless, despite an impressive armamentarium that includes cell cycle regulators, antiapoptotic molecules, signaling oncogenes, and angiogenesis activators,3,4,11,12 KSHV can cause KS only in certain settings. Moreover, several of the potentially pathogenic genes of KSHV appear to be expressed during an active viral replication/lytic cycle that should kill infected cells.4,13 In this issue ofBLOOD, Monini et al10 show that the inflammatory cytokines of the type upregulated in AIDS and found in KS lesions appear to reactivate KSHV infection. This observation is very important, because it links for the first time two proposed cofounding factors for AIDS-KS: immune-dysregulation leading to increased inflammatory cytokines and infection with KSHV. Furthermore, it could provide a link between inflammatory propathogenic environments, the expression of the lytic program of viral replication, and the concomitant expression of certain KSHV pathogenic genes.
Aoki et al9 show that transformed NIH3T3 cells are more tumorigenic when they express vIL-6. Because the tumors expressing vIL-6 are dramatically vascularized and express VEGF, they conclude that the increased tumorigenicity is due to augmented angiogenicity of vIL-6–expressing cells by induction of VEGF. The investigators also report that tumor-bearing mice show dysregulation of hematopoiesis in several lineages, including B-cell lineage alterations such as plasmacytosis, hepatosplenomegaly and hypergammaglobulinemia. To further define the mechanism of angiogenesis, the investigators demonstrate that vIL-6, like human IL-6, is able to induce VEGF expression, and that the angiogenocity observed in vitro by vIL-6–expressing cells is mediated by VEGF. These observations establish that vIL-6 is a pluripotential viral cytokine able to affect both B-cell lineage hematopoiesis and angiogenesis, thus pointing to a potentially pivotal role of vIL-6 in KS and in the lymphoproliferative KSHV-associated malignancies such as PEL and MCD. Further research will be needed to prove the direct effects of vIL-6 in hematopoiesis and the behavior of this viral cytokine in the context of human cells. The fact that KSHV carries three genes with potential for angiogenesis, vMIP-I and II,3 the KSHV-GPCR,12 and now the vIL-6, and that two of them, vIL-6 and KSHV-GPCR, can upregulate VEGF expression, suggest that angiogenesis and VEGF are important for KSHV biology, perhaps because major targets of infection are proliferating endothelial cells and angiogenesis-associated spindle cells.
The study of Monini et al10 represents the third part of a series of studies performed by the laboratories of Drs Ensoli and Gallo that were published in BLOOD. In the first two reports, they showed that inflammatory cytokines (IC) of the Th1 type, particularly γ-IFN, upregulated in KS lesions are able to induce a KS-like phenotype in endothelial cells and circulating cells and in animal models.14 The fact that in KS lesions the presence of infiltrating CD8+ T cells and γ-interferon (γ-IFN) precedes or is concurrent with detection of HHV-8,15prompted these investigators to suggest a causal relationship between the Th1 CD8+ T cells, the presence of HHV-8, and KS-pathogenesis. In the work by Monini et al10 in this issue of BLOOD, they perform experiments to study the effects of Th1 ICs in KSHV-infected PMBCs. They show that IC increases the KSHV viral load of PMBC from KS-patients and KS-risk groups in vitro and that this is coincident with the appearance of adherent cells having the phenotypic markers of KS-spindle cells. The investigators identify γ-IFN as the Th1 cytokine able to mediate both of these effects. More interestingly, they show that PMBC from some seropositive patients that were KSHV-negative by PCR became KSHV-positive upon incubation with ICs. They performed statistical analysis to demonstrate that this is not due to increased survival and enrichment in KSHV-infected cells, and they show that cells treated with IC express KSHV lytic cycle transcripts. This indicates that viral reactivation is the most likely mechanism for increase in viral load induced by ICs. The investigators propose that a similar mechanism operating in vivo in early KS lesions could be responsible of creating a microenvironment favoring the differentiation of KS-cell circulating progenitors and establishment of a KSHV infection in the lesion.
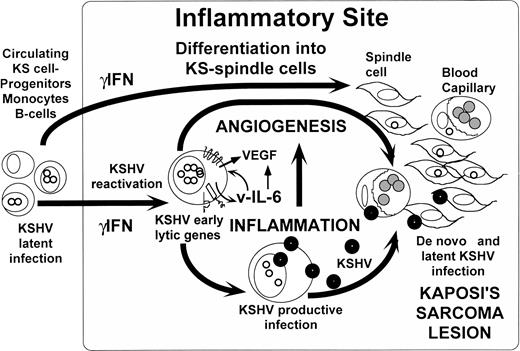
A possible scenario for KS pathogenesis emerging from our current knowledge and these provocative observations (Fig1) is that, when circulating KS-progenitors and cells latently infected by KSHV are exposed to inflammatory sites or increased IC environments, they differentiate into KS-like cells while the latent KSHV is reactivated. Reactivation of KSHV could lead to the expression of some of the potentially pathogenic early genes, such as vIL-6, KSHV-GPCR, and K1,3,4,11,12 and triggers the program of replication of the virus. The expression of pathogenic genes, and the production of virus, further fuels the creation of an inflammatory-angiogenic environment necessary to increase the availability of infectable cells such as endothelial cells8and spindle cells that will end up in the development of the KS lesion. Although most KS-spindle and endothelial cells are latently infected by KSHV, lytically infected spindle cells16 and productively infected monocytes were also found in KS lesions.17 It appears that constant reactivation and reinfection by the virus in the context of immunosupression and immune dysregulation seems to be necessary for sustaining the malignant state in KS, as suggested by the frequent regression of AIDS-KS that often accompanies HAART therapy.6,18 These initial observations by Monini et al10 provide an exciting working hypothesis to start to unveil the connections between the peculiar epidemiology and biology of KS and its etiologic agent KSHV.
Inflammatory reactivation and angiogenicity of KSHV/HHV8. A hypothesis for KS pathogenesis emerging from the observations by Aoki et al9 and Monini et al.10 When circulating KS-progenitors and cells latently infected with KSHV home to inflammatory sites, the exposure to ICs such as γIFN causes their differentiation into KS-like spindle cells and induces KSHV reactivation. Reactivation of KSHV could lead to the expression of potentially pathogenic early genes such as vIL-6 that can activate VEGF and induce angiogenesis. Viral lytic replication in the same cells can activate inflammation, which may also play a proangiogenic role. The creation of this inflammatory-angiogenic environment increases the availability of infectable cells such as endothelial cells and KS-spindle cells that will end up in the development of the KS lesion.
Inflammatory reactivation and angiogenicity of KSHV/HHV8. A hypothesis for KS pathogenesis emerging from the observations by Aoki et al9 and Monini et al.10 When circulating KS-progenitors and cells latently infected with KSHV home to inflammatory sites, the exposure to ICs such as γIFN causes their differentiation into KS-like spindle cells and induces KSHV reactivation. Reactivation of KSHV could lead to the expression of potentially pathogenic early genes such as vIL-6 that can activate VEGF and induce angiogenesis. Viral lytic replication in the same cells can activate inflammation, which may also play a proangiogenic role. The creation of this inflammatory-angiogenic environment increases the availability of infectable cells such as endothelial cells and KS-spindle cells that will end up in the development of the KS lesion.
Supported by Public Health Service Grant No. AI-39192 and a by a New York Community Trust Grant for Blood Disease.
Address reprints requests to Enrique A. Mesri, PhD, Laboratory of Viral Oncogenesis, Division of Hematology-Oncology, Department of Medicine, Weill Medical College of Cornell University, 1300 York Ave C-632, New York, NY 10021; e-mail: eamesri@mail.med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal