Abstract
A1 is an anti-apoptotic bcl gene that is expressed in endothelial cells (EC) in response to pro-inflammatory stimuli. We show that in addition to protecting EC from apoptosis, A1 inhibits EC activation and its associated expression of pro-inflammatory proteins by inhibiting the transcription factor nuclear factor (NF)-κB. This new anti-inflammatory function gives a broader dimension to the protective role of A1 in EC. We also show that activation of NF-κB is essential for the expression of A1. Taken together, our data suggest that A1 downregulates not only the pro-apoptotic and pro-inflammatory response, but also its own expression, thus restoring a quiescent phenotype to EC.
THE VASCULAR endothelium in its quiescent state promotes an anti-inflammatory and anticoagulant environment.1 This phenotype changes significantly when endothelial cells (EC) are subjected to pro-inflammatory stimuli such as tumor necrosis factor (TNF) or lipopolysaccharide (LPS) in vitro or in vivo, as can occur at sites of acute and chronic inflammation.2-5 This phenotypic change, referred to as EC activation, is characterized by transcriptional induction of a number of pro-inflammatory and procoagulant factors such as adhesion molecules, cytokines, and tissue factor.6 Even though this pro-inflammatory environment promotes the generation of damaging levels of reactive oxygen species and the activation of proteases, EC are usually resistant to cell death.7-9
EC resistance is not likely related to the prototypic members of thebcl family such as bcl-2 andbcl-xL.10-12 Previous studies, including our own, show that the constitutive expression ofbcl-2 (little to undetectable) and ofbcl-xL is not increased on stimulation of EC with pro-inflammatory cytokines.13,14 The more recent discovery of inducible anti-apoptotic genes in EC, such as the bcl gene A1,13,15 the zinc finger protein A20,16,17 and the newly described IAP genes,18 offers an explanation for the resistance of EC to inflammation-associated cell death.
A1 is a bcl family member that was originally identified as a hematopoietic-specific early response gene induced upon stimulation with granulocyte-macrophage colony-stimulating factor.19Further studies showed its expression in other cell types, ie, EC.15 A1 expression was also found in tumors associated with gastric cancers.20 In EC, A1 is induced through a protein kinase C–dependent pathway in response to pro-inflammatory stimuli and protects EC against TNF- and ceramide-mediated apoptosis.13,15 We show here that the cytoprotective effect of A1 is not limited to protection from apoptosis, but extends to downregulation of EC activation. The inhibitory effect of A1 on EC activation is due to inhibition of a key transcription factor required for the up-regulation of most genes associated with EC activation, namely nuclear factor (NF)-κB.21 22
We further show that NF-κB activation is required for A1 expression. Inhibition of NF-κB by overexpression of its specific inhibitor IκBα,23 abrogates TNF-mediated upregulation of A1. In addition, A1 is induced in EC overexpressing p65. Thus, A1 is the firstbcl gene shown to depend on NF-κB for its expression. This observation suggests that NF-κB is required not only for the upregulation of the pro-inflammatory molecules associated with EC activation, but also for the upregulation of “protective” molecules such as A1.
MATERIALS AND METHODS
Cell Culture
Human umbilical vein endothelial cells (HUVEC) were isolated and cultured as described.24 Bovine aortic endothelial cells (BAEC) and the 293 human embryonic kidney cell line (ATCC) were cultured in Dulbecco’s modified Eagle medium (DMEM) (GIBCO Life Technologies, Grand Island, NY) as described. All cells were cultured on tissue culture plates (Nunc; Marsh Products, Rochester, NY) precoated with 0.2% gelatin (Sigma Chemical Co, St Louis, MO) and grown at 37°C in a humidified incubator with a 5% CO2atmosphere.
Expression Plasmids and Reporter Constructs
A1 expression plasmid.
An N-terminally tagged A1 expression plasmid (HA-A1) was constructed by reverse transcription-polymerase chain reaction (RT-PCR) from TNF-induced HUVEC cDNA using the following primers: sense, 5′-TTGCTCGGATCCAGGCAGAAGATGACAG-3′ and anti-sense, 5′-GTGTGAATTCTGGTCAACAGTATTGC-3′. The PCR product was cloned in the pcDNA3 expression plasmid (Invitrogen, San Diego, CA) tagged with the hemagglutinin (HA) nonapeptide sequence YPYDVPDYA (kind gift of Dr J. Anrather, Immunobiology Research Center, Beth Israel/Deaconess Medical Center, Boston, MA). Sequence analysis of derived clones confirmed identity to the published human A1 sequence. The murine Bcl-2 cDNA is a kind gift of Dr T. Behrens (University of Minnesota, Minneapolis). This cDNA was subcloned in pAC expression vector. pAC is an 8.8-kb plasmid vector containing a cytomegalovirus promoter, a pUC19 polylinker site, and an SV40 splice/polyA site (a kind gift of Robert Gerard, University of Texas, Southwestern, Dallas, TX).
E-selectin, interleukin-8 (IL-8), ECI-6 (IκBα), pRc/RSV βeta galactosidase (β-gal), and NF-κB reporters were previously described.25 Human immunodeficiency virus (HIV)-long terminal repeat (LTR) wild type (wt) and HIV-LTR ΔκB reporter (HIV)-chloramphenicol acetyltransferase (CAT) and HIVΔκB-CAT) are a kind gift of Ernst Böhnlein (Systemix, Inc, Palo Alto, CA) and have been previously described; they represent nucleotides −117 bp up to the TATA box of HIV-1 LTR for the wt deleted from the NF-κB binding sites (for the ΔκB) cloned upstream of the CAT gene (CAT3N polylinker).26
p65 Expression plasmid.
The p65 plasmid was a kind gift of Dr J. Anrather and represents human RelA cDNA (amino acid 2-551) cloned in pcDNA3.C-Tat is an expression vector encoding the HIV-1 Tat protein (kind gift of Dr Ernst Böhnlein).26
Transient transfection assay.
2.105 BAEC were transfected with 1.5 μg/well of DNA (expression plasmids and reporter constructs) using lipofectamine (GIBCO) as previously described.25 In all experiments (except HIV-CAT) 0.3 μg of the β-gal reporter and 0.7 μg of the E-selectin, IL-8, IκBα, or NF-κB reporter was used with 0.001 to 0.5 μg of HA-A1 or pcDNA3 expression plasmids. In experiments inducing the NF-κB reporter by overexpression of p65 (RelA), 5 to 100 ng of p65 expression vector were added. For the HIV-CAT and HIV ΔκB-CAT reporter experiments, 0.2 μg of the β-gal reporter was transfected with 0.5 μg of HA-A1 or pcDNA3, 0.2 μg of the c-Tat expression plasmid, and 0.6 μg of the HIV-CAT or the HIVΔκB-CAT reporter. Cells were stimulated 48 hours after transfection with either human recombinant TNF (100 U/mL) (R&D, Minneapolis, MN) or LPS (100 ng/mL) (E coli 0B55; Sigma), harvested after 7 hours and assayed for β-galactosidase, luciferase, or CAT activity, as previously described.25 Luciferase and CAT activities were normalized for β-gal by using the formula: luciferase activity/β-gal activity ×1,000. Normalized luciferase activity is given in relative light units.
Western blot analysis.
Cytoplasmic extracts were prepared from transfected BAEC and lysed in RIPA buffer (10 mmol/L TRIS pH 7.5, 150 mmol/L NaCl, 1% Triton X-100) containing 0.5 μg/mL of aprotinin, leupeptin, and antipain, 0.5 mmol/L PMSF. Twenty μg of protein (determined by Bradford assay; BioRad, Hercules, CA) were resolved on a reducing 12.5% sodium dodecyl sulfate (SDS) polyacrylamide gel, and transferred onto Immobilon-P membranes (Millipore, Bedford, MA). Membranes were blocked in BLOTTO (5% nonfat dry milk in 0.1% Tween 20 phosphate-buffered saline). HA-A1 protein expression was detected by incubating 1/1,000 dilution of anti-HA antibody (Boehringer Mannheim, Indianapolis, IN) followed by a 1/3,000 dilution of peroxidase-conjugated goat anti-mouse secondary antibody (Pierce, Rockford, IL) and revealed by enhanced chemiluminescence (ECL; Amersham, Arlington Hights, IL).
Recombinant adenoviral-mediated gene transfer.
HUVEC were infected with a recombinant adenovirus (rAd) expressing either A20 (gift of Dr V.M. Dixit, Genentech Inc, San Francisco, CA), porcine IκBα (gift of Dr C. Wrighton, Therexys Ltd, Keele University, Keele, Staffordshire, UK),27 or, as a control, β-gal (gift of Dr R. Gerard), at a moiety of infection (MOI) of 100 plaque-forming units/cell, previously shown to infect more than 90% of the cells.27 HUVEC were assayed 48 hours after the infection.
Northern blot analysis.
HUVEC, with or without rAd-mediated gene transfer, were stimulated for 3 hours with TNF (100 U/mL), LPS (100 ng/mL), or phorbol-12-myristate-13-acetate (PMA) (5 × 10−8mol/L) (Sigma). Total cellular RNA was extracted with Trizol (GIBCO) and 10 μg of RNA were separated on a 1.3% agarose-formaldehyde gel and transferred onto nylon membrane (Amersham). RNA was hybridized with α-[32P]-dATP (NEN) labeled probes as indicated (Stratagene, La Jolla, CA). Membranes were stripped by boiling in 0.1% SDS before reprobing with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to correct for unequal sample loading.
RT-PCR.
BAEC were transfected with 1.5 μg of pcDNA3 or 0.9 μg of pcDNA3 along with 0.6 μg of the p65 expression plasmid. Eighteen hours after transfection, RNA was extracted with Trizol. Five micrograms of total RNA were used for cDNA synthesis using the superscript reverse transcriptase (GIBCO) and an oligodT primer. The cDNA were then amplified for A1 expression using primers derived from the human A1 sequence: sense primer 5′-AGATGACAGACTGTGAATTTGG-3′ and anti-sense primer 5′-TGGTCAACAGTATTGCTTCAGG-3′. After an initial denaturation for 5 minutes at 94°C, the cDNA were amplified in a Peltier thermocycler 200 (MJ Research Inc, Incline Village, NV) for 35 cycles with each cycle programmed for a denaturation at 94°C for 60 seconds, annealing at 50°C for 60 seconds, and elongation at 72°C for 60 seconds. The elongation time of the last cycle was extended to 5 minutes. Amplified bovine A1 PCR product was sequenced using the DNA cycle sequencing kit from Perkin Elmer (Norwalk, CT). To reliably compare the amount of amplified product, cDNA samples were amplified with housekeeping GAPDH primers which amplified products were recovered at 15, 20, 25, 30, and 35 cycles (Clontech, Palo Alto, CA).
RESULTS
A Novel Function for A1: Inhibition of EC Activation
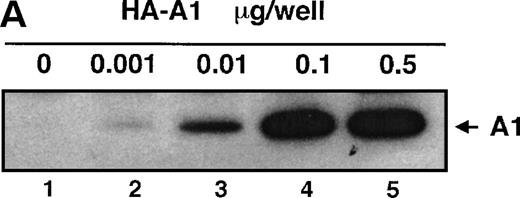
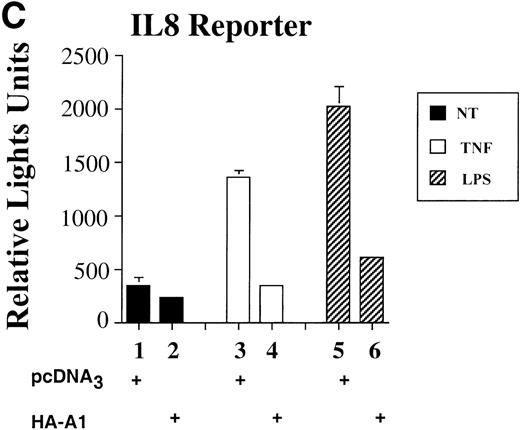
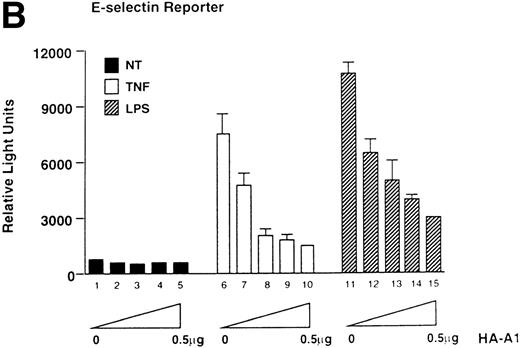
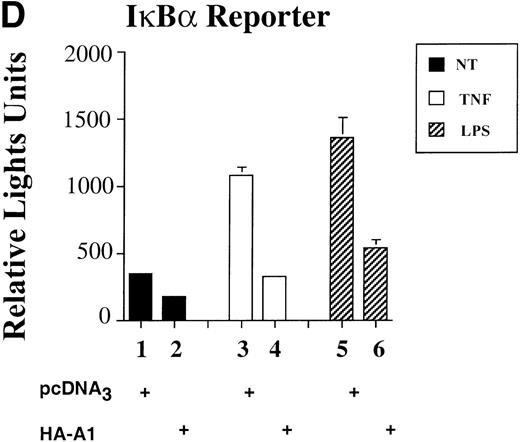
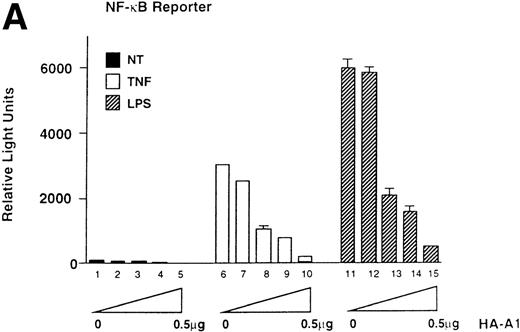
To assess whether A1 could have an effect on EC activation, we used an E-selectin reporter construct as a read-out for EC activation. E-selectin is an EC-specific adhesion molecule that is upregulated in response to pro-inflammatory stimuli.28 BAEC were transfected with the E-selectin reporter together with either the HA-A1 expression plasmid or pcDNA3 in amounts ranging from 10 ng to 0.5 μg. Transfection with increasing amounts of the HA-A1 plasmid correlated with increased A1 protein expression as evaluated by Western blot using an antibody against HA (Fig 1A). Forty-eight hours after transfection, BAEC were stimulated for 7 hours with TNF or LPS, then harvested and assayed for luciferase activity. In BAEC transfected with pcDNA3, treatment with TNF and LPS led, respectively, to 10- and 14.5-fold induction of E-selectin reporter activity (Fig 1B, lanes 6 and 11 v 1). Coexpression of A1 inhibited TNF- or LPS-induced luciferase activity in a dose-dependent manner (Fig 1B, lanes 7 through 10 and lanes 12 through 15). Transfection of 0.5 μg of HA-A1 inhibited TNF-induced reporter activity by 80% (Fig 1B, lane 10 v 6) and LPS-induced reporter activity by 70% (Fig 1B, lane 15 v 11). Transfection with as little as 1 ng of HA-A1 still inhibited reporter activity by 40% with either TNF or LPS stimulation (Fig 1B, lane 7 v 6 and lane 12v 11). In the next set of experiments, we chose reporters constructed with promoters of genes that, like E-selectin, are upregulated during EC activation: IL-8 and porcine IκBα (ECI-6). A1 expression inhibited the 3- and 4.5-fold induction of the IL-8 reporter following stimulation with TNF or LPS (Fig 1C, lane 4 v 3 and lane 6 v 5). Similar results were obtained with the IκBα reporter. A1 expression inhibited the 2.5- and 3.5-fold induction of the IκBα reporter following stimulation with TNF or LPS (Fig 1D, lane 4 v 3 and lane 6 v 5).
A1 expression inhibits E-selectin, IL-8, and IκB reporter activity. (A) Western blot analysis of A1 expressing BAEC. A1 protein was detected using anti-HA antibody at the expected molecular weight of 20 kD, with as little as 0.001 μg (lane 2) of transfected HA-A1 expression plasmid and expression was near optimal with 0.1 μg (lane 4) (B) BAEC were transfected with the HA-A1 expression plasmid titrated (0, 1 ng, 10 ng, 0.1 μg, or 0.5 μg) with pcDNA3 to equal 0.5 μg of DNA together with the E-selectin (0.7 μg) and β-gal (0.3 μg) reporters. Forty-eight hours after transfection, cells were stimulated with either TNF (100 U/mL) (lanes 6 through 10) or LPS (100 ng/mL) (lanes 11 through 15) for 7 hours. Results are given in relative light units. Data shown are representative of six experiments. Error bars are ± SE (C and D) BAEC were transfected with 0.5 μg of the HA-A1 expression plasmid together with the IL-8 or IκB (0.7 μg) and β-gal (0.3 μg) reporters. BAEC were then stimulated with TNF (100 U/mL) (lanes 3 through 4) or LPS (100 ng/mL) (lanes 5 through 6). Results are given in relative light units. Data shown are representative of three experiments.
A1 expression inhibits E-selectin, IL-8, and IκB reporter activity. (A) Western blot analysis of A1 expressing BAEC. A1 protein was detected using anti-HA antibody at the expected molecular weight of 20 kD, with as little as 0.001 μg (lane 2) of transfected HA-A1 expression plasmid and expression was near optimal with 0.1 μg (lane 4) (B) BAEC were transfected with the HA-A1 expression plasmid titrated (0, 1 ng, 10 ng, 0.1 μg, or 0.5 μg) with pcDNA3 to equal 0.5 μg of DNA together with the E-selectin (0.7 μg) and β-gal (0.3 μg) reporters. Forty-eight hours after transfection, cells were stimulated with either TNF (100 U/mL) (lanes 6 through 10) or LPS (100 ng/mL) (lanes 11 through 15) for 7 hours. Results are given in relative light units. Data shown are representative of six experiments. Error bars are ± SE (C and D) BAEC were transfected with 0.5 μg of the HA-A1 expression plasmid together with the IL-8 or IκB (0.7 μg) and β-gal (0.3 μg) reporters. BAEC were then stimulated with TNF (100 U/mL) (lanes 3 through 4) or LPS (100 ng/mL) (lanes 5 through 6). Results are given in relative light units. Data shown are representative of three experiments.
A1 Inhibits EC Activation Through Inhibition of NF-κB: Inhibition Occurs Upstream of p65-Mediated Transactivation
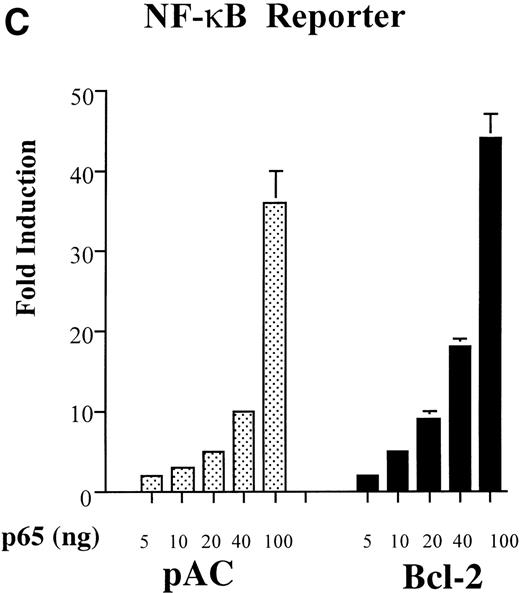
The transcription factor NF-κB plays a key role in the up-regulation of pro-inflammatory genes such as E-selectin, IL-8, and IκBα.21 Therefore, we questioned whether inhibition by A1 of these reporters reflects an effect of A1 on NF-κB activity. BAEC were cotransfected with the HA-A1 expression plasmid in amounts ranging from 1 ng to 0.5 μg together with a reporter plasmid whose expression is strictly dependent on activation of NF-κB. In BAEC transfected with pcDNA3, TNF and LPS, respectively, led to a 30- and 60-fold induction of NF-κB luciferase activity (Fig2A, lanes 6 and 11 v 1). A1 expression inhibited TNF- or LPS-induced luciferase activity in a dose-dependent manner (Fig 2A, lanes 7 through 10 and lanes 12 through 15). In the presence of 10 ng of the A1 expression plasmid, inhibition of induction reached 60% with TNF or LPS stimulation (Fig 2A, lane 8v 6 and lane 13 v 11). Transfection with 0.5 μg of A1 expression plasmid repressed TNF or LPS induction of NF-κB reporter activity by greater than 95% (Fig 2A, lane 10 v 6 and lane 15v 11).
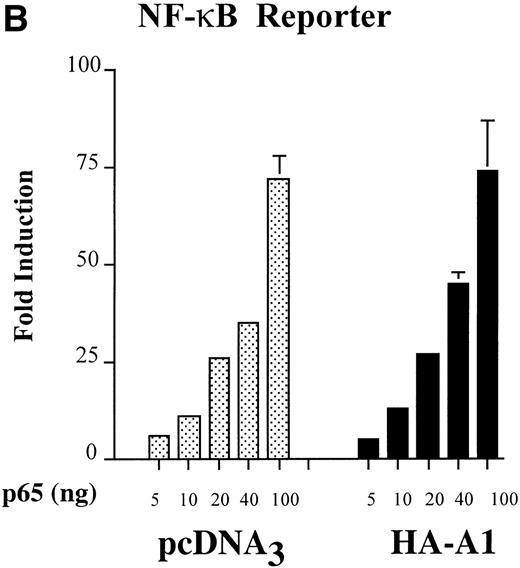
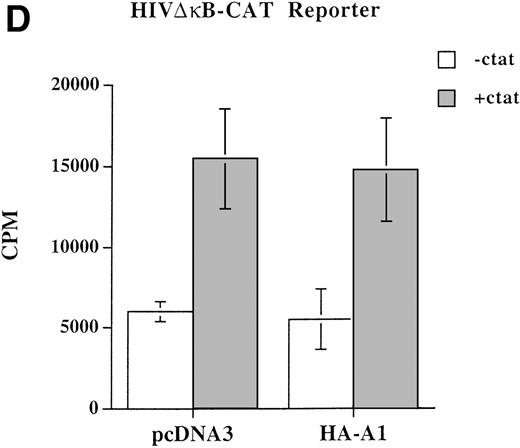
A1 inhibits NF-κB reporter activity. (A) Inhibition is upstream of p65-mediated transactivation, (B) and is comparable to that achieved with Bcl-2; (C) A1 expression does not inhibit the NF-κB–independent reporter, HIV-▵κB CAT (D). (A) BAEC were transfected with the HA-A1 expression plasmid titrated (0, 1 ng, 10 ng, 0.1 μg, or 0.5 μg) with pcDNA3 to equal 0.5 μg of DNA together with the NF-κB (0.7 μg) and β-gal (0.3 μg) reporters. Forty-eight hours after transfection, cells were stimulated with either TNF (100 U/mL) (lanes 6 through 10) or LPS (100 ng/mL) (lanes 11 through 15) for 7 hours. Data shown are representative of six experiments. (B) BAEC were cotransfected with 0.5 μg of pcDNA3 or HA-A1 expression plasmid along with NF-κB reporter (0.7 μg), β-gal reporter (0.3 μg) and in the presence of increasing amounts of p65 expression plasmid ranging from 0 to 100 ng. Data shown represent the fold induction of the NF-κB reporter luciferase activity by increasing amount of cotransfected p65. Data shown are representative of three experiments performed. (C) BAEC were cotransfected with 0.5 μg of pAC or murine Bcl-2 expression plasmid along with NF-κB reporter (0.7 μg), β-gal reporter (0.3 μg), and increasing amounts of p65 expression plasmid ranging from 0 to 100 ng. Data shown represent the fold induction of the NF-κB reporter luciferase activity by increasing amount of cotransfected p65. Data shown are representative of two experiments performed. (D) BAEC were cotransfected with 0.5 μg of pcDNA3 or HA-A1 together with 0.6 μg of an HIV-▵κB CAT reporter in the absence (−) or presence of 0.2 μg (+) of the viral protein c-Tat. Results are given in cpm ± SE. All error bars are ± SE. Data shown are representative of three experiments performed.
A1 inhibits NF-κB reporter activity. (A) Inhibition is upstream of p65-mediated transactivation, (B) and is comparable to that achieved with Bcl-2; (C) A1 expression does not inhibit the NF-κB–independent reporter, HIV-▵κB CAT (D). (A) BAEC were transfected with the HA-A1 expression plasmid titrated (0, 1 ng, 10 ng, 0.1 μg, or 0.5 μg) with pcDNA3 to equal 0.5 μg of DNA together with the NF-κB (0.7 μg) and β-gal (0.3 μg) reporters. Forty-eight hours after transfection, cells were stimulated with either TNF (100 U/mL) (lanes 6 through 10) or LPS (100 ng/mL) (lanes 11 through 15) for 7 hours. Data shown are representative of six experiments. (B) BAEC were cotransfected with 0.5 μg of pcDNA3 or HA-A1 expression plasmid along with NF-κB reporter (0.7 μg), β-gal reporter (0.3 μg) and in the presence of increasing amounts of p65 expression plasmid ranging from 0 to 100 ng. Data shown represent the fold induction of the NF-κB reporter luciferase activity by increasing amount of cotransfected p65. Data shown are representative of three experiments performed. (C) BAEC were cotransfected with 0.5 μg of pAC or murine Bcl-2 expression plasmid along with NF-κB reporter (0.7 μg), β-gal reporter (0.3 μg), and increasing amounts of p65 expression plasmid ranging from 0 to 100 ng. Data shown represent the fold induction of the NF-κB reporter luciferase activity by increasing amount of cotransfected p65. Data shown are representative of two experiments performed. (D) BAEC were cotransfected with 0.5 μg of pcDNA3 or HA-A1 together with 0.6 μg of an HIV-▵κB CAT reporter in the absence (−) or presence of 0.2 μg (+) of the viral protein c-Tat. Results are given in cpm ± SE. All error bars are ± SE. Data shown are representative of three experiments performed.
Having established that A1 interfered with NF-κB activation, we questioned whether this inhibition targets RelA/p65-mediated transactivation of NF-κB. BAEC were cotransfected with 0.5 μg of HA-A1 or pcDNA3 as well as the NF-κB reporter construct. Cotransfection of the p65 expression plasmid with amounts ranging from 5 to 100 ng induced a dose-dependent increase in the luciferase activity of the NF-κB reporter. A maximal induction of 40-fold was achieved with the highest dose of p65 (100 ng). RelA/p65-mediated induction of the NF-κB reporter was not modified by expression of A1 (Fig 2B). This result indicates that A1 does not interfere with p65-mediated transactivation in EC but rather exerts its effect further upstream in the signaling cascade. Bcl-2 had a similar effect to A1 in EC; expression of Bcl-2 in BAEC did not affect the p65-mediated increase in the luciferase activity of the NF-κB reporter (Fig 2). Comparable induction of the luciferase activity was achieved in the control pAC or the Bcl-2–transfected BAEC reaching again a 40-fold increase with the highest p65 dose.
To determine whether the inhibitory effect of A1 on the activity of NF-κB–dependent reporters was selective, we evaluated whether A1 expression affects the induction of a c-Tat driven Sp1.26Induction of the wt HIV-CAT reporter by coexpression of c-Tat was not modified by A1 expression (data not shown). To further confirm that NF-κB binding sites were not involved in the induction of the HIV-CAT reporter, a similar experiment was performed using the HIV ΔκB-CAT reporter that has been deleted from its two κB binding sites but that still has all the Sp1 binding sites. Here again, A1 expression did not alter the 2.5- to 3-fold induction of the reporter observed on stimulation with c-Tat (Fig 2D). Although much lower than the induction achieved in human cells, the 3-fold c-Tat–induced activation of the HIV-LTR reporter in BAEC was consistently reproducible. Difference in induction might well relate to interspecies variations. This result indicates that, in EC, A1 does not have an overall inhibitory effect on transcription nor does it inhibit the transactivation properties of Sp1.
NF-κB Activation Is Required for A1 Expression in HUVEC
To examine how the expression of A1 is regulated by pro-inflammatory stimuli, RNA was extracted from HUVEC before and 3 hours after treatment with 100 U/mL of TNF (lane 2), 100 ng/mL of LPS (lane 3) or 5 × 10−8 mmol/L PMA (lane 4). In consensus with recent literature reports, Northern blot analysis of A1 mRNA showed that A1 is not expressed constitutively, but is induced on stimulation with TNF or PMA (Fig 3A).15 We show, in addition, that A1 is induced following LPS stimulation (Fig 3A). In contrast, constitutive mRNA levels of bcl-xL in EC, were not modified by treatment with TNF, LPS, or PMA (Fig 3A).
A1 expression is NF-κB–dependent. (A) HUVEC were stimulated with TNF (100 U/mL), LPS (100 ng/mL), or PMA (5 × 10−8 mol/L) for 3 hours. A1 or Bcl-XL mRNA was detected using -[32P]-dATP–labeled specific cDNA probes. (B) HUVEC were noninfected (NI) or infected with rAd.A20, rAd.IκB or rAd.β-gal. Forty-eight hours after infection, HUVEC were either left nontreated (−) or were stimulated with TNF (+) (100 U/mL). A1, A20, and IκB mRNA levels were detected by Northern blot analysis using their specific -[32P]-dATP–labeled cDNA probes. GAPDH was used to confirm equal quantity of RNA. Result shown is representative of three independent experiments. (C) BAEC were either nontransfected (NT) or transfected with a control plasmid (pcDNA3) or the p65 expression plasmid (+p65). A1 mRNA was only induced in p65 transfected BAEC (lane 5 v 3 and 4). Lanes 1 and 2 correspond to negative (C) and positive controls (TNF treated). GAPDH amplification (0.45 kb) was similar in all samples for all cycles tested. Only the 20 cycles’ amplification results are shown.
A1 expression is NF-κB–dependent. (A) HUVEC were stimulated with TNF (100 U/mL), LPS (100 ng/mL), or PMA (5 × 10−8 mol/L) for 3 hours. A1 or Bcl-XL mRNA was detected using -[32P]-dATP–labeled specific cDNA probes. (B) HUVEC were noninfected (NI) or infected with rAd.A20, rAd.IκB or rAd.β-gal. Forty-eight hours after infection, HUVEC were either left nontreated (−) or were stimulated with TNF (+) (100 U/mL). A1, A20, and IκB mRNA levels were detected by Northern blot analysis using their specific -[32P]-dATP–labeled cDNA probes. GAPDH was used to confirm equal quantity of RNA. Result shown is representative of three independent experiments. (C) BAEC were either nontransfected (NT) or transfected with a control plasmid (pcDNA3) or the p65 expression plasmid (+p65). A1 mRNA was only induced in p65 transfected BAEC (lane 5 v 3 and 4). Lanes 1 and 2 correspond to negative (C) and positive controls (TNF treated). GAPDH amplification (0.45 kb) was similar in all samples for all cycles tested. Only the 20 cycles’ amplification results are shown.
Most of the early phase response genes that are induced with EC activation are dependent on activation of NF-κB.21 To assess whether NF-κB is also involved in the induction of A1, HUVEC cultures were infected with an rAd expressing IκBα, the specific inhibitor of NF-κB, at an MOI of 100. Forty-eight hours after infection, HUVEC were stimulated with TNF for 3 hours, and total RNA was extracted and assayed for A1 mRNA upregulation. A1 mRNA was not induced in response to TNF in HUVEC overexpressing IκBα (Fig 3B, lane 6 v 5), whereas high levels were detected in noninfected HUVEC (Fig 3B, lane 2 v 1), or in HUVEC infected with the control rAd β-gal (Fig 3B, lane 8 v 7). Comparably, adenoviral-mediated gene transfer of A20, an anti-apoptotic gene that inhibits activation of NF-κB in porcine aortic EC, also resulted in inhibition of A1 induction by TNF (Fig 3B, lane 4 v 3). To confirm that in HUVEC A20 retains its inhibitory effect on NF-κB activation, electrophoretic mobility shift assay (EMSA) analysis (data not shown) of nuclear extracts of HUVEC infected with rAdA20 showed no NF-κB binding activity following stimulation with TNF.29
We then questioned whether p65 (Rel A) (the major transactivating subunit within the Rel family members) was directly involved in A1 gene induction. Total RNA was extracted from BAEC transfected with the p65 expression plasmid and the same amount (5 μg) from each sample was then used for cDNA synthesis. Five microliters of the reverse transcriptase reaction was then amplified by PCR for A1 or GAPDH expression. Results showed that A1 mRNA expression is only induced when p65 is overexpressed in BAEC but not in nontransfected or pcDNA3-transfected cells (Fig 3C, lane 5 v 3 and 4). Positive and negative control were samples taken from quiescent and TNF-stimulated BAEC (Fig 3C, lanes 1 and 2). The identity of the amplified bovine A1 PCR product was confirmed by sequencing (data not shown).
DISCUSSION
EC are usually resistant to TNF-mediated death unless RNA or protein synthesis is inhibited,9 which indicates that de novo protein synthesis is required to protect from apoptosis. A1, A20, and the IAP proteins are TNF-inducible genes that belong to this category of newly synthesized anti-apoptotic proteins in EC.13,15-18,30 We recently showed that A20 has a broader cytoprotective role than merely inhibiting apoptosis; overexpression of A20 also inhibits the up-regulation of genes associated with EC activation by inhibiting the transcription factor NF-κB.25,29 A1 is a bcl family member that, unlike the other anti-apoptotic bcl family members, ie, bcl-2 and bcl-xL, is induced in response to pro-inflammatory stimuli in EC.15 The described function of A1 is to protect EC against TNF- or ceramide-mediated apoptosis.13 More recently, it has also been implicated as part of the cytoprotective response of EC against LPS.31
In this paper we show that in EC, A1 has the same cytoprotective properties as A20 ie protection from apoptosis and inhibition of activation. As shown, overexpression of A1 inhibits the upregulation of E-selectin, a specific marker for EC activation.32 In lieu of a protein measurement of E-selectin, this result was obtained using a reporter containing the full porcine E-selectin promoter that has been thoroughly characterized. This E-selectin reporter has been used in several studies and shown to always correlate with the regulation of the endogenous E-selectin.25,33-35 A1-mediated inhibition of EC activation relates, at least in part and perhaps totally, to the inhibition of the transcriptional factor NF-κB. Like E-selectin, the regulatory regions of most of the pro-inflammatory genes induced during EC activation share a common feature; they contain at least one binding site for NF-κB.21 We also show that A1 equally suppresses the expression of other NF-κB–dependent pro-inflammatory genes ie, IL-8 and IκBα. Taken together, these data suggest that A1 expression would markedly curb the inflammatory response associated with EC activation.
The exact mechanism by which A1 inhibits activation of NF-κB remains to be determined. In EC, the major active form of NF-κB is a heterodimer of p50/NF-κB1 and p65/RelA proteins. RelA/p65 contributes to transcriptional activation whereas NF-κB1/p50 is involved in DNA binding.36 In quiescent EC, NF-κB is held in the cytoplasm by its inhibitory protein, IκBα.23Stimulation of EC with agonists such as TNF, IL-1, PMA, or LPS initiate a signaling cascade that results in the phosphorylation,37ubiquitination,38 and degradation39,40 of IκBα. NF-κB is then able to translocate to the nucleus, bind to its DNA sequence element, and initiate transcription of its target genes. Our data shows that A1 does not interfere with p65-mediated transactivation of NF-κB; induction of NF-κB reporter activity by p65 expression is not altered by A1. This result contrasts with a previous report studying Bcl-2. Bcl-2 was reported to inhibit NF-κB activation in 293 cells by downmodulating the transactivating potential of nuclear p65.41 This difference does not relate to variances in function between A1 and Bcl-2. We show here that, like A1, expression of Bcl-2 in EC inhibited NF-κB activation56without altering p65-mediated transactivation. The difference in the effect of the Bcl proteins upon p65-mediated transactivation is more likely explained by cell-type specific function(s) of the bclgenes as we were able to reproduce the inhibitory effect of Bcl-2 upon p65-mediated transactivation in 293 cells (data not shown). Given that the yield of transfected BAEC using lipofectamine does not usually exceed 10% to 15% of the cells, analysis of NF-κB translocation to the nucleus or of IκBα degradation following cytokine treatment could not be achieved. Generation of a replication defective adenovirus expressing A1 that would have the ability of transducing a high percentage of cultured EC is currently in progress and would help address these questions.
In summary, our data show that the inhibitory effect of A1 on NF-κB activation is at a level upstream of p65-mediated transactivation, however, the molecular basis of this novel function for A1 is still not defined. One avenue of research is to evaluate whether the inhibitory effect of A1 on NF-κB activation requires its interaction with other cellular proteins. Like Bcl-2 and Bcl-xL, A1 can heterodimerize with pro-apoptotic bcl family members, namely Bax.42 Whether interaction of A1 with Bax occurs in EC and is equally as important for the inhibitory effect of A1 on NF-κB activation as for regulation of apoptosis needs to be tested. Alternatively, A1 may interrupt the pathway leading to NF-κB activation by interacting with key signaling molecules. In favor of this hypothesis are the data in the literature showing that Bcl-2 interacts with molecules such as p21Ras, p23Ras, Raf-1 kinase, and calcineurin.43-47 For instance, the interaction between Bcl-2 and calcineurin is critical for the inhibitory effect of Bcl-2 on the transcription factor NF-AT (nuclear factor for activated T cells) in T cells.47 One can speculate, giving the high homology between Bcl-2 and A1, namely within the BH4 domain (necessary for all interactions mentioned above), that A1 will also interact with similar proteins to inhibit NF-κB activation in EC. Further studies are addressing this issue.
As previously stated, most of the early response genes associated with EC activation are dependent on NF-κB; this includes A20 whose expression also requires NF-κB.48,49 We questioned whether activation of NF-κB is also a prerequisite for A1 expression. Using rAd, we overexpressed in HUVEC two inhibitors of NF-κB: IκBα, the specific inhibitor of NF-κB, or A20.25HUVEC expressing either one of these inhibitors no longer upregulated A1 mRNA after TNF stimulation. In lieu of a complete promoter analysis, this result indicates that A1 expression, in EC, requires activation of NF-κB. The direct demonstration that A1 expression is induced in BAEC by overexpression of RelA/p65 confirmed these data. A1 is, to the best of our knowledge, the first bcl gene whose expression is dependent on NF-κB.
In conclusion, A1 appears to belong to the same category of cytoprotective molecules in EC as the nonrelated anti-apoptotic protein, A20.50 When EC are confronted with pro-inflammatory stimuli, cytoprotective genes are induced as part of the activation process and help protect EC from apoptosis as well as limiting EC activation through inhibition of the transcription factor NF-κB. These cytoprotective genes require NF-κB for their expression and therefore would downregulate not only the expression of pro-inflammatory proteins, but also their own expression. This negative feedback loop would then bring the cells back to their original quiescent phenotype. That NF-κB is necessary for inducing protective proteins is supported by data from our own laboratory in EC, and others showing that inhibition of NF-κB by overexpression of IκBα or by knocking out p65/RelA sensitizes the cells to TNF-mediated apoptosis.51-54 It is clear that to achieve this anti-inflammatory function, A1 and related proteins such as A20 need to accumulate over time and probably attain a critical level to block the activating pathway leading to NF-κB activation. Further studies are now performed to address this latter question, namely a tight study of their kinetic of expression and their half life.
From a therapeutic point of view, blockade of NF-κB has been suggested as a possible approach to prevent pro-inflammatory consequences of EC activation, which have been implicated in several pathological conditions, including allografts and xenografts rejection.2-5 To achieve this goal, a method to block NF-κB is needed that will not sensitize the cells to TNF-induced apoptosis and even protect them against it. Indeed, EC loss will expose the subendothelial matrix, which is equally as detrimental as the consequences of EC activation itself. Expression of a gene such as A1 that inhibits inflammatory reactions and still protects the cells from death may achieve this purpose. We propose that A1 will have the same potential as A20 of being a potent inhibitor of NF-κB without sensitizing to TNF-mediated apoptosis,55 as occurs when NF-κB is inhibited with IκBα.51-54 Present studies are addressing whether A1 and A20 have different intracellular targets and thus would provide additive or synergistic protection in EC.
ACKNOWLEDGMENT
We thank Dr J. Anrather for his helpful discussions and resources, namely the pcDNA3 HA-tagged expression plasmid and the p65 expression vector; Dr S.T. Grey for critical review of this manuscript; Dr V. Dixit for providing the rAdA20, Dr Robert Gerard for the rAd β-gal control adenovirus; and E. Czismadia for her skilled technical assistance in cell culture.
D.M.S. and A.Z.B. contributed equally to this work.
This is manuscript 738 from our laboratories. F.H.B. is a paid consultant of Novartis.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Christiane Ferran MD, PhD, Immunobiology Research Center, Beth Israel Deaconess Medical Center, 99 Brookline Ave, Boston, MA 02215; e-mail:cferran@caregroup.harvard.edu.

![Fig. 3. A1 expression is NF-κB–dependent. (A) HUVEC were stimulated with TNF (100 U/mL), LPS (100 ng/mL), or PMA (5 × 10−8 mol/L) for 3 hours. A1 or Bcl-XL mRNA was detected using -[32P]-dATP–labeled specific cDNA probes. (B) HUVEC were noninfected (NI) or infected with rAd.A20, rAd.IκB or rAd.β-gal. Forty-eight hours after infection, HUVEC were either left nontreated (−) or were stimulated with TNF (+) (100 U/mL). A1, A20, and IκB mRNA levels were detected by Northern blot analysis using their specific -[32P]-dATP–labeled cDNA probes. GAPDH was used to confirm equal quantity of RNA. Result shown is representative of three independent experiments. (C) BAEC were either nontransfected (NT) or transfected with a control plasmid (pcDNA3) or the p65 expression plasmid (+p65). A1 mRNA was only induced in p65 transfected BAEC (lane 5 v 3 and 4). Lanes 1 and 2 correspond to negative (C) and positive controls (TNF treated). GAPDH amplification (0.45 kb) was similar in all samples for all cycles tested. Only the 20 cycles’ amplification results are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3803/4/m_blod41127003aw.jpeg?Expires=1769085370&Signature=a9Ksg22RM8WSzsj0Q5a3cj43gM25OhGXkEw4~kW0vLf5CesrvF8wLsaIDBVvIZ2SEMlmCEdoHkDrRgT98N9QRnvd9xgKEdC3jh12hau4YMpQ9C8yO5cyU0tawMQhmSpEpX5IEWEXljJNg6nXhvnlYZ1LlGw8cEcBDyjWiCNWjCotjI3EbCB~r~Xs9yjt6lnH1lALZdAkdxhetah196MKCUhcri3rWWRmQI-pQcPG0Sv-9smka2RI9zAZ8AW5LTQ-J2HfzVXm0q8OWBS5HlZqgIsVsHjuGoN~muxNOZVD23pfNRwKd-xfV8rr8VpeqTmUGGJjJKaqOse4PU9e16Xw8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. A1 expression is NF-κB–dependent. (A) HUVEC were stimulated with TNF (100 U/mL), LPS (100 ng/mL), or PMA (5 × 10−8 mol/L) for 3 hours. A1 or Bcl-XL mRNA was detected using -[32P]-dATP–labeled specific cDNA probes. (B) HUVEC were noninfected (NI) or infected with rAd.A20, rAd.IκB or rAd.β-gal. Forty-eight hours after infection, HUVEC were either left nontreated (−) or were stimulated with TNF (+) (100 U/mL). A1, A20, and IκB mRNA levels were detected by Northern blot analysis using their specific -[32P]-dATP–labeled cDNA probes. GAPDH was used to confirm equal quantity of RNA. Result shown is representative of three independent experiments. (C) BAEC were either nontransfected (NT) or transfected with a control plasmid (pcDNA3) or the p65 expression plasmid (+p65). A1 mRNA was only induced in p65 transfected BAEC (lane 5 v 3 and 4). Lanes 1 and 2 correspond to negative (C) and positive controls (TNF treated). GAPDH amplification (0.45 kb) was similar in all samples for all cycles tested. Only the 20 cycles’ amplification results are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3803/4/m_blod41127003bw.jpeg?Expires=1769085370&Signature=GrgMZqsV5tMVwsyH6jTX7MhemW0jwP0YxRTiviI0RY9dzlsBq2T6AhlHXMWjDTETEF4WDmPqnrMDV1neqXiO6WexEDeJTQdIJ8dSG7zyf4q5gp3yp9y~v7b3667uzqG5y8WwC~GslkekzkANZKpi2Fr7NgSURhzXI3PFaFLW0xA23MX7iJqAmDsR9pkhpM9ugIpi2PBPL3RnFMWj7V0-I6gjlMsab52zsZtRxpw~x3BYP-V8fgQECk3eDutgcKbdG4nR5u-N76er1B~WL6q-E9ZZ69Fz3-MN3tsFfqDnJ4f7x25SXq9-TYkrYLcLUrWUKNP~b8fQtXBnT2A808sqCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. A1 expression is NF-κB–dependent. (A) HUVEC were stimulated with TNF (100 U/mL), LPS (100 ng/mL), or PMA (5 × 10−8 mol/L) for 3 hours. A1 or Bcl-XL mRNA was detected using -[32P]-dATP–labeled specific cDNA probes. (B) HUVEC were noninfected (NI) or infected with rAd.A20, rAd.IκB or rAd.β-gal. Forty-eight hours after infection, HUVEC were either left nontreated (−) or were stimulated with TNF (+) (100 U/mL). A1, A20, and IκB mRNA levels were detected by Northern blot analysis using their specific -[32P]-dATP–labeled cDNA probes. GAPDH was used to confirm equal quantity of RNA. Result shown is representative of three independent experiments. (C) BAEC were either nontransfected (NT) or transfected with a control plasmid (pcDNA3) or the p65 expression plasmid (+p65). A1 mRNA was only induced in p65 transfected BAEC (lane 5 v 3 and 4). Lanes 1 and 2 correspond to negative (C) and positive controls (TNF treated). GAPDH amplification (0.45 kb) was similar in all samples for all cycles tested. Only the 20 cycles’ amplification results are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3803/4/m_blod41127003cw.jpeg?Expires=1769085370&Signature=iM5Rqe-wuEv1Bolct2s1RJyCcJJaSxCUfvhUxQu0HqxcY3icDBy1RK-nr9PMmrovjvXKgCo8y5uckaJGMKYE0kgs0sNn~iS2N1DoRsfPIGmDKzy7f-W7PB0~qkdFRlJFLdxNiinUfF46sR8EjS0WTlFA4sb1MCTDa1gCJin~HGvT5RzB2A0s25kUR2PiRjv6u6sDdWMKWtDi3gFVRc-aX9vmH-XsZMuGMYEN9fBIx9WFxYpHOB-2YVRTUJoISBSzBEY3bbXU~ZOpfmG3Tg6RTXweElUmAwMOieW8aCTqkiu59r3qPm8dkinynuWSSyGVLumtpWUMtQ4jjd807dQxYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal