To the Editor:
Recent studies by Yamamoto et al1 and Benis and Schneider2 in Blood suggest that Gc-globulin (also known as vitamin D–binding protein) may have an important role in macrophage activation and osteoclast differentiation from monocytes and so may control bone morphogenesis and remodeling.1-5Deglycosylation of Gc-globulin (removal of galactose and sialic acid from the trisaccharide leaving N-acetyl-galactosamine [GalNAc]) produces a potent macrophage-activating factor (Gc-MAF).1In osteopetrotic rat and mice models4,5 and in a single human study,1 indirect data suggest a defect in lysophospholipid-inducible Gc-MAF, although direct estimation of this factor in normal and disease state has never been performed.
In this report, we extend earlier observations by showing a basal level of Gc-MAF generation in normal healthy human subjects, its absence in a patient with infantile autosomal recessive osteopetrosis (IOP), and supression in IOP carriers. The patient, a female infant of related (second cousin) South Asian parents, presented with seizures on the eighth day of life. Investigation showed severe hypocalcemia (plasma calcium, 1.25 mmol/L; albumin 36 g/L), with elevated parathyroid hormone level (208 pg/L reference, 10 to 54 pg/mL). Bone marrow aspirate and biopsy were consistent with a diagnosis of osteopetrosis. The extended family was highly consanguineous, with several marriages between cousins, and a history of osteopetrosis in two distant cousins. A skeletal survey demonstrated uniformly dense bones with associated metaphyseal lucent bands in keeping with osteopetrosis. The father was a genotypical HLA match, and the patient underwent a conditioned unmanipulated bone marrow transplant at the age of 10 months. By day 28 post bone marrow transplant, she had an increasing lymphocyte count with serum Ca2+ 2.29 mmol/L, phosphate 1.27 mmol/L, and albumin 36 g/L. Molecular DNA studies confirmed engrafting.
The measurement of exposed N-acetyl-galactosamine (GalNac) component of Gc-MAF was performed by lectin-based immunoassay using a combination of antibody monospecific to Gc-globulin protein (Diasorin, Berkshire, UK), and Helix pomatia lectin conjugated to horseradish peroxidase (HRP) (Sigma, Dorset, UK), which specifically binds to GalNac. The specificity and reproducibility of the assay used was confirmed using appropriate positive and negative controls.
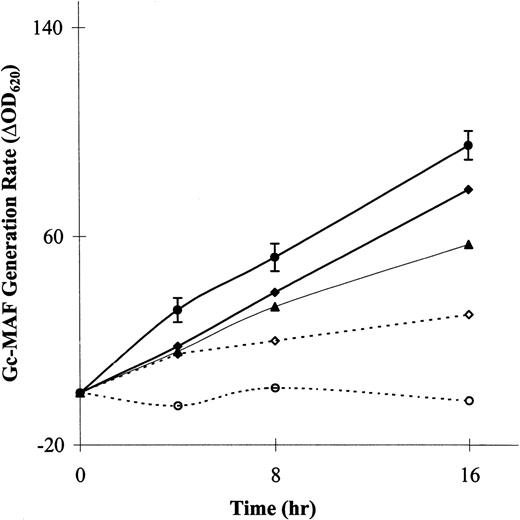
The rates of generation of the factor for the patient, her parents, and for male control subjects (n = 9) are shown in Fig1. All control subjects showed basal noninduced generation of Gc-MAF when lymphocytes were incubated with the plasma Gc-globulin (ΔOD620 range, 84 to 180; mean ± SD, 95 ± 18). The patient showed complete lack of generation of Gc-MAF pretransplantation, despite prolonged incubation of lymphocytes and plasma, lasting 16 hours. After transplantation, Gc-MAF generation was shown. The patient’s parents showed only 60% to 80% of the Gc-MAF generation of controls, findings in keeping with this recessive trait. The generation of activating factor was constant over the first 8 hours; thereafter, there was slight decline in the rate of production. The plasma Gc-globulin concentrations in the control subjects (range, 25 to 47; mean ± SD, 35 ± 5.3 mg/100 mL), the patient (37 mg/100 mL), and the carriers (24 and 27 mg/100 mL for mother and father, respectively) were quite similar. All levels were found to be within the reference range (male, 24 to 47 mg/100 mL and female 20 to 47 mg/100 mL).
Noninducible basal Gc-MAF generation from plasma Gc-globulin by lymphocytes in male control subjects, osteopetrotic patient, and her parents. The patient showed complete lack of generation of Gc-MAF before transplantation, but after transplantation Gc-MAF generation was evident. (•), Controls; (⧫), father; (▴), mother; (○), op/op; (◊), op/op (RX).
Noninducible basal Gc-MAF generation from plasma Gc-globulin by lymphocytes in male control subjects, osteopetrotic patient, and her parents. The patient showed complete lack of generation of Gc-MAF before transplantation, but after transplantation Gc-MAF generation was evident. (•), Controls; (⧫), father; (▴), mother; (○), op/op; (◊), op/op (RX).
Previous studies had assumed that Gc-MAF generation and, therefore, the enzymes involved in its production are only induced as part of an inflammatory response.4,5 In these investigations only lysophospholipid-inducible enzymes (B-lymphocyte β-galactosidase and T-lymphocyte sialidase) and Gc-MAF generation were measured and inducible Gc-MAF generation was thought to be defective. The results of these studies suggest that lymphocytes play an important role in modulating osteoclast activity. This is not altogether surprising because osteoclasts are members of the macrophage/monocyte lineage. The modulation of macrophage function by T lymphocytes is well recognized, and a defect in T lymphocytes is responsible for uncontrolled macrophage proliferation in Griscelli and Chediak-Hegashi syndromes.6 Our own experience has shown that osteopetrotic patients have a surprisingly higher incidence of pre and post bone marrow transplant viral infections than other patients (unpublished observations, March 1997). Further studies of T- and B-cell function in IOP are needed as well as work on the precise contribution of Gc-MAF in the activation of other cells of macrophage/monocyte and osteoclast lineages. It is also of note that infants born with absent T cells (T-negative severe combined immunodeficiency) do not show features of osteopetrosis, so other cell lineages must also generate Gc-MAF. These observations imply that Gc-MAF may have a hitherto unrecognized role in normal bone morphogenesis and remodeling. A recent unpublished observation suggests that lymphocytes may have a prominent role in osteoclastogenesis from monocytes (R.M. Kanan and H.K. Datta, February 1999). The putative role of lymphocytes in osteoclast activation and differentiation may in fact be mediated by Gc-MAF acting in concert with recently described cytokine TRANCE/RANKL (tumor necrosis factor–related activated-induced cytokine).7 The results of this study and other investigations in animal models suggest that the phenotypic manifestation of osteopetrosis may arise from defective Gc-MAF generation. Infusion of Gc-MAF into affected individuals may mitigate the life-threatening effect of IOP.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal