Abstract
Under normal conditions, platelets do not adhere to endothelium. However, when platelets or endothelial cells are stimulated by thrombin or cytokines, respectively, platelets bind avidly to endothelium. Because there is accumulating evidence that endothelial cells may become apoptotic under certain proinflammatory or prothrombotic conditions, we investigated whether endothelial cells undergoing apoptosis may become proadhesive for nonactivated platelets. Human umbilical vein endothelial cells (HUVEC) were induced to undergo apoptosis by staurosporine, a nonspecific protein kinase inhibitor, or by culture in suspension with serum-deprivation. After treatment of HUVEC or platelets with different receptor antagonists, nonactivated, washed human platelets were allowed to adhere to HUVEC for 20 minutes. To exclude matrix involvement, platelet binding was measured in suspension by using flow cytometry. Independent of the method of apoptosis induction, there was a marked increase in platelet binding to apoptotic HUVEC. Although HUVEC exhibited maximal adhesiveness for platelets after 2 to 4 hours, complete DNA fragmentation of HUVEC occurred only several hours later. Adhesion assays after blockade of different platelet receptors showed only involvement of β1-integrins. Platelet binding to apoptotic HUVEC was inhibited by more than 70% when platelets were treated with blocking anti-β1 antibodies. Treatment of apoptotic HUVEC with blocking antibodies to different potential platelet receptors, including known ligands for β1-integrins, did not affect platelet binding. As assessed by determination of β-thromboglobulin and platelet factor 4 in the supernatants, platelets bound to apoptotic HUVEC became slightly activated. However, significant expression of platelet P-selectin (CD62P) was not found. These data provide further evidence that endothelial cells undergoing apoptosis may contribute to thrombotic events.
UNPERTURBED endothelial cells provide potent anticoagulant properties, preventing the adhesion of platelets as well as the initiation of coagulation. However, when exposed to proinflammatory stimuli the endothelium is rapidly transformed into a proadhesive and procoagulant surface, promoting formation of thrombi.1,2 Several lines of evidence indicate that, under similar conditions, endothelial cells may also undergo apoptosis. Vascular cell death has been observed primarily in the environment of hypertension, inflammation, and arteriosclerosis.3,4 Thus, it is suggestive that apoptotic endothelial cells may contribute to the pathophysiology of thrombosis. In a recent in vitro study, we have found that endothelial cells undergoing apoptosis became highly procoagulant because of the loss of anticoagulant membrane components and the exposure of negatively charged phospholipids, which accelerated activation of coagulation factors.5 In addition, two other reports showed that endothelial cells markedly increased cell surface tissue factor activity on induction of apoptosis.6,7Vascular smooth muscle cells have similarly been found to promote thrombin generation during the process of cell death.8Hence, it is conceivable that, in vivo, an area of vascular cells undergoing apoptosis may contribute to the initiation of thrombosis.
For years it has been shown that activated platelets readily adhere to normal endothelium.9 We have recently elucidated the receptors responsible for this binding mechanism and showed that platelet adhesion is mediated by a bridging mechanism involving platelet GPIIbIIIa (CD41a/CD61), different adhesive proteins, and three different endothelial cell-counter receptors.10Perturbation of endothelial cells has similarly been found to alter their reactivity with platelets. In vivo studies have shown that nonactivated platelets roll on stimulated endothelium, thereby interacting with endothelial P-selectin and E-selectin.11,12 Consequently, stimulated, but structurally intact, endothelium has been suggested to contribute significantly to the formation of thrombosis.13
The purpose of this study was to determine whether endothelial cells undergoing apoptosis would become proadhesive for nonactivated platelets and whether platelets would become activated through contact with apoptotic endothelial cells. With a flow cytometry-based binding assay, we show that nonactivated platelets bind to apoptotic human umbilical vein endothelial cells (HUVEC) to the same extent as thrombin-activated platelets bind to normal HUVEC. [The mechanism by which HUVEC apoptosis was induced was not critical, since the extent of nonactivated platelet adhesion was similar using either staurosporine-treated or suspended and starved HUVEC.] Binding was inhibited only by treating platelets with anti–β1-integrin antibodies, indicating that platelet β1 receptors were primarily responsible for adhesion. The β1-receptor ligands on apoptotic HUVEC remain undefined, but collagen, fibronectin, and RGD-dependent ligands are not involved. Platelets bound to apoptotic HUVEC became slightly activated as demonstrated by the release of β thromboglobulin and platelet factor 4, but P-selectin was not expressed significantly. These data provide further evidence that endothelial cells undergoing apoptosis may acquire prothrombotic properties.
MATERIALS AND METHODS
Cell culture.
HUVEC were obtained by collagenase treatment of umbilical cord veins as previously described.14 Cells were cultured on gelatin-coated dishes and propagated in RPMI 1640 medium supplemented with 20% bovine calf serum, 90 μg/mL heparin (Sigma Chemical Co, St Louis, MO), and 50 μg/mL endothelial cell growth factor prepared from bovine hypothalamus.15 For flow cytometry assays, HUVEC derived from passage two or three were allowed to grow to confluence in 12-well dishes. Before the adhesion assay, HUVEC were washed twice with RPMI medium and incubated with receptor antagonists in phenol red–free RPMI medium for 30 minutes at 37°C. Where indicated, HUVEC were stimulated with 0.5 U/mL human thrombin (Sigma) for 15 minutes at 37°C.
Apoptosis induction.
Apoptosis of HUVEC was induced either by 200 nmol/L staurosporine (Sigma) in growth medium or by culture in suspension in the absence of heparin, serum, and growth factors. To prevent cells from adherence, tissue culture plates were coated with a 2% agarose gel. Both methods induce apoptosis in about 50% of the cells within 6 to 8 hours.5
Platelet preparation.
Blood was obtained by venipuncture from healthy adult volunteers according to a protocol approved by the Human Subjects Division of the University of Washington, Seattle. The volunteers did not take any drugs for the previous 10 days. Isolation of platelets was performed as described by Baenziger and Majerus.16 In brief, blood was drawn into polypropylene syringes containing one-tenth volume of 0.11 mol/L sodium citrate and centrifuged at 1,000g for 4 minutes to obtain platelet-rich plasma. Platelets were sedimented by centrifugation at 2,000g for 10 minutes and washed twice with 10 mL of HEPES buffer (10 mmol/L HEPES, 0.5 mmol/L MgCl2, 130 mmol/L NaCl, 4 mmol/L KCl, 1 mmol/L CaCl2, 5 mmol/L glucose, pH 7.4). To pellet erythrocytes selectively, platelets were resuspended in the same buffer and centrifuged twice at 120g for 3 minutes. Subsequently, the content of erythrocytes was less than 1% as calculated with a hemocytometer. Platelets were stained with 2.5 μmol/L calcein-acetoxymethyl ester (Molecular Probes, Eugene, OR) in the dark for 15 minutes. To avoid platelet activation, all centrifugations were done at room temperature (RT) and in the presence of 1 μmol/L prostaglandin E1 (Alprostadil, Prostin VR Pediatric; Upjohn, Kalamazoo, MI). After a washing step, platelets were adjusted to a final concentration of 2 × 109/mL and incubated with receptor antagonists for 30 minutes at RT. Where indicated, platelets were activated with 0.5 U/mL human thrombin for 10 minutes. Thrombin was then inactivated with 2 U/mL hirudin (Sigma) for 10 minutes.
Antibodies and other receptor antagonists.
To block platelet or HUVEC receptors, the following monoclonal antibodies (MoAbs) were used (the corresponding references refer to studies in which the MoAbs were found to have blocking functions): P4C10 (β1 integrin, CD29, IgG1) (Life Technologies Inc, Gaithersburg, MD)17; 2A11 (β1 integrin, CD29) produced in our laboratory (B.R. Schwartz, unpublished observations); P1E6 (α2, CD49b, IgG1) (Chemicon International, Inc, Temecula, CA)18; P1D6 (α5, CD49e, ascites fluid) (Life Technologies Inc)19; GoH3 (α6, CD49f, IgG2A) (Endogen Inc, Woburn, MA)20; LM609 (αvβ3 integrin, CD51/CD61, IgG1) (Chemicon)21; P2 (GPIIbIIIa, CD41a/CD61, IgG1) (Immunotech Inc, Westbrook, ME)22; SZ2 and 2E4 (GPIbα, CD42b, IgG1) (Immunotech and Dr G.J. Roth, Veterans Affairs Medical Center, Seattle, WA, respectively)23; FA6.152 (GPIV, CD36, IgG1) (Immunotech); R6.5 and 2D5 (ICAM-1, CD54, IgG1) provided by Dr R. Rothlein, Department of Immunologic Diseases, Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT and Dr D.C. Altieri, The Boyer Center for Molecular Medicine, Yale University School of Medicine, New Haven, CT, respectively24,25; WAPS12.2 and G1 (P-selectin, CD62P, IgG1) (Endogen Inc and Biosource Int, Camarillo, CA, respectively26,27; 1.2B6 and H18-7 (E-selectin, CD62E) (Endogen Inc and Becton Dickinson, San Jose, CA, respectively)28,29; E1-6 and 1G11 (VCAM-1; CD106, IgG1) (Becton Dickinson and Immunotech, respectively).29,30 JC70A and M89D3 (PECAM-1, CD31, IgG1) (Accurate Chemicals Co, Westbury, NY, and Dr M.R. Zocchi, Laboratory of Adoptive Immunotherapy, Milan, Italy, respectively); and anti-human fibronectin MoAb type 2 from Calbiochem (La Jolla, CA).31 All of these MoAbs were used at a final concentration of 30 μg/mL. The anti-human collagen IV MoAb COL-94 (IgG1; Sigma) and the anti-human von Willebrand factor (vWF) rabbit polyclonal antibody (IgG fraction; Sigma) were used at a final dilution of 1:250.10 A nonspecific isotype control was performed with a mouse IgG1k MoAb (Sigma). Gly-Arg-Gly-Asp-Ser (GRGDS) and Gly-Arg-Gly-Glu-Ser (GRGES) peptides (Peninsula Laboratories, Belmont, CA) and recombinant annexin V (provided by Dr J.F. Tait, Department of Laboratory Medicine, University of Washington, Seattle, WA) were used at a final concentration of 50 μg/mL and 5 μmol/L, respectively.
Adherence assay.
HUVEC (either adherent or suspended) were washed twice with RPMI medium and incubated with 600 μL of HEPES buffer (same buffer as described above, but with 2 mmol/L CaCl2) and 40 μL of the suspension of stained platelets for 20 minutes at 37°C. The final platelet concentration was 1.25 × 108/mL. When suspended HUVEC were used, the adhesion assay was performed in a 1.5-mL Eppendorf tube placed on a shaking table to avoid sedimentation. Adherent HUVEC were harvested mechanically by vigorous pipetting. After washing, HUVEC were fixed immediately with 80% ethanol on ice for 30 minutes. To differentiate HUVEC from residual unbound platelets, HUVEC were stained with the DNA binding dye, propidium iodide. The cells were resuspended in 200 μL phosphate-buffered saline pH 7.4 and 0.1% Triton-X 100 (Sigma) containing 5 μg/mL propidium iodide (Sigma) and 50 μg/mL ribonuclease A (R-6513; Sigma). Subsequently, specimens were analyzed by a FACScan (Becton Dickinson, Mountain View, CA). At least 10,000 cells that stained positive for propidium iodide (FL-2-H) were evaluated. Platelet adhesion to HUVEC was expressed by the median fluorescence (FL-1-H) of the entire HUVEC population. Based on the distribution of the DNA content (FL-2-H), HUVEC were routinely tested for apoptosis (designated A0 region below G0/G1 peak) and homotypic aggregate formation (region above G2/M peak). On average, less than 5% of the cells exhibited more than 4C DNA, indicating that they were clumped. Statistical significance was determined by using Student’st-test.
Determination of P-selectin expression.
Suspended HUVEC, platelets, or platelet-HUVEC aggregates were incubated with the anti–P-selectin MoAb WAPS12.2 (IgG1; Endogen Inc) at a final concentration of 10 μg/mL for 30 minutes at RT. After washing twice, the secondary fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Caltag Lab, San Francisco, CA) was incubated for 30 minutes (final dilution of 1:200). At least 10,000 cells were then analyzed by a FACScan (Becton Dickinson). Cells treated with FITC-conjugated isotype-specific IgG were used as negative controls.
Determination of release of platelet factor 4 and β-thromboglobulin from platelets.
After incubation of platelets with staurosporine-treated and washed HUVEC in HEPES-buffer (see above) for 20 minutes at 37°C, the supernatants were carefully removed and centrifuged at 2,000gfor 5 minutes to remove residual unbound platelets. Before centrifugation, a stop solution was added to a final concentration of 5 mmol/L EDTA (Sigma), 1 μmol/L prostaglandin E1 (see above), and 3% paraformaldehyde (Sigma) to prevent centrifuge-induced secretion from the residual unbound platelets. β-thromboglobulin and platelet factor 4 were then determined by using enzyme-linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (Asserachrom BTG and Asserachrom PF4, Diagnostica Stago, Asnieres-Sur-Seine, France).
RESULTS
Apoptotic HUVEC bind platelets.
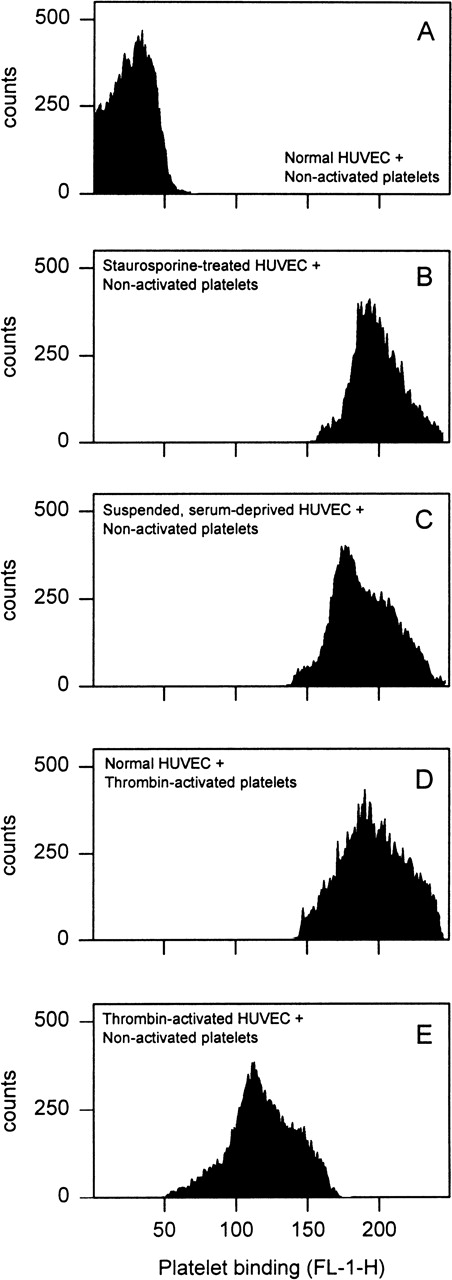
Because platelets adhere primarily to matrix proteins, determination of platelet adhesion to adherent HUVEC may produce spurious results if the monolayer is not completely confluent. This may be a particular problem with apoptotic HUVEC, because endothelial cell death is accompanied by retraction and also detachment. To avoid this problem, HUVEC were mechanically detached after the binding assay and platelet binding was determined in suspension with flow cytometry. As we have previously shown, this method allows us to completely exclude involvement of matrix proteins.10 Unstimulated live HUVEC did not bind nonactivated platelets in a significant manner (Fig1A). However, HUVEC treated with staurosporine, a nonspecific protein kinase inhibitor, for 8 hours (Fig 1B) or kept in suspension and serum deprived also for 8 hours (Fig1C) bound nonactivated platelets to the same degree as normal HUVEC incubated with thrombin-activated platelets (Fig 1D). Within this period of time, both methods induce cell death in at least 50% of the cells as demonstrated by the occurrence of apoptosis-associated DNA degradation.5 Platelet adhesion to apoptotic HUVEC was not further increased when thrombin-activated platelets were used (data not shown). On stimulation with 0.5 U/mL human thrombin for 15 minutes, HUVEC also exhibited an increased fluorescence which, however, was not as high as with apoptotic HUVEC (Fig 1E). This proadhesive effect of thrombin could be blocked by treating HUVEC with 2 U/mL hirudin for 15 minutes before adding the platelets (data not shown). Higher thrombin concentrations (up to 2 U/mL) did not increase further platelet adhesion (data not shown). These data indicate that HUVEC undergoing apoptosis may become more proadhesive for platelets than on direct stimulation with a prothrombotic stimulus.
Platelet binding to HUVEC. Nonactivated or thrombin-activated, washed, and calcein-loaded platelets were allowed to adhere to normal, apoptotic or thrombin-stimulated HUVEC for 20 minutes. Apoptosis was induced either by staurosporine or by suspending cells with serum deprivation. Platelet binding was then determined by flow cytometry to exclude involvement of subendothelial matrix proteins. Binding is expressed as the median fluorescence (FL-1-H) of the entire HUVEC population. Apoptotic HUVEC bound nonactivated platelets as effective as normal HUVEC bound thrombin-activated platelets. A representative experiment of three performed is shown.
Platelet binding to HUVEC. Nonactivated or thrombin-activated, washed, and calcein-loaded platelets were allowed to adhere to normal, apoptotic or thrombin-stimulated HUVEC for 20 minutes. Apoptosis was induced either by staurosporine or by suspending cells with serum deprivation. Platelet binding was then determined by flow cytometry to exclude involvement of subendothelial matrix proteins. Binding is expressed as the median fluorescence (FL-1-H) of the entire HUVEC population. Apoptotic HUVEC bound nonactivated platelets as effective as normal HUVEC bound thrombin-activated platelets. A representative experiment of three performed is shown.
Time course of platelet binding.
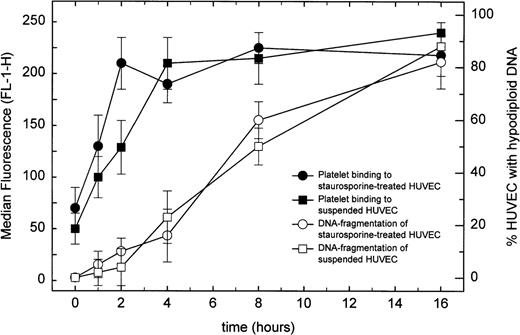
To differentiate HUVEC from residual unbound platelets in flow cytometry, HUVEC were stained with the DNA-binding dye propidium iodide after the adhesion assay. This procedure allowed us to also determine the percentage of HUVEC with a hypodiploid DNA content that is characteristic of apoptosis. Thus, it was possible to correlate the time course of the increase in platelet adhesion with the occurrence of apoptotic DNA fragmentation. As shown in Fig2, the onset of platelet adhesion did not correlate with DNA fragmentation, but preceded it by several hours. The maximal increase in platelet adhesion was observed after 2 to 4 hours by using either staurosporine or suspension/starvation for apoptosis induction, whereas complete DNA fragmentation was observed only after 16 or more hours. These results indicate that during the course of HUVEC apoptosis important functionalchanges occur several hours before structural alterations of the nucleus are completed.
Time-course of platelet binding to apoptotic HUVEC. Washed and calcein-loaded nonactivated platelets were allowed to adhere to HUVEC undergoing apoptosis induced either by staurosporine or by keeping cells in suspension with serum-deprivation. After the adhesion assay, HUVEC were permeabilized and stained for DNA with propidium iodide. HUVEC were then assayed by flow cytometry to determine platelet binding and, simultaneously, apoptosis-induced DNA fragmentation. Platelet binding is expressed as the median fluorescence (FL-1-H) of the entire HUVEC population. DNA fragmentation is expressed as percentage of cells with hypodiploid DNA. A representative experiment of three performed is shown.
Time-course of platelet binding to apoptotic HUVEC. Washed and calcein-loaded nonactivated platelets were allowed to adhere to HUVEC undergoing apoptosis induced either by staurosporine or by keeping cells in suspension with serum-deprivation. After the adhesion assay, HUVEC were permeabilized and stained for DNA with propidium iodide. HUVEC were then assayed by flow cytometry to determine platelet binding and, simultaneously, apoptosis-induced DNA fragmentation. Platelet binding is expressed as the median fluorescence (FL-1-H) of the entire HUVEC population. DNA fragmentation is expressed as percentage of cells with hypodiploid DNA. A representative experiment of three performed is shown.
Platelet receptors.
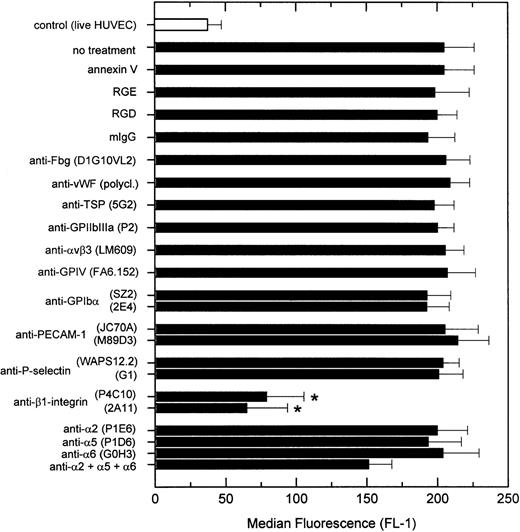
To determine the platelet receptors involved in the binding of nonactivated platelets to apoptotic HUVEC, platelets were incubated with different blocking MoAbs for 30 minutes before the adhesion assay. Apoptosis of HUVEC was induced by treating the cells with staurosporine for 6 hours. Inhibition of platelet adhesion was observed only when platelets were treated with the anti–β1-integrin antibodies P4C10 or 2A11 (Fig 3), both of which inhibited platelet binding by more than 70%. To clarify further which type of platelet β1-integrin (α2β1, α5β1, or α6β1) would be involved in the binding, platelets were treated with blocking moAbs to either the α2-, α5-, or α6-subunit. However, platelet binding was not reduced significantly when one of the α-subunits was blocked. Significant inhibition of platelet binding (about 20%) was observed only when all three anti-α-MoAbs were used in combination. RGD peptides also were without effect at concentrations that inhibit GPIIaIIIb-dependent adhesion of thrombin-activated platelets to HUVEC.10 Blockade of other important receptors for platelet-subendothelium interactions, including GPIbα, GPIV, and αvβ3-integrin, as well as activation-dependent receptors, including GPIIbIIIa and P-selectin, did not inhibit platelet binding to staurosporine-treated HUVEC. Furthermore, incubation of platelets with annexin V to block negatively charged phospholipids did not affect platelet adherence.
Platelet adhesion to apoptotic HUVEC after blockade of platelet receptors. Nonactivated platelets treated with different receptor antagonists were allowed to adhere to a HUVEC monolayer treated with staurosporine for 6 hours. Platelet binding was determined by flow cytometry as described above. The following concentrations were used: 5 μmol/L annexin V, 50 μg/mL RGE and RGD peptides, 30 μg/mL MoAb, and a dilution of 1:250 for polyclonal antibodies. Results are expressed as the mean ± SD of the median fluorescence (FL-1-H) of at least three experiments. *P < .01 by Student’st-test, compared with adhesion of nonactivated platelets treated with the isotype-specific control MoAb.
Platelet adhesion to apoptotic HUVEC after blockade of platelet receptors. Nonactivated platelets treated with different receptor antagonists were allowed to adhere to a HUVEC monolayer treated with staurosporine for 6 hours. Platelet binding was determined by flow cytometry as described above. The following concentrations were used: 5 μmol/L annexin V, 50 μg/mL RGE and RGD peptides, 30 μg/mL MoAb, and a dilution of 1:250 for polyclonal antibodies. Results are expressed as the mean ± SD of the median fluorescence (FL-1-H) of at least three experiments. *P < .01 by Student’st-test, compared with adhesion of nonactivated platelets treated with the isotype-specific control MoAb.
HUVEC receptors.
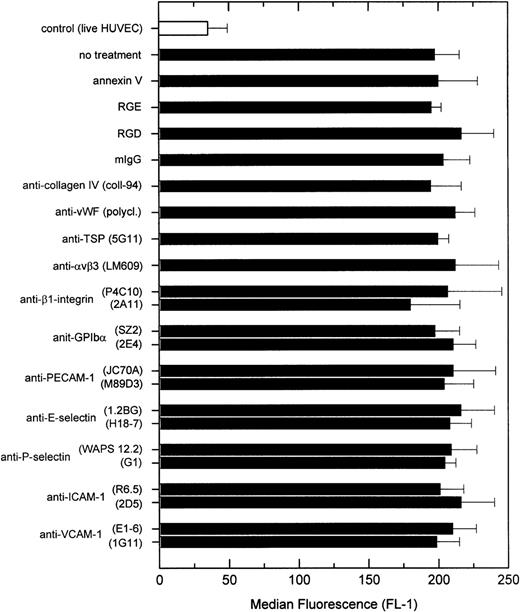
To determine a counter receptor on apoptotic HUVEC, cells were treated with a variety of blocking MoAbs to different adhesion receptors known to be important for the binding of leukocytes or activated platelets. However, none of the tested MoAbs was found to inhibit adherence significantly. Blockade of both constitutively expressed receptors (αvβ3-integrin, β1-integrin, GPIbα, ICAM-1, and PECAM-1) and inducible receptors (VCAM-1, E-selectin, and P-selectin) did not affect platelet binding (Fig4). In accordance with this finding, we found that HUVEC undergoing apoptosis induced by staurosporine did not show increased expression of ICAM-1 or induction of expression of E-selectin or VCAM-1 (B.R. Schwartz, unpublished observations). Based on the fact that platelet β1-integrins mediated adhesion to apoptotic HUVEC, it was conceivable that HUVEC would expose particular platelet-binding matrix proteins such as collagen or fibronectin that promoted binding. However, treatment of apoptotic HUVEC with blocking antibodies to collagen IV or fibronectin did not reduce adhesion. Because a previous report has shown that nonactivated platelet adhesion to virally infected endothelial cells was mediated by endothelial exposure of von Willebrand factor (vWF),32 platelet adhesion was also tested after treatment of apoptotic HUVEC with a blocking polyclonal anti-vWF antibody. However, platelet binding was not altered, consistent with the fact that vWF is not a known ligand for β1-integrins.
Platelet adhesion to apoptotic HUVEC after blockade of HUVEC receptors. Nonactivated platelets were allowed to adhere to HUVEC treated with staurosporine for 6 hours. Before the adhesion assay, HUVEC were treated with different receptor antagonists. Platelet binding was determined by flow cytometry as described above. The following concentrations were used: 5 μmol/L annexin V, 50 μg/mL RGE and RGD peptides, 30 μg/mL MoAb, and a dilution of 1:250 for polyclonal antibodies. Results are expressed as the mean ± SD of the median fluorescence (FL-1-H) of at least two experiments.
Platelet adhesion to apoptotic HUVEC after blockade of HUVEC receptors. Nonactivated platelets were allowed to adhere to HUVEC treated with staurosporine for 6 hours. Before the adhesion assay, HUVEC were treated with different receptor antagonists. Platelet binding was determined by flow cytometry as described above. The following concentrations were used: 5 μmol/L annexin V, 50 μg/mL RGE and RGD peptides, 30 μg/mL MoAb, and a dilution of 1:250 for polyclonal antibodies. Results are expressed as the mean ± SD of the median fluorescence (FL-1-H) of at least two experiments.
HUVEC-bound platelets become slightly activated.
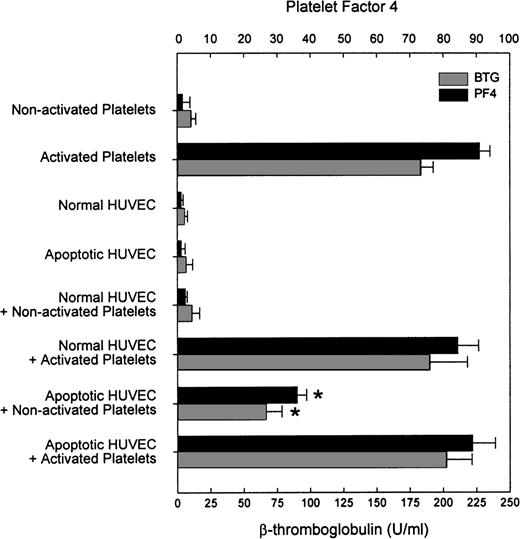
The question of whether platelets would become activated through the contact with apoptotic HUVEC was also addressed. As shown in Fig5, the supernatants of apoptotic HUVEC with bound platelets contained a significant amount of β-thromboglobulin and platelet factor 4 as compared with the supernatants of normal or apoptotic HUVEC alone, nonactivated platelets alone, or normal HUVEC incubated with nonactivated platelets. However, the concentration of β-thromboglobulin and platelet factor 4 was almost three times higher when HUVEC were incubated with thrombin-activated platelets.
Release of activation markers of HUVEC-bound platelets. To determine whether platelets were activated through the contact with apoptotic HUVEC, the supernatants were assayed for the presence of platelet-specific activation markers. After incubation of the platelets with HUVEC for 20 minutes, the supernatants were tested for β-thromboglobulin and platelet factor 4 using an ELISA assay. Apoptosis of HUVEC was induced by treatment with staurosporine for 6 hours. Results are expressed as the mean ± SD of at least three experiments. *P < .01 by Student’s t-test, compared with the release of activation markers by nonactivated platelets incubated with normal HUVEC.
Release of activation markers of HUVEC-bound platelets. To determine whether platelets were activated through the contact with apoptotic HUVEC, the supernatants were assayed for the presence of platelet-specific activation markers. After incubation of the platelets with HUVEC for 20 minutes, the supernatants were tested for β-thromboglobulin and platelet factor 4 using an ELISA assay. Apoptosis of HUVEC was induced by treatment with staurosporine for 6 hours. Results are expressed as the mean ± SD of at least three experiments. *P < .01 by Student’s t-test, compared with the release of activation markers by nonactivated platelets incubated with normal HUVEC.
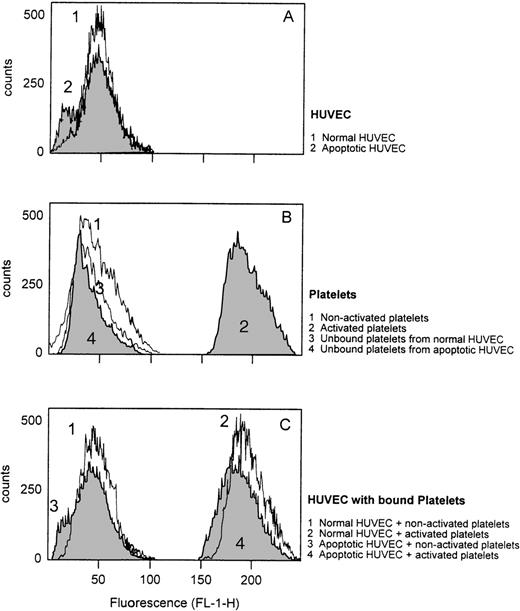
Likewise, normal or apoptotic HUVEC alone, nonactivated platelets alone, or normal HUVEC incubated with nonactivated platelets did not express P-selectin, as determined by flow cytometry (Fig 6A-C). Complexes between nonactivated platelets and apoptotic HUVEC as well as residual unbound platelets derived from the supernatant of apoptotic HUVEC were not found to express P-selectin in any significant manner (Fig 6B and C). Only when preactivated with thrombin, platelets or platelet-HUVEC complexes exhibited increased expression of P-selectin (Fig 6B and C). These data indicate that platelets do indeed become slightly activated on binding to apoptotic HUVEC. This is evidenced by release of a small quantity of activation markers, although expression of P-selectin is not significant.
Expression of P-selectin of platelets and HUVEC. To determine the degree of platelet activation through the contact with apoptotic HUVEC, platelets were tested for the expression of P-selectin. After incubation of the platelets with HUVEC for 20 minutes, the cells (whether adherent or unbound) were stained separately with anti–P-selectin MoAb (WAPS 12.2) followed by an FITC-conjugated anti-mouse MoAb. Cells were then assayed by flow cytometry to determine P-selectin expression. As controls, nonactivated and activated platelets and HUVEC were also measured separately. A representative experiment of three performed is shown.
Expression of P-selectin of platelets and HUVEC. To determine the degree of platelet activation through the contact with apoptotic HUVEC, platelets were tested for the expression of P-selectin. After incubation of the platelets with HUVEC for 20 minutes, the cells (whether adherent or unbound) were stained separately with anti–P-selectin MoAb (WAPS 12.2) followed by an FITC-conjugated anti-mouse MoAb. Cells were then assayed by flow cytometry to determine P-selectin expression. As controls, nonactivated and activated platelets and HUVEC were also measured separately. A representative experiment of three performed is shown.
DISCUSSION
Whereas endothelium normally controls platelet reactivity, recent in vitro and in vivo studies have showed that, under certain conditions, endothelial cells may develop properties that render them adhesive for platelets. Once activated, platelets are well known to rapidly adhere to both microvascular and macrovascular endothelial cells, recruit additional platelets, and eventually form increasing larger aggregates.9,10,33 Recently, we further characterized the adhesion of activated platelets to endothelial cells, showing that it is mediated by adhesive proteins involving platelet GPIIbIIIa and different endothelial cell counter receptors.10Furthermore, platelet-endothelial interactions may also occur when the endothelium, but not the platelets, are activated. Two recent in vivo studies have showed that nonactivated platelets roll on the endothelium when it is activated with proinflammatory stimuli.11,12Stimulation of the endothelium by laser light has similarly been shown to induce adhesion of nonactivated platelets.13 This report shows that during the process of apoptotic cell death, endothelial cells also become proadhesive for nonactivated platelets.
Independent of the method of apoptosis induction, both adherent HUVEC and HUVEC kept in suspension dramatically increased adhesion of nonactivated platelets. Interestingly, the amount of binding was similar to that of normal HUVEC incubated with thrombin-activated platelets. Because we have previously shown that activated platelets bound to normal HUVEC in the entire circumference,10 it is conceivable that apoptotic HUVEC may be covered with platelets to a similar extent. This hypothesis is supported by the fact that incubation of apoptotic HUVEC with thrombin-activated platelets did not further increase the fluorescence.
As shown in Fig 2, the acquisition of an adhesive phenotype during the process of apoptosis preceded DNA fragmentation by several hours. This is not a surprising finding, as it is well known that the translocation of negatively-charged phospholipids to the external leaflet of the cell membrane is an early event in apoptosis.5,34 Furthermore, we have previously shown that apoptosis-induced changes of the HUVEC surface membrane occurred well before detectable alterations of the nucleus.5 Hence, the rapid binding of platelets to apoptotic HUVEC may reflect early structural modifications of the cell membrane such as the expression of new, presynthesized molecules or the loss of constitutively expressed, platelet-repellent components. In particular, treatment of valvular endothelial cells with heparinase or neuraminidase has been found to render the cells proadhesive for nonactivated platelets, suggesting that the loss of membrane sialyl residues and heparan sulfate may induce a prothrombotic phenotype in endothelial cells.35
So far, several receptors have been shown to be involved in the interaction of live and apoptotic cells. Macrophages and other phagocytic cells recognize and bind apoptotic cells primarily via αvβ3-integrin or the CD36 receptor. Binding is mediated by thrombospondin, which is secreted by the macrophages, forming a bridge to a so far unidentified counter receptor.36 Binding of apoptotic cells by phagocytes has also been found to involve CD14 and the fibronectin receptor α5β1-integrin, which is thought to be regulated by αvβ3-integrin.37,38 In our studies, blockade of platelet αvβ3-integrin or the thrombospondin receptor CD36 (GPIV) did not inhibit binding to apoptotic HUVEC. As well, platelet adhesion was not reduced by treatment with an antithrombospondin MoAb. However, when platelets were treated with anti-β1 MoAbs, binding to apoptotic HUVEC was inhibited by more than 70%. The fact that only the combination of all three anti-α-MoAbs but not any one anti-α-MoAb alone revealed a blocking effect suggests that all three α-subunits might recognize a ligand(s) on apoptotic HUVEC. It is important to note that the anti-β1-MoAbs blocked platelet binding more effectively than the combination of the three anti-α-MoAbs. Importantly, all three anti-α–MoAbs (P1E6, P1D6, and GoH3) have been found to block platelet adhesion to matrix proteins.18-20 However, because treatment of HUVEC with blocking MoAbs to fibronectin or collagen IV or addition of RGD peptides did not reduce platelet binding, it is not likely that matrix ligands were involved. Based on the lack of evidence in the literature, we believe that another α-subunit is involved.39 Furthermore, studies in our laboratory have shown that adhesion of leukocytes to endothelial cells undergoing apoptosis is similarly mediated by multiple β1-integrins (B.R. Schwartz, A. Karsan, T. Bombeli, J.M. Harlan, submitted). Possibly, platelet binding to the apoptotic ligand(s) on HUVEC uses regions of the α-subunits different from those used for known ligands.
Unfortunately, our attempts failed to determine the ligand(s) on apoptotic HUVEC to which nonactivated platelets bind. Blockade of the HUVEC receptors ICAM-1, αvβ3-integrin, and GPIbα, which we have previously found to mediate binding of thrombin-activated platelets,10 did not reduce platelet binding. In addition, we did not find any evidence for the involvement of endothelial vWF or PECAM-1, which have been suggested to be responsible for the binding of nonactivated platelets to endothelial cells stimulated with cytokines or laser light.32,40 Recent in vivo studies have shown that nonactivated platelets roll on postischemic or inflamed endothelium.11,12,41 These interactions were found to be dependent on endothelial P-selectin and E-selectin, whereas the counter receptors on platelets have, so far, not been characterized. In our studies, treatment of apoptotic HUVEC with blocking anti–P-selectin or E-selectin MoAbs did not reduce nonactivated platelet binding. When compared with leukocyte-endothelial interactions, these findings are not surprising because rolling is known to involve different receptors than firm adhesion.42 Therefore, we expected other endothelial adhesion receptors to be involved, possibly VCAM-1 (binds β1-integrins) and ICAM-1. Both receptors have recently been suggested to be responsible for the hyperadhesiveness of apoptotic HUVEC for monocytic cells.43 However, blockade of these receptors did not affect platelet adhesion to apoptotic HUVEC. Moreover, we did not find any evidence that VCAM-1 and ICAM-1 became significantly upregulated on induction of apoptosis (B.R. Schwartz, unpublished observations). It has further been demonstrated that binding and phagocytosis of apoptotic cells can be mediated by phosphatidylserine, which is rapidly upregulated on induction of apoptosis.44 45 However, treatment of apoptotic HUVEC with saturating concentrations of the phosphatidylserine-binding protein annexin V did not reduce platelet adhesion. These findings indicate that nonactivated platelet adhesion to HUVEC undergo-ing apoptosis involves a, so far, unidentified endothelial cell ligand(s) recognized by multiple β1-integrins.
When bound to subendothelial matrix proteins, platelets become readily activated as characterized by the shape change, the release reaction, and the activation of adhesion receptors. Thus, it seemed important to evaluate whether binding of platelets to apoptotic endothelial cells would similarly induce activation of the platelets. Based on the increase of β-thromboglobulin and platelet factor 4 in the supernatant of apoptotic HUVEC with bound platelets, it is evident that platelets become activated. However, apoptotic HUVEC seem to be a relatively weak agonist, because the bound platelets did not significantly express P-selectin. This slight activation may be explained by the observation that only one type of platelet receptors (β1-integrins) is involved and that the ligand(s) on HUVEC is probably not a matrix protein. Platelet binding to matrix proteins and subsequent activation involves several receptors.46 Hence, apoptotic HUVEC appear to bind nonactivated platelets as effectively as the subendothelium. However, after binding of a single layer, the recruitment of additional platelets into the evolving thrombus would likely be less effective, as compared with subendothelial matrix proteins. Based on our previous findings that apoptotic endothelial cells also become highly procoagulant,5 it is tempting to speculate that endothelium undergoing apoptosis may support thrombotic events.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr R. Rothlein (Department of Immunologic Diseases, Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT), Dr D.C. Altieri (The Boyer Center for Molecular Medicine, Yale University School of Medicine, New Haven, CT), Dr M.R. Zocchi (Laboratory of Adoptive Immunotherapy, Milan, Italy), and Dr G.J. Roth (Veterans Affairs Medical Center, Seattle, WA) for providing antibodies. We also thank Dr J.F. Tait (Department of Laboratory Medicine, University of Washington, Seattle, WA) for the generous gift of recombinant annexin V and Tom Eunson for preparing cell cultures.
Supported by the United States Public Health Service Grant Nos. HL 18645 and HL 03174. T.B. was supported by the Swiss Foundation for Medical and Biological Grants of Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Thomas Bombeli, MD, Division of Hematology, Department of Medicine, Raemistrasse 100, University Hospital of Zurich, 8091 Zurich, Switzerland; e-mail: haembomb@usz.unizh.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal