Abstract
Thrombotic thrombocytopenic purpura (TTP) after bone marrow transplantation (BMT) differs from classic TTP in its clinical course and therapy. A characteristic of classic TTP is the inhibition of a plasma protease that specifically cleaves von Willebrand factor (vWF), thus reducing its multimeric size. We investigated whether this protease was also inhibited in BMT-associated TTP. Plasma from patients with classic or BMT-associated TTP was incubated with recombinant vWF R834Q, a vWF mutant with enhanced sensitivity to the protease. The proteolysis of vWF multimers was analyzed and quantified on Western blot. Metalloprotease activity was strongly inhibited in the classic TTP patient group. However, metalloprotease activity was normal in the BMT-associated TTP patient group. The difference in activity between the two patient groups was highly significant (P = .0016). The results indicate that the etiologies of classic and BMT-associated TTP are indeed different and provide an explanation for the lack of success of plasma exchange in BMT-associated TTP.
THROMBOTIC thrombocytopenic purpura (TTP) is characterized by thrombocytopenia, microangiopathic hemolytic anemia, fever, neurological symptoms, and renal impairment resulting from the formation of platelet thrombi within the microvasculature. TTP may develop spontaneously, but it can also occur as a dangerous complication after a bone marrow transplantation (BMT).1
It has been hypothesized that the presence of unusually high multimers of von Willebrand factor (vWF) is the central cause of TTP.2 vWF is a plasma glycoprotein that is involved in platelet adhesion and aggregation.3 The vWF molecule is present in plasma as a series of multimers, which may consist of up to and over 80 monomers.4 It is generally believed that the higher multimers are more active in hemostasis.
Multimeric vWF is specifically cleaved by a 300-kD plasma metalloprotease between positions 842-843.5 In vitro, this protease is not active towards native vWF, but it is active towards vWF that has been denatured by urea or towards vWF containing a von Willebrand disease type 2A mutation.6 7 Although the exact function of this protease is unknown, it is likely that it is necessary for controlling the multimeric size of vWF and thereby its biological activity.
Furlan et al8 found that the activity of this metalloprotease is decreased in patients with chronic relapsing classic TTP. They hypothesized that inhibition of the metalloprotease causes diminished proteolysis of vWF multimers, resulting in the presence of unusually high multimers of vWF in the circulation. These unusually high multimers of vWF then induce spontaneous platelet aggregation and thrombus formation in the vasculature. Recently, inhibiting antibodies against the metalloprotease have been found in patients with classic TTP.9 Therapeutic plasma exchange is a very effective treatment of classic TTP. It is likely that plasma exchange removes the inhibitors, the inhibited metalloprotease, and the unusual high multimers of vWF, replacing them by active metalloprotease and vWF with a normal multimeric pattern.
TTP may also develop as a complication after BMT.1Recognition of this BMT-associated TTP has increased in recent years.10 The reported incidence of TTP is 14% in allograft and 7% in autograft recipients.1 Although the clinical picture is the same as for classic TTP, plasma exchange as treatment is generally unsuccessful.10-13 This suggests that plasma-derived factors like vWF and the vWF degrading metalloprotease are not important in the etiology of BMT-associated TTP. To investigate whether this suggestion is true, we measured the activity of the vWF degrading metalloprotease in TTP patients.
MATERIALS AND METHODS
Patients.
Thirteen patients with classic or BMT-associated TTP were studied. The diagnosis of TTP was made if the patient had thrombocytopenia (defined as a platelet count <100 × 109/L), microangiopathic hemolytic anemia as indicated by red blood cell fragmentation present in a peripheral blood smear and elevated lactate dehydrogenase (LDH), without an identifiable cause for the thrombocytopenia or microangiopathic hemolytic anemia (eg, sepsis, disseminated intravascular coagulation, carcinoma, eclampsia).
The clinical data of all patients are shown in Table 1. Five patients with classic TTP were studied. All patients with classic TTP were successfully treated by plasma exchange. Patients received a minimum of seven plasma exchanges over 9 days with assessment concerning further treatment made at the end of this treatment cycle. One and a half plasma volume of the patient was removed daily and replaced by the same volume of fresh frozen plasma.
Eight patients with BMT-associated TTP were studied. The mean time between the BMT and the development of TTP was 7 months (range, 3 to 13). In one patient, TTP developed after 18 months, when additional T cells were infused because of relapse of acute lymphoblastic leukemia.
Progressive disease during plasma exchange was observed in two autologous BMT patients (patients 8 and 9) and one allogeneic BMT patient (patient 11) (further decline in renal function, no improvement of hemoglobin, LDH, and platelet count). Subsequently, patients 8 and 9 received cyclosporin as alternative treatment. In another two autologous BMT patients (patients 6 and 7), cyclosporin was given as a first treatment. The TTP responded in all four patients.
All four patients with allogeneic BMT received oral cyclosporin and suffered from graft-versus-host disease (GVHD) at the moment that TTP developed. In three of these patients, cyclosporin was stopped followed by a gradual improvement of the peripheral blood counts and disappearance of TTP. However, all allogeneic BMT patients developed lethal complications unrelated to TTP (pneumonia [two patients], veno-occlusive disease of the liver, intracerebral hemorrhage).
Blood collection.
Unless otherwise indicated, samples were taken before treatment was started. Blood was collected by vacutainer system in 3.1% citrate (1:10). To obtain platelet-free plasma, the blood was centrifuged for 15 minutes at 4°C at 2,000g; the supernatant was removed and centrifuged a second time. Samples were stored at −80°C.
Characterization of vWF and cellular fibronectin (FN) in plasma.
The following parameters were determined for each plasma sample: vWF antigen concentration (vWF:Ag) and the vWF:ristocetin cofactor activity (vWF RiCof).14 The multimeric structure of endogenous patients’ vWF was determined by agarose gel electrophoresis followed by Western blotting as described by Lawrie et al.15 For detection, horseradish peroxidase-labeled polyclonal antibodies to human vWF were used (DAKOpatts, Glostrup, Denmark).
Cellular FN was determined as described previously.16
Preparation of recombinant vWF.
The construction, expression, and purification of recombinant human vWF containing the mutation R834Q (vWF R834Q) was described previously.6
vWF proteolysis assay.
The activity of the vWF degrading metalloprotease was determined by a modified version of the method described by Furlan et al.5 8 This method assays the activity of the protease in plasma. The plasma is diluted so far that endogenous vWF is not detectable, and purified vWF is then added as a substrate. Recombinant vWF R834Q, which has enhanced sensitivity to proteolysis by the metalloprotease, was used.
The experiments were performed as follows: citrate plasma (4 μL) was mixed with 26 μL low ionic strength buffer (5 mmol/L Tris-HCl) containing 1 mmol/L Pefabloc (Boehringer Mannheim, Almere, The Netherlands). To activate the metalloprotease, 1 μL of 300 mmol/L BaCl2 was added, and the mixture was incubated for 5 minutes at 37°C. Subsequently, 10 μL vWF R834Q was added (final concentration approximately 14 μg/mL). The mixture was transferred onto a hydrophilic filter membrane (VSWP, 25-μm diameter; Millipore, Bedford, PA), floating on the surface of 50 mL dialysis buffer (1.5 mol/L urea and 5 mmol/L Tris-HCl, pH = 8.0) in a screw-cap plastic tube. The tube was closed and the mixture was incubated overnight at 37°C. Samples were taken and mixed with 3 vol of sample buffer. The multimeric structure of substrate vWF after incubation was determined by agarose gel electrophoresis followed by Western blotting according to Lawrie.15 Equal amounts of incubation mixture were loaded on gel.
The extent of proteolysis of vWF by patient plasma was compared with the proteolysis of vWF by normal plasma from a healthy volunteer. The activity of the metalloprotease in control plasma was assessed without EDTA (maximal proteolysis of vWF) or in the presence of EDTA (no proteolysis of vWF). EDTA (1 mmol/L final concentration) was added to both the incubation mixture and the dialysis buffer.
Data analysis.
To quantify the results, blots were scanned and analyzed with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). The integrated pixel intensity was determined for the two lowest visible vWF multimer bands (“LMW-vWF”) and for all vWF visible in a lane (“total vWF”). Values were corrected for background. The fraction LMW-vWF was calculated by dividing the integrated pixel density of LMW-vWF through the integrated pixel density of total vWF. The breakdown was then calculated as the relative change in the fraction LMW-vWF, expressed as a normalized ratio. The value for fraction LMW-vWF of the control incubation with EDTA was taken as 0 and the value of the control without EDTA was taken as 1 (note that this method may yield values below 0 and above 1). For statistical analysis, the Mann-Whitney test was used.
RESULTS
The vWF:Ag, vWF:RiCof, and cellular FN plasma concentration of each patient are shown in Table 1. The cellular FN concentrations in plasma from patients with TTP were significantly higher compared with control (2.9 μg/mL ± 1.1 in TTP; compared with 0.9 μg/mL ± 0.2 in plasma from 40 healthy controls; P < .001). The levels in BMT-associated TTP were not significantly different from those in classic TTP. The concentration of vWF antigen was normal in patients with classic TTP, but was increased in patients with BMT-associated TTP (normal range, 70% to 130%). The multimeric distribution of endogenous vWF of the patients was similar to the multimeric distribution of vWF in normal pooled plasma (data not shown).
Clinical Data of the Patients Included in This Study
| . | Age/Sex . | Disease . | Type of BMT . | Plasma Exchange . | Cyclosporin Treatment . | Result of Treatment . | Survival Time (mo) . | LDH (U/L) . | Creatinin (μmol/L) . | Hb (mmol/L) . | Platelets (×109/L) . | CellularFibronectin (ng/mL) . | vWF:Ag (%) . | vWF:RiCof (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary TTP Patients | ||||||||||||||
| 1 | 43/F | Yes | Good | 22 | 1,311 | 62 | 5, 1 | 16 | 2,943 | 81 | 53 | |||
| 2 | 25/F | Yes | Good | 39 | 1,482 | 76 | 6, 4 | 5 | 2,221 | 88 | 71 | |||
| 3 | 35/F | Yes | Good | 44 | 2,086 | 188 | 6, 1 | 44 | 2,331 | 129 | 67 | |||
| 4 | 29/F | Yes | Good | 4 | 4,901 | 75 | 2, 9 | 11 | 3,219 | 196 | 132 | |||
| 5 | 29/F | Yes | Good | 4 | 1,777 | 86 | 5, 2 | 30 | 1,988 | 82 | 47 | |||
| BMT-Associated TTP Patients | ||||||||||||||
| 6 | 57/M | MM | Autologous | Started | Good | 50 | 658 | 121 | 4, 1 | 64 | 5,153 | 261 | 137 | |
| 7 | 40/M | Hodgkin’s | Autologous | Started | Good | 59 | 768 | 155 | 5, 0 | 49 | 2,932 | 181 | 104 | |
| 8 | 52/F | NHL | Autologous | Yes* | Started | On dialysis | 72 | 1,276 | 152 | 4, 6 | 24 | 2,244 | 263 | 185 |
| 9 | 16/M | NHL | Autologous | Yes* | Started | Good | 56 | 999 | 201 | 4, 6 | 44 | 1,825 | 148 | 90 |
| 10 | 37/F | ALL | Allogeneic | Stopped | Good† | 2 | 1,016 | 86 | 5, 9 | 17 | 2,212 | 320 | 238 | |
| 11 | 49/F | MDS | Allogeneic | Yes* | † | 1 | 1,959 | 209 | 5, 3 | 43 | 5,275 | 485 | 125 | |
| 12 | 35/M | ALL | Allogeneic | Stopped | Good† | 17 | 3,570 | 345 | 5, 5 | 19 | 3,444 | 470 | 243 | |
| 13 | 53/M | AA | Allogeneic | Stopped | Good† | 1 | 4,300 | 210 | 5, 0 | 5 | 3,010 | 620 | 300 | |
| . | Age/Sex . | Disease . | Type of BMT . | Plasma Exchange . | Cyclosporin Treatment . | Result of Treatment . | Survival Time (mo) . | LDH (U/L) . | Creatinin (μmol/L) . | Hb (mmol/L) . | Platelets (×109/L) . | CellularFibronectin (ng/mL) . | vWF:Ag (%) . | vWF:RiCof (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary TTP Patients | ||||||||||||||
| 1 | 43/F | Yes | Good | 22 | 1,311 | 62 | 5, 1 | 16 | 2,943 | 81 | 53 | |||
| 2 | 25/F | Yes | Good | 39 | 1,482 | 76 | 6, 4 | 5 | 2,221 | 88 | 71 | |||
| 3 | 35/F | Yes | Good | 44 | 2,086 | 188 | 6, 1 | 44 | 2,331 | 129 | 67 | |||
| 4 | 29/F | Yes | Good | 4 | 4,901 | 75 | 2, 9 | 11 | 3,219 | 196 | 132 | |||
| 5 | 29/F | Yes | Good | 4 | 1,777 | 86 | 5, 2 | 30 | 1,988 | 82 | 47 | |||
| BMT-Associated TTP Patients | ||||||||||||||
| 6 | 57/M | MM | Autologous | Started | Good | 50 | 658 | 121 | 4, 1 | 64 | 5,153 | 261 | 137 | |
| 7 | 40/M | Hodgkin’s | Autologous | Started | Good | 59 | 768 | 155 | 5, 0 | 49 | 2,932 | 181 | 104 | |
| 8 | 52/F | NHL | Autologous | Yes* | Started | On dialysis | 72 | 1,276 | 152 | 4, 6 | 24 | 2,244 | 263 | 185 |
| 9 | 16/M | NHL | Autologous | Yes* | Started | Good | 56 | 999 | 201 | 4, 6 | 44 | 1,825 | 148 | 90 |
| 10 | 37/F | ALL | Allogeneic | Stopped | Good† | 2 | 1,016 | 86 | 5, 9 | 17 | 2,212 | 320 | 238 | |
| 11 | 49/F | MDS | Allogeneic | Yes* | † | 1 | 1,959 | 209 | 5, 3 | 43 | 5,275 | 485 | 125 | |
| 12 | 35/M | ALL | Allogeneic | Stopped | Good† | 17 | 3,570 | 345 | 5, 5 | 19 | 3,444 | 470 | 243 | |
| 13 | 53/M | AA | Allogeneic | Stopped | Good† | 1 | 4,300 | 210 | 5, 0 | 5 | 3,010 | 620 | 300 | |
Abbreviations: AA, aplastic anemia; MDS, myelodysplastic syndrome; NHL, non-Hodgkin’s lymphoma; ALL, acute lymphoblastic leukemia.
No improvement after plasma exchange.
Developed lethal complications unrelated to TTP.
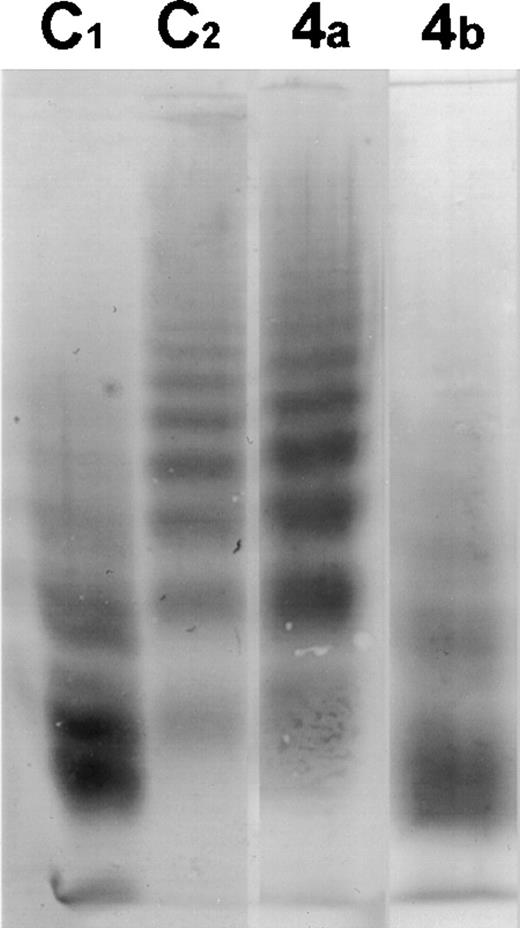
In Fig 1, the proteolysis of vWF by the metalloprotease in plasma of patient 4 before and after plasma exchange is shown. There is no active protease present in the plasma sample taken before treatment, while after the start of plasma exchange, there is active protease present in the plasma; vWF R834Q was then proteolysed by plasma from this patient to an extent comparable with control plasma. This is in agreement with the results of Furlan et al.9
Western blot of the multimeric pattern of vWF R834Q after incubation with plasma from patient 4 suffering from classic TTP before (4A) and after (4B) plasma exchange. C1, plasma from a healthy donor incubated with vWF R834Q. C2, plasma from a healthy donor incubated with vWF R834Q in the presence of EDTA (1 mmol/L).
Western blot of the multimeric pattern of vWF R834Q after incubation with plasma from patient 4 suffering from classic TTP before (4A) and after (4B) plasma exchange. C1, plasma from a healthy donor incubated with vWF R834Q. C2, plasma from a healthy donor incubated with vWF R834Q in the presence of EDTA (1 mmol/L).
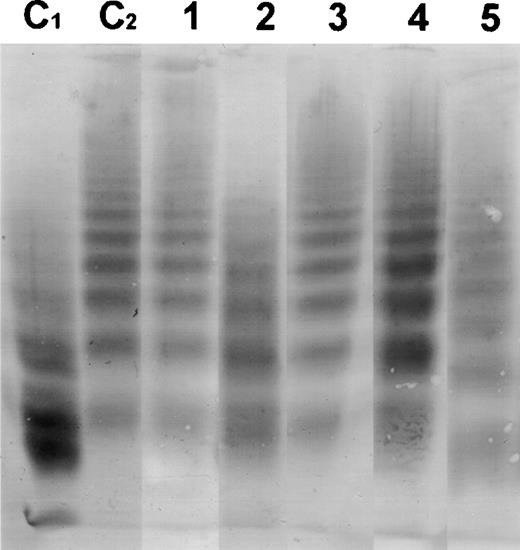
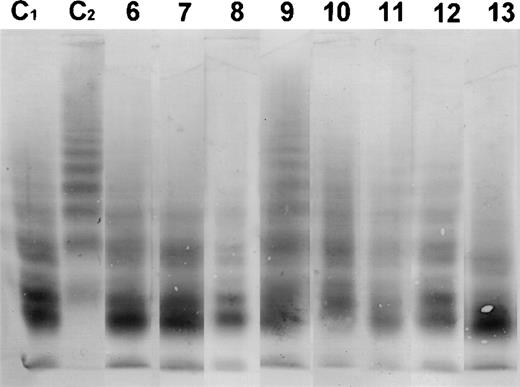
In Figs 2 and3, the Western blots are shown from the incubation of all patient plasmas with purified vWF R834Q. A qualitative assessment of these Western blots shows that the multimer size of vWF is not affected by incubation with plasma from patients with classic TTP, but is strongly reduced after incubation with plasma from patients with BMT-associated TTP, indicating that there is a clear difference in vWF breakdown between the patient groups.
Western blot of the multimeric pattern of vWF R834Q after incubation with plasma from patients suffering from classic TTP. C1, plasma from a healthy donor incubated with vWF R834Q. C2, plasma from a healthy donor incubated with vWF R834Q in the presence of EDTA (1 mmol/L). Lanes 1 through 5, patient plasmas (numbers as in Table 1).
Western blot of the multimeric pattern of vWF R834Q after incubation with plasma from patients suffering from classic TTP. C1, plasma from a healthy donor incubated with vWF R834Q. C2, plasma from a healthy donor incubated with vWF R834Q in the presence of EDTA (1 mmol/L). Lanes 1 through 5, patient plasmas (numbers as in Table 1).
Western blot of the multimeric pattern of vWF R834Q after incubation with plasma from patients suffering from BMT-associated TTP. C1, plasma from a healthy donor incubated with vWF R834Q. C2, plasma from a healthy donor incubated with vWF R834Q in the presence of EDTA (1 mmol/L). Lanes 6 through 13, patient plasmas (numbers as in Table1).
Western blot of the multimeric pattern of vWF R834Q after incubation with plasma from patients suffering from BMT-associated TTP. C1, plasma from a healthy donor incubated with vWF R834Q. C2, plasma from a healthy donor incubated with vWF R834Q in the presence of EDTA (1 mmol/L). Lanes 6 through 13, patient plasmas (numbers as in Table1).
The results were quantified by scanning the Western blots and expressing the extent of breakdown as a normalized ratio as is described in Materials and Methods. A ratio of zero signifies that the extent of breakdown is identical to the control in the presence of EDTA, while a ratio of 1 signifies that the extent of breakdown is the same as observed for normal plasma. The results of the quantitative analysis are shown in Table 2. The mean breakdown of vWF is 0.086 ± 0.094 for the classic TTP patient group and 0.91 ± 0.092 for the BMT-associated TTP patient group. Each patient group has one outlier with an intermediate normalized ratio. One patient with classic TTP (patient 2) had a normalized ratio of 0.42 and one patient with BMT-associated TTP (patient 9) had a normalized ratio of 0.48. The difference between the two patient groups, not excluding both patients with an intermediate ratio, is highly significant (Mann-Whitney test: P = .0016). The mean vWF breakdown in the classic TTP group was not significantly different from control with EDTA, which implies that this patient group has completely inhibited metalloprotease activity. The mean vWF breakdown in the BMT-associated TTP was not significantly different from control without EDTA, which implies that this patient group has normal metalloprotease activity.
Quantitative Analysis of vWF Breakdown
| Classic TTP . | BMT-Associated TTP . | ||
|---|---|---|---|
| Patient . | vWF Breakdown (normalized ratio) . | Patient . | vWF Breakdown (normalized ratio) . |
| 1 | −0.13 | 6 | 0.76 |
| 2 | 0.42 | 7 | 0.78 |
| 3 | −0.04 | 8 | 1.06 |
| 4 | 0.10 | 9 | 0.48 |
| 5 | 0.08 | 10 | 0.71 |
| 11 | 1.11 | ||
| 12 | 1.24 | ||
| 13 | 1.10 | ||
| Mean (±SEM) | 0.086 ± 0.094 | 0.91 ± 0.092 | |
| Classic TTP . | BMT-Associated TTP . | ||
|---|---|---|---|
| Patient . | vWF Breakdown (normalized ratio) . | Patient . | vWF Breakdown (normalized ratio) . |
| 1 | −0.13 | 6 | 0.76 |
| 2 | 0.42 | 7 | 0.78 |
| 3 | −0.04 | 8 | 1.06 |
| 4 | 0.10 | 9 | 0.48 |
| 5 | 0.08 | 10 | 0.71 |
| 11 | 1.11 | ||
| 12 | 1.24 | ||
| 13 | 1.10 | ||
| Mean (±SEM) | 0.086 ± 0.094 | 0.91 ± 0.092 | |
DISCUSSION
Furlan et al8 have shown that the activity of a vWF degrading plasma metalloprotease is decreased in patients with classic TTP. Inhibition of this protease was postulated as a general phenomenon in TTP. However, the observation that plasma exchange is ineffective in patients with BMT-associated TTP10-13 suggests that this plasma protease may not be involved in this form of TTP.
We assayed the activity of this metalloprotease in plasma from patients with classic or BMT-associated TTP. Most (seven of eight) patients who developed TTP after a BMT had a metalloprotease activity comparable to normal control. The metalloprotease activity in one patient was partially inhibited. In contrast, the metalloprotease activity was virtually absent in plasmas from patients with classic TTP, again with one exception of partial inhibition. The difference between these two patient groups is statistically significant.
Plasma exchange is very effective in the treatment of classic TTP, and we confirm in this report that plasma exchange results in restoration of plasma metalloprotease activity.9 However, the patients with BMT-associated TTP described in this and other reports10-13 did not improve after plasma exchange. This difference is explained by the normal metalloprotease activity in BMT-associated TTP. Apparently, plasma factors like the vWF degrading metalloprotease are not involved in BMT-associated TTP.
The etiology of BMT-associated TTP remains unclear. In allografted BMT patients, cyclosporin seems to be the most important risk factor for the development of TTP. The underlying mechanism is not completely understood, but cyclosporin is known to cause endothelial damage in vivo17 and activation and tissue factor expression on endothelial cells in vitro.18 It is possible that extensive endothelial damage, microangiopathy, and other effects caused by cyclosporin may finally lead to TTP.1 10 Discontinuing cyclosporin administration may thus result in disappearance of TTP. However, GVHD may also be involved in the development of endothelial damage and pathogenesis of TTP.
In autografted BMT patients, the TTP develops while patients are not receiving cyclosporin and surprisingly, cyclosporin is an effective treatment of TTP in these patients.11 There are two possible explanations for this observation. First, cyclosporin stimulates production of nitric oxide by endothelial cells,19 which may result in inhibition of platelet activation and aggregation. Second, cyclosporin is an immunosuppressive drug and may inhibit immune-mediated mechanisms in TTP.20The balance between the opposing effects of cyclosporin, endothelial damage versus inhibition of platelet activation, and antibody production apparently differs between autologous and allogeneic BMT patients. The immunosuppressive action of cyclosporin also explains the observation that it can be beneficial in classic TTP.21
The plasma concentration of cellular FN, a marker for endothelial damage, was elevated in both classic and BMT-associated TTP. Endothelial cell damage is probably a common feature of all forms of TTP. The increased vWF concentration in patients with BMT-associated TTP may be a factor in the development of TTP. At least it is a marker of the endothelial damage present. Surprisingly, the vWF concentration is in the normal range in patients with classic TTP, while it is expected to be increased because of the endothelial damage and the reduced vWF proteolysis. Apparently, the consumption of unusually high vWF multimers during the formation of platelet thrombi counteracts this effect.
We confirm that the metalloprotease that is involved in the proteolysis of vWF multimers is inhibited in patients with classic TTP, and we show that plasma exchange results in restoration of its activity. We observed that this metalloprotease is normally active in all patients with BMT-associated TTP. The present findings provide an explanation why plasma exchange is not effective in patients with BMT-associated TTP. It is, of course, of interest to study whether the vWF degrading metalloprotease is inhibited in other forms of secondary TTP, like pregnancy-, human immunodeficiency virus (HIV)-, cancer-, and drug-induced TTP.
Supported by Grant No. 902-26-193 from the Netherlands Organization for Scientific Research (NWO) and by the University Medical Centre Utrecht.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R. Martijn van der Plas, MSc, Department of Haematology, Room No. G03.647, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail: j.vd.velde@digd.azu.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal