Abstract
We have investigated the expression and functional competence of folate receptor (FR) isoforms on human hematopoietic cells. Using immunofluorescence and reverse transcriptase-polymerase chain reaction (RT-PCR) methodology, we find that a substantial fraction of low-density mononuclear and CD34+ cells express both the β and γ isoforms of FR. The isoform of FR (the form most commonly found on cancer cells) was surprisingly absent from all hematopoietic cells examined. Compared with KB cells (a human cell line known for its elevated expression of FR-), the abundance of FR-β on CD34+ cell surfaces was relatively low (≈8% of KB cell levels). Because many antifolates and folic acid-linked chemotherapeutic agents enter malignant cells at least partially via FR endocytosis, it was important to evaluate the ability of FR on CD34+ cells to bind folic acid (FA). Based on three FR binding assays, freshly isolated CD34+ cells were found to display no affinity for FA. Thus, regardless of whether steps were taken to remove endogenous folates before receptor binding assays, FR on primitive hematopoietic cells failed to bind 3H-FA, fluorescein isothiocyanate (FITC)-linked FA, or FA-derivatized liposomes. In contrast, analogous studies on KB cells showed high levels of receptor binding for all three FR probes. These studies show that although multipotent hematopoietic progenitor cells express FR, the receptor does not transport significant amounts of FA. Consequently, antifolates and FA-linked chemotherapeutic agents that can be engineered to enter malignant cells exclusively through the FR should not harm progenitor/stem cell function.
THE FOLATE RECEPTOR (FR) is a single-chain cell-surface glycoprotein that binds folic acid (FA) and mediates uptake of the vitamin by receptor-mediated endocytosis.1-4 Three isoforms of the FR have been identified and cloned to date. These are FR-α from KB cells,5 CaCo-2 cells,6 and placenta7; FR-β from placenta8; and FR-γ and its truncated cogener, FR-γ’, from malignant hematopoietic cells.9 These three FR isoforms share ≈70% sequence identity,8 but FR-α and FR-β are attached to the cell surface by a glycosylphosphatidylinositol anchor, while FR-γ is secreted due to lack of an efficient signal for glycosylphosphatidylinositol modification.9 10 FR-γ is also generally expressed at levels much lower than FR-α and FR-β.
Despite divergence in their carboxyterminal sequences, FR-α and FR-β display relatively similar affinities for FA, with Kd values of ≈10−10 mol/L and 10−9 mol/L, respectively.11 However, FR-α and FR-β differ in their stereospecificities for reduced folate coenzymes, with FR-α having a significantly higher affinity (50-fold) than FR-β for the physiologic (6S) diastereoisomer of N5-methyltetrahydrofolate.11-13 Importantly, even the α isoform of FR binds FA much more avidly (≈10 times) than it does any of the more reduced forms of the vitamin.11
FR isoforms are not evenly distributed among the various tissues and cell types of the body. Rather, FR-α is primarily expressed on normal epithelial cells and upregulated in malignant tissues deriving from the same cell types.14-17 Similarly, FR-β, which is less well characterized than FR-α, may be overexpressed primarily in neoplastic tissues of nonepithelial origin.15 As a consequence of this upregulation, FR has not only been exploited clinically as a diagnostic marker for tumor tissue,14-17 but it has further been used preclinically as a targeting receptor for delivery of imaging and therapeutic agents to cancer cells.18-20 Thus, when FA is covalently linked to a molecule via its γ-carboxyl group, its affinity for cell-surface FR remains essentially unaltered, and the cell internalizes the FA-conjugate in a manner similar to FA. Based on this strategy, FR has been used to target FA-conjugated toxins,21 FA-linked imaging agents,18,22FA-tethered liposomes,23,24 FA-drug conjugates,20 FA-linked genes,25,26FA-derivatized antibodies,27,28 and various antifolates29 to cancer cells.
A major concern with any form of chemotherapy or radiation therapy centers on the toxicity of the therapeutic agent to the cancer relative to its toxicity to the bone marrow. Within the bone marrow are hematopoietic stem and progenitor cells that exhibit the ability to proliferate and differentiate into multiple hematopoietic lineages. Because FA-linked therapeutic agents do not discriminate among the various FR-expressing cells, the relative abundance and functional activity of FR on malignant cells versus hematopoietic stem cells may be decisive in determining which tumors can be targeted with acceptable selectivity over stem cells. Although no information is currently available on FR levels in CD34+ cells (ie, the hematopoietic cell population containing stem and progenitor cells), FR have been identified on mature erythrocytes, albeit in inactive form,30 and also on erythroid hematopoietic progenitor cells (burst-forming unit-erythroid [BFU-E]).31 In addition, FR have been detected on pluripotential (colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte [CFU-GEMM]) and myeloid (colony-forming unit–granulocyte-macrophage [CFU-GM]) hematopoietic progenitors,31 as well as differentiated myelocytic cells.32 In this study, we have undertaken to evaluate the abundance and functional activity of FR on CD34+ bone marrow cells. We report here that only the β isoform and not the α isoform of FR is measurably expressed on CD34+ cells, albeit at lower levels than those observed for FR-α on cultured cancer cells. We further show that FR-β on CD34+ cells is unable to bind FA, even though the β-isoform is shown to readily mediate uptake of 3H-FA and FA-conjugates after transfection into A549 cells (an FR-negative cell line).
MATERIALS AND METHODS
Cells.
KB cells, a human nasopharyngeal epidermal carcinoma cell line, and A549 cells, a human lung carcinoma cell line (Purdue Cancer Center, West Lafayette, IN), were cultured at 37°C in a humidified atmosphere containing 5% CO2. The cells were grown continuously as a monolayer in folate-deficient Dulbecco’s modified Eagle’s medium (FDMEM) (GIBCO-BRL, Gaithersburg, MD) supplemented with 10% heat inactivated fetal bovine serum (Hyclone Laboratories, Logan, UT), penicillin (50 U/mL), streptomycin (50 μg/mL), and 2 mmol/L L-glutamine. The normal complement of endogenous folates in the fetal bovine serum brings the net folate concentration in the growth medium to the low end of the physiologic range of folate concentrations found in human plasma.
Bone marrow cells were obtained in heparinized syringes from healthy adult donors. Low-density mononuclear cells (LDMNC) were prepared by centrifugation on Ficoll-Hypaque (density 1.077 g/mL; Sigma Chemical Company, St Louis, MO) for 30 minutes at 25°C. The institutional review boards at Indiana and Purdue University have approved all experiments.
Specificity of the anti-FR antiserum.
The human placental FR was isolated to apparent homogeneity based on its migration as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analytical gel filtration chromatography.33 This purified protein was used to generate polyclonal rabbit anti-human placental FR antiserum. Subsequent studies8 have indicated that the purified human placental FR used to generate the polyclonal antiserum contains both FR-α and FR-β isoforms. Consistent with this observation, we have found that the anti-FR antiserum cross-reacts with both α and β FR isoforms. Additional data on the homogeneity of the placental FR antigen used to generate the antiserum has been provided by immunodiffusion studies.34
Studies that demonstrate that the strong fluorescence on the surface of LDMNC from normal human bone marrow is the result of interaction of a single species with the anti-FR antiserum have also been published.34 In addition, following the surface iodination of 9 × 107 LDMNC, only a single species of glycosylated protein at ≈44 kD was immunoprecipitated with anti-FR antiserum. Moreover, immunoprecipitation of particulate membrane proteins from 4 × 108 LDMNC similarly identified only a single glycosylated FR. Therefore, these data show that anti-FR antiserum recognizes only a single species on the surface of LDMNC.
Flow cytometric analysis of LDMNC and KB cells.
To phenotypically define which subsets of CD34+ cells express FR, 2 × 106 LDMNC were stained with anti-CD34-allophycocyanin (APC) and anti-CD38-phycoerythrin (PE) or anti-CD15-PE (PharMingen, San Diego, CA) for 20 minutes at 4°C and washed twice before anti-FR incubation. For cell-surface labeling of FR, 2 × 106LDMNC or KB cells were incubated with either 2 μL rabbit preimmune serum or rabbit anti-human FR antibody for 30 minutes on ice. The cells were then washed, incubated similarly with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG, washed again three times, and analyzed by flow cytometry on a FACScan (Becton Dickinson, San Jose, CA).
Purification of CD34+ cells by immunomagnetic selection.
CD34+ cells were initially purified by immunomagnetic selection from LDMNC as previously described.35 Briefly, mouse anti-human CD34 antibody (9C5; Baxter, Santa Anna, CA) was added to cells (0.5 mg/106 LDMNC) and incubated on ice for 15 minutes. Cells were incubated with immunomagnetic microspheres coated with sheep anti-mouse IgG1 (Dynal, Lake Success, NY) for 30 minutes at 4°C. The cell mixture was placed on a Dynabead magnet (Dynal). In some experiments, adherent cells were enzymatically cleaved from microspheres with chymopapain (Baxter, McGaw Park, IL). The magnet was reapplied and CD34+ cells were collected from the nonadherent fraction. The CD34+ cells were placed in folate-deficient RPMI (GIBCO-BRL) containing 2% fetal calf serum and either used immediately or stimulated overnight in the presence of 200 U/mL recombinant interleukin-6 (Boehringer Mannheim, Indianapolis, IN) and 100 ng/mL recombinant stem cell factor (a generous gift from Amgen, Thousand Oaks, CA). In other experiments, CD34+ cells were purified using the MACS system (Miltyeni Biotech, Auburn, CA), which does not require the use of chymopapain. Results were similar using either system of CD34+ purification. CD34+ cell purity was determined by fluorescence cytometry and ranged from 87% to 95% after immunomagnetic selection. When desired, CD34+cells were washed with 1 mL phosphate-buffered saline (PBS) two times and once with 1 mL 0.15 mol/L NaCl, 10 mmol/L Na-acetate, adjusted to pH 3.5 to strip any externally bound folate from the cell surface. After folate elution, the cells were returned to FDMEM.
Purification of CD34+ cells for reverse transcriptase-polymerase chain reaction (RT-PCR).
The adherent cells were incubated with mouse anti-human CD34 conjugated with PE (PharMingen, San Diego, CA). Cells were sorted by FACS (Becton Dickinson) to obtain pure populations of hematopoietic cells for RT-PCR. Bone marrow samples contained 1 to 2 × 106CD34+ cells. Postsort analysis of cells was performed using fluorescence cytometry to ensure purity.
RT-PCR.
Total RNA was isolated from CD34+ cells using the Tri-reagent method exactly as specified by the manufacturer (Molecular Research Center, Cincinnati, OH). A total of 0.5 μg of RNA was reverse transcribed using a reverse transcription system as specified by the manufacturer (Promega, Madison, WI). Mouse RNA was used as a negative control for the reverse transcription.
Amplification of total cellular RNA from each hematopoietic cell population was performed using primers and conditions that have been previously reported.9 The primers that were used and the expected size of RNA amplified from each isoform are listed below.
α 5′CATGGCTGCAGCATAGAACCTCGC3′ (sense) 639 bp
5′GTAGTAGGGGAGGCTCAGACAAGG 3′ (antisense)
β 5′CATGGCTGCAGCATAGAACCTCGC3′ (sense) 501 bp
5′CACAGCCAGCACCAGCCAGGAGCTG3′ (antisense)
γ 5′AGCGCATTCTGAACGTGCCCCTG3′ (sense) 357 bp
5′CAGGAATCAATAATCCCACGAGACCG3′ (antisense)
β-actin 5′TGACGGGGTCACCCACACTGTGCCCATCTA3′ (sense) 361 bp
5′CTAGAAGCATTGCGGTGGACGATGGAGGG3′ (antisense)
A total of 20 μL of PCR reaction mixture was electrophoresed on a 2% agarose gel and transferred to a nylon filter (Gene Screen Plus, New England Nuclear Corp, Boston, MA). The filter was hybridized using the appropriate FR isoform cDNA labeled with 32P deoxycytidine triphosphate (dCTP). Hybridization was performed at 68°C for 24 hours. Filters were washed at 55°C for 15 minutes and exposed to Kodak XAR film (Eastman-Kodak, Rochester, NY) at −80°C for 24 hours. RNA samples were amplified without reverse transcription to ensure there was no contaminating genomic DNA. cDNAs for the α, β, and γ FR isoforms were used as positive PCR controls. β-Actin was used as an internal control.
Liposome preparation.
FA-derivatized liposomes were constructed by conjugating FA to distearoylphosphatidylethanolamine (DSPE) via a 250Å long polyethyleneglycol (PEG) spacer (FA-PEG-PE), and incorporating the FA tethered lipid at 2 mol% in a 1:1 molar mixture of egg phosphatidylcholine and cholesterol.23 24 Control (nontargeted) liposomes were similarly formulated using nontargeted PEG-PE in place of FA-PEG-PE. Briefly, 50 mg of egg phosphatidylcholine, 16.5 mg of cholesterol (Avanti Polar Lipids, Alabaster, AL), and 5 mg of FA-PEG-DSPE or PEG-DSPE were dissolved in 3 mL of chloroform. The lipids were dried under reduced pressure to form a thin film and then rehydrated in 0.5 mL of 10 mmol/L calcein in PBS, pH 7.4. The suspension was then subjected to 10 cycles of freezing and thawing and extruded 10 times through a 100-nm polycarbonate membrane (Nucleopore, Pleasanton, CA). The resulting liposomes encapsulating the fluorescent dye were then separated from free calcein on a Sepharose CL-4B column (Pharmacia, Uppsala, Sweden) preequilibrated with PBS. The final lipid concentration was ≈67 mg/mL.
Uptake of FA-PEG-liposomes by cells.
FA-PEG-liposomes or PEG-liposomes encapsulating calcein (50 μL) were diluted in 1 mL FDMEM and added to CD34+ cells (1 × 105) or to monolayers of KB cells (5 × 105). The cells were then incubated at 37°C for 4 hours. The cells were washed three times with PBS and analyzed by fluorescence cytometry on a FACScan (Becton Dickinson). From every sample, 10,000 gated events (light scatter gates were used to discard cellular debris) were collected in the list-mode. List-mode data were then analyzed using PC-LYSY5 analysis software (Becton Dickinson).
Retroviral packaging cell line construction.
The retroviral backbone MSCVneo was obtained from Dr Robert Hawley (Sunnybrook Health Science Centre, Toronto, Ontario, Canada).36 All molecular biology reagents were obtained from Boehringer Mannheim unless otherwise stated. MSCVneo plasmid was digested with XhoI restriction endonuclease. The cDNA for the FR-β isoform, generously provided by Dr Manohar Ratnam (Medical College of Ohio, Toledo), was adapted withXhoI linkers and ligated into the retroviral backbone using T4 DNA ligase. The ligated product was transformed into competent DH5α cells (GIBCO-BRL), plated onto ampicillin plates, and colony hybridization was performed on resistant colonies using the full cDNA as a probe. The completed retrovirus plasmid was transfected as previously described37,38 into GP+Am12 packaging cells provided by Dr Arthur Bank (Columbia University, New York, NY).39 Supernatant was collected from individual clones and titered on NIH3T3 cells for neomycin resistance as previously described.37 38 The clone chosen for all further experimentation had a neomycin resistance titer of 1 × 106.
Expression of FR-β in A549 cells.
A549 cells were transfected with recombinant retroviral supernatants from the above high titer FR-β clone. Transduced A549/FR-β cells were selected for neomycin resistance gene expression by growing in 1.0 mg geneticin/mL for 4 weeks. Untransduced A549 cells were used as the negative control in all experiments.
Synthesis of FA-conjugated I125-labeled bovine serum albumin (BSA).
Folic acid (10 mg; Sigma Chemical Co) was dissolved in anhydrous dimethyl sulphoxide (DMSO) and incubated under stirring with 25 mg 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide for 30 minutes at room temperature. The solution was then added to 150 mg BSA in 2 mL PBS, pH 7.4. After a 2-hour incubation with stirring at room temperature, the reaction mixture was passed through a PD-10 desalting column (Bio-Rad Laboratories, Hercules, CA) equilibrated in PBS to separate the conjugated protein from excess free FA. The extent of FA conjugation was determined to be approximately 2.5 FAs per BSA molecule.40 To label the FA-BSA conjugate with I125, FA-BSA was dissolved in PBS and added to prewashed Iodobeads (Pierce, Rockford, IL). NaI125 (Amersham, Arlington Heights, IL) was then added and the mixture was incubated for 30 minutes at room temperature. Free I125 was separated from FA-BSA-I125 using a PD-10 desalting column equilibrated in PBS, pH 7.4.
Measurement of 3H-FA or I125-BSA-FA uptake.
Cells were plated in 35-mm culture dishes at 5 × 105cells per dish 48 hours before each experiment. Cells were incubated with 100 nmol/L I125-BSA-FA or 3H-FA in 1 mL of folate-deficient medium for 2 hours at 37°C. Each dish was then washed either with 1 mL PBS three times to remove unbound FA or FA-conjugate, or with 1 mL PBS two times and once with 1 mL 0.15 mol/L NaCl, 10 mmol/L Na-acetate, adjusted to pH 3.5 to remove both unbound material and strip any externally bound FA/FA-conjugates from the surface. Cells were then lysed in 1% Triton X-100. The number of molecules taken up/endocytosed per cell was calculated from the measured radioactivity in the lysis medium.
3H-FA binding assay for secreted FR.
KB cells (5 × 105) or CD34+ cells (5 × 106) were washed twice with 1 mL PBS and incubated with 1 mL of FDMEM containing 50 pmol of 3H-FA at 37°C for either 2 hours or 20 hours. The medium was then collected and any suspended cells were removed by centrifuging at 5,000g for 15 minutes. The supernatant was then fractionated on a Sephadex G-25 column equilibrated in PBS to separate free 3H-FA from protein bound 3H-FA. The radioactivity in each 0.25-mL fraction was measured in a liquid scintillation counter.
RESULTS
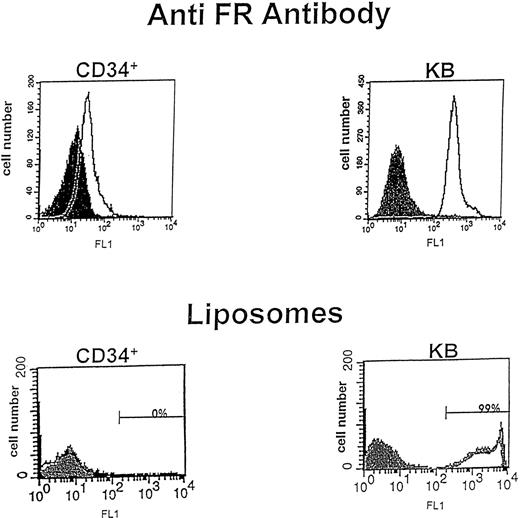
To obtain an initial indication of whether CD34+hematopoietic cells might express an FR, a polyclonal antibody that recognizes both FR-α and FR-β was incubated with purified CD34+ cells and quantitated using fluorescence cytometry. As shown in Fig 1A, a measurable fraction of CD34+ cells expressed a cross-reactive polypeptide, albeit at a relatively low level. Thus, using a gate that excluded greater than 98% of cells labeled with preimmune serum, ≈20% of CD34+ cells were FR positive as detected by the FR antibody. These data suggest that a significant number of hematopoietic stem/progenitor cells synthesize an isoform of FR and express FR on the cell surface.
Flow cytometric analysis for expression of the FR. (Top) CD34+ cells and KB cells were incubated with rabbit preimmune serum or rabbit polyclonal antibody to placental FR for 30 minutes, washed, and incubated with secondary antibody (goat anti-rabbit IgG-FITC) for 30 minutes to evaluate FR expression on each cell population. Cells were then analyzed by fluorescence flow cytometry, where log cell fluorescence is plotted on the x-axis and cell number on the y-axis. The filled peak corresponds to cells treated only with preimmune serum, while the open peak shows cells labeled with anti-FR IgG. The results indicate that both CD34+ cells and KB cells express FR on their cell surfaces. (Bottom) CD34+ cells and KB cells were incubated with FA-PEG-liposomes or PEG-liposomes containing calcein for 4 hours, washed, and analyzed by fluorescence flow cytometry. The filled peak represents PEG liposome staining and the open peak represents FA-PEG-liposome staining. For CD34+ cells, the peaks for FA-PEG-liposomes and PEG-liposomes overlay one another, showing no detectable binding to FR. In contrast, FA-PEG-liposomes bind well to KB cells.
Flow cytometric analysis for expression of the FR. (Top) CD34+ cells and KB cells were incubated with rabbit preimmune serum or rabbit polyclonal antibody to placental FR for 30 minutes, washed, and incubated with secondary antibody (goat anti-rabbit IgG-FITC) for 30 minutes to evaluate FR expression on each cell population. Cells were then analyzed by fluorescence flow cytometry, where log cell fluorescence is plotted on the x-axis and cell number on the y-axis. The filled peak corresponds to cells treated only with preimmune serum, while the open peak shows cells labeled with anti-FR IgG. The results indicate that both CD34+ cells and KB cells express FR on their cell surfaces. (Bottom) CD34+ cells and KB cells were incubated with FA-PEG-liposomes or PEG-liposomes containing calcein for 4 hours, washed, and analyzed by fluorescence flow cytometry. The filled peak represents PEG liposome staining and the open peak represents FA-PEG-liposome staining. For CD34+ cells, the peaks for FA-PEG-liposomes and PEG-liposomes overlay one another, showing no detectable binding to FR. In contrast, FA-PEG-liposomes bind well to KB cells.
For FR to be useful as a port of entry for the selective uptake of antifolates and FA-conjugated chemotherapeutic drugs by cancer cells, the level of FR expression on cancer cells must significantly exceed its level on bone marrow cells. To obtain a preliminary estimate of the relative magnitude of FR expression on CD34+ cells and cancer cells, the same analysis was conducted on KB cells, a human nasopharyngeal epidermal carcinoma cell line known for its elevated expression of FR-α.5 As seen in Fig 1, the mean fluorescence intensity of the sorted KB cells was ≈12 times higher than the corresponding value for CD34+ cells. However, because human ovarian cancer tissue expresses FR at ≈1/20 the level of cultured KB cells (Mary Jo Turk, et al, unpublished observations, May 1998), we conclude that FR expression on CD34+ cells is comparable to its expression in at least one human cancer tissue.
Two additional experiments were performed in an attempt to more accurately quantitate the number of FR on the surface of CD34+ cells. First, FA-targeted liposomes containing encapsulated calcein as a fluorescent marker were incubated with both the CD34+ and KB cells to allow liposome binding and the consequent quantitation of FR abundance. As shown previously23 24 and reconfirmed by flow cytometry in Fig 1, KB cells bind the fluorescent FA-linked liposomes avidly, shifting several log units along the fluorescence axis after incubation with the liposomes containing FA-PEG-PE as their targeting ligand. Furthermore, nontargeted liposomes, containing PEG-PE instead of FA-PEG-PE, predictably display no affinity for the FR+ cultured cells. In contrast, CD34+ cells, which exhibited clear immunologic evidence for cell-surface FR expression, show no capacity to bind FA-linked liposomes (Fig 1), suggesting that FR on CD34+cells are unable to bind ligand. To confirm the refractory nature of the FR on CD34+ cells, the same cells were incubated with either 100 nmol/L 3H-FA or a FA-FITC conjugate shown previously to bind avidly to FR-expressing cells. Consistent with the liposome data, no significant binding of either FA compound is observed (Fig 2). Finally, to ensure that even very low levels of FR were not escaping detection, we examined binding with a high specific activity I125-labeled FA-BSA conjugate and again found no detectable binding to CD34+ cells (data not shown). These data suggest that the FR on freshly isolated CD34+ cells exists in a state that binds neither FA nor its conjugates.
Comparison of binding of three FR ligands and FR antiserum to KB and CD34+ cells. KB cells (□) and CD34+ cells (▨) were incubated with fluorescent FA-derivatized liposomes (FA-liposome; 4 hours at 37°C), FA labeled with fluorescein (FA-FITC; 1 hour at 37°C), 3H-FA (3H-FA; 2 hours at 37°C), or rabbit antiserum to placental FR (anti-FR; 30 minutes at 4°C). Fluorescent ligand binding to cells was quantitated by FACS, while 3H-FA binding was determined by scintillation counting. Relative uptake of fluorescent ligands was calculated from the product of average cell fluorescence intensity and percentage of positively gated cells. To display the number of 3H-FA molecules taken up per cell on the same axis as the fluorescent ligands, 3H-FA/cell values were divided by a factor of 20.
Comparison of binding of three FR ligands and FR antiserum to KB and CD34+ cells. KB cells (□) and CD34+ cells (▨) were incubated with fluorescent FA-derivatized liposomes (FA-liposome; 4 hours at 37°C), FA labeled with fluorescein (FA-FITC; 1 hour at 37°C), 3H-FA (3H-FA; 2 hours at 37°C), or rabbit antiserum to placental FR (anti-FR; 30 minutes at 4°C). Fluorescent ligand binding to cells was quantitated by FACS, while 3H-FA binding was determined by scintillation counting. Relative uptake of fluorescent ligands was calculated from the product of average cell fluorescence intensity and percentage of positively gated cells. To display the number of 3H-FA molecules taken up per cell on the same axis as the fluorescent ligands, 3H-FA/cell values were divided by a factor of 20.
One possible explanation for the inability of CD34+ cells to bind FA and its conjugates is that the stem/progenitor cells could secrete significant quantities of FR, which would then compete for binding to FR on the cell surface. To address this possibility, CD34+ and KB cells were incubated for 2 hours or 20 hours in their normal growth medium (FDMEM) containing 50 nmol/L3H-FA to allow 3H-FA binding to any competing form of FR that might have been secreted/released into the medium. After incubation, the cell culture medium was collected and analyzed by gel filtration chromatography for protein bound 3H-FA. As shown in Fig 3, considerable FA binding activity was observed in the conditioned medium from the 2-hour incubated KB cells, but no increase in 3H-FA binding could be detected in either the 2-hour or 20-hour CD34+ cell supernatant above the level already present in the unmodified FDMEM. These data suggest that neither FR-γ nor any other isoform of the FR is secreted into the medium of CD34+ cells over at least the 20-hour duration of the experiment. In contrast, the supernatant from the KB cells (which numbered 10-fold less than the number of CD34+ cells used) contained a substantial amount of folate binding protein not found in the medium in which the cells were grown. This soluble protein could be a released form of FR-α, which could have been generated by the action of either a membrane-associated metalloprotease or an endogenous phospholipase.41 42
Gel filtration analysis of FR secretion by KB and CD34+ cells. KB cells (▿) and CD34+ cells (▾, ○) were incubated for 2 hours (▿, ▾) or 20 hours (○) in FDMEM containing 50 nmol/L 3H-FA. After incubation, the growth medium was collected, and protein bound 3H-FA was separated from free 3H-FA by gel filtration chromatography on a Sephadex G-25 column. For comparison, fresh (unconditioned) FDMEM was treated with 3H-FA and evaluated similarly (•). Protein bound 3H-FA eluted at ≈3 mL, while free3H-FA eluted beyond 5 mL. Although considerable protein-bound 3H-FA was apparently generated during the 2-hour incubation of KB cells, no measurable increase in3H-FA binding capacity was detected in either the 2-hour or 20-hour supernatant of the CD34+ cells above the level present in the unconditioned FDMEM.
Gel filtration analysis of FR secretion by KB and CD34+ cells. KB cells (▿) and CD34+ cells (▾, ○) were incubated for 2 hours (▿, ▾) or 20 hours (○) in FDMEM containing 50 nmol/L 3H-FA. After incubation, the growth medium was collected, and protein bound 3H-FA was separated from free 3H-FA by gel filtration chromatography on a Sephadex G-25 column. For comparison, fresh (unconditioned) FDMEM was treated with 3H-FA and evaluated similarly (•). Protein bound 3H-FA eluted at ≈3 mL, while free3H-FA eluted beyond 5 mL. Although considerable protein-bound 3H-FA was apparently generated during the 2-hour incubation of KB cells, no measurable increase in3H-FA binding capacity was detected in either the 2-hour or 20-hour supernatant of the CD34+ cells above the level present in the unconditioned FDMEM.
An alternative explanation for the inability of FR on CD34+cells to bind FA proposes that during overnight stimulation of the cells in folate-deficient medium, the receptor might be degraded by endogenous proteinases. To explore this issue, freshly isolated CD34+ cells were immediately examined for 3H-FA binding before they were stimulated with cytokines or processed further in any manner. As previously seen in Fig 2, the cells were again found to be totally refractory to the added FA (data not shown). These data confirm that the FR isoform on the surface of CD34+ cells does not participate in FA binding.
FR expression increases with differentiation of CD34+cells.
CD34+ antigen has been used to phenotypically characterize hematopoietic stem/progenitor cells. CD34+ cells can be further fractionated into subpopulations of immature and more differentiated cells by evaluation of coexpression with other antigens. For example, CD34+CD38+ cells contain more mature progenitors and differentiated cells than CD34+CD38− cells, which contain immature stem/progenitor cells. In addition, coexpression of CD15 and CD34 defines a more differentiated population of myeloid cells. To determine which subpopulations of CD34+ cells express FR, three-color flow cytometry was conducted. As shown in Fig 4A, both CD34+CD38− and CD34+CD38+ cells express FR. Interestingly, CD34+CD38+ cells exhibit a slightly higher level of FR expression compared with CD34+CD38− cells. When CD34+CD15+ cells were similarly evaluated, FR expression was seen to be even further enhanced, as shown by the increased fluorescence intensity and increased proportion of cells expressing the FR (Fig 4B). From these data, it is clear that multiple subpopulations of CD34+ cells express FR, including the most immature population evaluated (CD34+CD38−). Further, these data show that as CD34+ cells become more differentiated, FR expression increases.
Comparison of FR expression on multiple subpopulations of CD34+ cells. LDMNC were stained with anti–CD34-APC and either (A) anti–CD38-PE or (B) anti–CD15-PE (20 minutes at 4°C). After washing, the cells were incubated with either rabbit preimmune serum or anti-FR (30 minutes at 4°C), washed, and incubated with the secondary antibody, goat anti-rabbit-FITC (30 minutes at 4°C) before analyzing the cells by three-color fluorescence cytometry. (A) FR expression (FL1 on x-axis) after anti-FR staining is shown for CD34+CD38− (light gray) and CD34+CD38+ (black) cells. CD34+ cells that were incubated with preimmune serum are represented by the dark gray peak in both (A and B). These data indicate that a significant proportion of CD34+ cells express FR and that CD34+CD38+ cells have slightly higher levels of FR expression than CD34+CD38− cells. (B) FR expression levels on CD34+CD15− (black) and CD34+CD15+ (light gray) cells are shown on this histogram. The results show that the more differentiated CD34+CD15+ cells have an increased proportion that express FR. In addition, CD34+CD15+ cells are shifted to the right further than any other population evaluated, suggesting that these cells have an increased number of FR/cell.
Comparison of FR expression on multiple subpopulations of CD34+ cells. LDMNC were stained with anti–CD34-APC and either (A) anti–CD38-PE or (B) anti–CD15-PE (20 minutes at 4°C). After washing, the cells were incubated with either rabbit preimmune serum or anti-FR (30 minutes at 4°C), washed, and incubated with the secondary antibody, goat anti-rabbit-FITC (30 minutes at 4°C) before analyzing the cells by three-color fluorescence cytometry. (A) FR expression (FL1 on x-axis) after anti-FR staining is shown for CD34+CD38− (light gray) and CD34+CD38+ (black) cells. CD34+ cells that were incubated with preimmune serum are represented by the dark gray peak in both (A and B). These data indicate that a significant proportion of CD34+ cells express FR and that CD34+CD38+ cells have slightly higher levels of FR expression than CD34+CD38− cells. (B) FR expression levels on CD34+CD15− (black) and CD34+CD15+ (light gray) cells are shown on this histogram. The results show that the more differentiated CD34+CD15+ cells have an increased proportion that express FR. In addition, CD34+CD15+ cells are shifted to the right further than any other population evaluated, suggesting that these cells have an increased number of FR/cell.
β and γ, but not α FR isoforms are expressed on CD34+ cells.
When total RNA from various cell types was reverse transcribed, amplified by PCR and hybridized using the appropriate 32P dCTP-labeled FR isoform cDNA, the FR-α, FR-β, and FR-γ transcripts gave the expected cDNA fragments of 639, 501, and 357 bp, respectively. RT-PCR of LDMNC and CD34+ cells from three individual bone marrow specimens also yielded reproducible FR isoform expression patterns. Figure 5 illustrates results obtained from one representative experiment. The data show that LDMNC do not express FR-α, but do express FR-β and FR-γ. Similarly, CD34+ cells express both the β and γ FR isoforms. Because FR-γ is secreted and not retained on the cell surface,9 10 it will not have contributed to the flow cytometry analysis in Figs 1 and 4. Taken together, these data confirm the expression of FR in CD34+ cells.
RT-PCR of FR isoforms in hematopoietic cells. RNA was isolated from LDMNC and CD34+ cells. RNA was reverse transcribed into cDNA and sequences unique to the , β, and γ FR isoforms were amplified. PCR reactions were separated on a 2% agarose gel, transferred to a nylon filter, and hybridized using the appropriate FR isoform cDNA. cDNAs for each FR isoform were used as positive controls and mouse RNA was used as a negative control. LDMNC and CD34+ cells express the FR-β and FR-γ isoforms but not FR-.
RT-PCR of FR isoforms in hematopoietic cells. RNA was isolated from LDMNC and CD34+ cells. RNA was reverse transcribed into cDNA and sequences unique to the , β, and γ FR isoforms were amplified. PCR reactions were separated on a 2% agarose gel, transferred to a nylon filter, and hybridized using the appropriate FR isoform cDNA. cDNAs for each FR isoform were used as positive controls and mouse RNA was used as a negative control. LDMNC and CD34+ cells express the FR-β and FR-γ isoforms but not FR-.
Evaluation of FR-β binding in a transfected cell line.
The lack of detectable FA uptake by FR on CD34+ cells was not anticipated. Indeed, FA binding to both α and β isoforms of FR on other cell types has already been documented,11 and endocytosis by FR-α receptors is equally well established.1 A very recent study has also suggested that FR-β can mediate endocytosis of 3H-FA.43Nevertheless, to confirm that FR-mediated endocytosis of FA and its conjugates can indeed be catalyzed by β isoform receptors, two additional studies were conducted. First, a cultured cell line (A549 cells) lacking measurable FR was transduced with FR-β and then shown to express the FR transgene product on its cell surface (compare FACS of the parent and FR-β transduced A549 cells in Fig 6A). Examination of 3H-FA uptake by the same cells (Fig 6B) shows that the A549/FR-β cells bind significantly more of the labeled ligand (1.1 × 106molecules/cell) than the A549 controls (2.1 × 105molecules/cell). Further, when the cells are briefly subjected to low pH treatment to remove externally bound folate, 7.6 × 105 molecules of 3H-FA remain associated with the A549/β-FR cells. Because this fraction of acid resistant ligand is generally accepted as the internalized population of3H-FA, we conclude that A549 cells expressing FR-β are capable of both binding and internalizing 3H-FA.
Evaluation of the binding and endocytosis of3H-FA and FA-labeled bovine serum albumin by cultured cells lacking (A549) or expressing (A549/FR-β) the β isoform of the FR. (A) A549 cells, a human lung carcinoma cell line expressing no detectable FR, were transfected with the β isoform of FR (A549/β-FR) and examined by flow cytometry for expression of the β isoform, as described in Materials and Methods. The filled peaks correspond to cells treated only with preimmune serum, while the open peaks show cells labeled with anti-FR IgG. (B) Cells were incubated with 100 nmol/L 3H-FA for 2 hours at 37°C and then washed three times in PBS, pH 7.4, to remove unbound ligand (□) or three times in sodium acetate buffer, pH 3.5, to strip all externally bound ligand from the cells (▨). (C) Binding and endocytosis of 125I-labeled serum albumin (100 nmol/L) was conducted similarly, except to distinguish specific from nonspecific binding, uptake was also evaluated in the presence of a 10,000 times molar excess of free FA to competitively block all folate-specific sites (▩).
Evaluation of the binding and endocytosis of3H-FA and FA-labeled bovine serum albumin by cultured cells lacking (A549) or expressing (A549/FR-β) the β isoform of the FR. (A) A549 cells, a human lung carcinoma cell line expressing no detectable FR, were transfected with the β isoform of FR (A549/β-FR) and examined by flow cytometry for expression of the β isoform, as described in Materials and Methods. The filled peaks correspond to cells treated only with preimmune serum, while the open peaks show cells labeled with anti-FR IgG. (B) Cells were incubated with 100 nmol/L 3H-FA for 2 hours at 37°C and then washed three times in PBS, pH 7.4, to remove unbound ligand (□) or three times in sodium acetate buffer, pH 3.5, to strip all externally bound ligand from the cells (▨). (C) Binding and endocytosis of 125I-labeled serum albumin (100 nmol/L) was conducted similarly, except to distinguish specific from nonspecific binding, uptake was also evaluated in the presence of a 10,000 times molar excess of free FA to competitively block all folate-specific sites (▩).
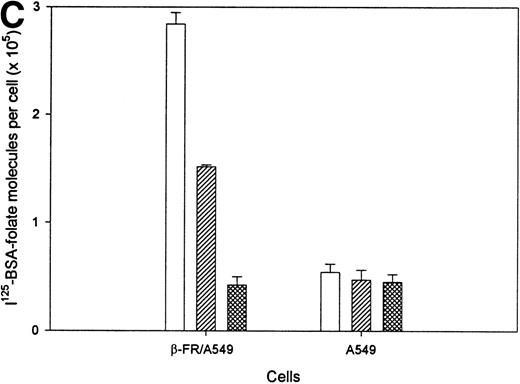
Because 3H-FA, but not FA-linked macromolecules, can also enter cells via a reduced folate carrier,44 a second experiment was conducted to unequivocally establish the ability of FR-β to mediate internalization of FA/FA-conjugates by an endocytic mechanism. For this purpose, 125I-labeled bovine serum albumin was either derivatized covalently with FA or left unmodified, and its association with A549/FR-β cells was quantitatively examined. As seen in Fig 6C, FA-derivatized albumin is taken up by A549/FR-β cells in a manner that is competitively blocked by excess free FA, indicating that its cell association is mediated by the FR. Retention of roughly half of this bound radioactivity after removal of surface-associated material by low pH wash further documents that part of the FA-derivatized 125I-labeled serum albumin has been endocytosed. In contrast, neither binding nor endocytosis of FA-linked serum albumin is observed to exceed nonspecific binding (binding not competitively blocked by free FA) by untransduced A549 cells. These data firmly establish the ability of FR-β to facilitate uptake of FA and its covalent conjugates by receptor-mediated endocytosis, and they also suggest that CD34+ cells must process or present the receptor differently to display it at the cell surface in a nonbinding state.
DISCUSSION
We have shown that a subpopulation of CD34+ hematopoietic cells expresses the β isoform of FR on its cell surfaces, and that although this isoform can mediate endocytosis of FA and its conjugates in other cell types, it is unable to facilitate detectable FA transport in CD34+ cells. Unfortunately, despite several attempts to clarify this anomalous behavior, the absence of folate binding activity by FR on CD34+ remains unexplained. Indeed, several possibilities were considered: first, because FR-β is anchored to the membrane by a glycosylphosphatidylinositol-anchor, it was possible that the small amounts of glycosylphosphatidylinositol-specific phospholipase C/D that are normally present in fetal calf serum could have cleaved and released the FR from the membrane. However, no FR could be detected in the conditioned medium from CD34+cells (Fig 3), and furthermore, had FR been released, no FR antigen would have been observed on the CD34+cells, because the anti-FR antiserum was raised against a C-terminally truncated (metalloprotease-generated) FR protein and does not recognize the glycosylphosphatidylinositol-anchor or the C-terminal residues of FR. A second possibility is that the chymopapain used during processing of some preparations of CD34+ cells (see Materials and Methods) could have somehow altered folate binding without releasing the protein antigen from the cell surface. To resolve this issue, we performed the LDMNC sorting/processing in the absence of chymopapain and still obtained similar results. The third possibility was that the endogenous folate binds so tightly to the FR that conventional acid-stripping methods failed to release it from the cell surface. This is, however, unlikely based on the lower affinity of FR-β when compared with FR-α (which is easily stripped of bound folate by acid treatment). Thus, none of these trivial possibilities can explain our data.
While the absence of FA binding activity may at first seem perplexing, it is at least not inconsistent with recent observations on other hematopoietic cells. Thus, Antony et al30 have previously described the expression of an inactive FR on erythrocytes and their precursor cells, and Ratnam et al will soon be reporting a similar phenomenon for neutrophils (M. Ratnam, personal communication, September 1998). Although Antony et al31,34have also provided evidence that a functional FR is essential for normal differentiation of LDMNC into their mature forms, their investigations were conducted on cells that had been aggressively stimulated to differentiate for 7 to 14 days and not on the freshly isolated quiescent CD34+ cells used here. It is, therefore, conceivable that a change in the functionality of FR could ensue in response to a change in proliferation/differentiation of hematopoietic cells. This hypothesis is consistent with our observations that CD34+CD38+ cells and CD34+CD15+ cells exhibited progressively increasing FR expression when compared with more immature subpopulations of CD34+ cells and with the earlier data.34 It will obviously be important to define the stimuli that might promote such a change in the activity of hematopoietic cell surface FRs.
Given the above observation on FR functionality in freshly isolated CD34+ cells, the question naturally arises as to why the primitive hematopoietic cells express any FR at all. Three tentative explanations can be envisioned. First, FR-β may serve a function in CD34+ cells that is distinct from its role in folate uptake. Thus, IGF-2 receptors also mediate internalization of mannose-6-phosphate containing ligands,45 and integrin receptors participate both in signal transduction and cell adhesion processes.46 If FR-β were also to perform an alternative structural or catalytic function, its refractory response to folates may, in fact, be incidental. Second, as speculated above, the inactive FR-β could be a precursor or product of the active receptor that will be/was required for cell function during another stage of cellular development. Thus, a number of well-characterized receptors require stimulation before they express their biologic activity (eg, integrin IIb/IIIa during platelet activation), and many others can be desensitized/inactivated after execution of their normal function. Finally, the inactive FR-β on CD34+ cells may, in fact, serve no function, but simply represent a gene product whose expression is not well regulated.
Finally, the apparent absence of a functional FR on the surfaces of hematopoietic progenitor and/or stem cells raises the possibility that the main route for FA uptake by these cells could be through the reduced folate carrier. Given the frequent overexpression of FR-α on epithelial cancers14,15 and FR-β on nonepithelial cancers,15 the opportunity would seem to exist to design a chemotherapeutic agent that could avoid bone marrow toxicity. Thus, by constructing an antifolate or FA-chemotherapeutic agent conjugate that could enter cells only by FR-mediated endocytosis, FR-expressing cancer cells could be targeted leaving FR-inactive hematopoietic cells unharmed. Several antifolates under current development display a preference for FR-mediated over carrier-catalyzed uptake,47and to the best of our knowledge, all FA-drug conjugates enter cells exclusively via FR-mediated endocytosis. Consequently, this latter class of drugs should be nondetrimental to hematopoietic cells.
ACKNOWLEDGMENT
We thank Dr M. Ratnam for his generous gifts of an antibody to FR-β and the cDNA for FR-β and YingJuan Lu for providing us with FA-FITC.
Supported in part by Grants No. GM24417, P50DK49218, IF32, and HL09851-01 from the National Institutes of Health, Bethesda, MD, and by Endocyte, Inc, West Lafayette, IN.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Philip S. Low, PhD, Department of Chemistry, 1393 Brown Bldg, Purdue University, West Lafayette, IN 47907-1393.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal