Abstract
E-selectin, an endothelial-specific adhesion molecule best known for its role in leukocyte adhesion, is not detected in quiescent endothelial cells, but is induced by inflammatory stimuli. However, E-selectin is also expressed in proliferating endothelial cells under noninflammatory conditions in vivo and in vitro, suggesting that E-selectin is also regulated by growth signals. To investigate E-selectin expression in lipopolysaccharide-stimulated versus nonstimulated proliferating cells, we analyzed the distribution of E-selectin–positive human microvascular endothelial cells in G0/G1, S, and G2/M phases of the cell cycle under both conditions. Lipopolysaccharide treatment resulted in uniformly increased E-selectin expression in cells in G0/G1, S, and G2/M. In contrast, levels of E-selectin in nonstimulated proliferating cells showed a linear correlation with the percentage of cells in G2/M. E-selectin in proliferating endothelial cells was not reduced by addition of soluble tumor necrosis factor-–receptor or soluble interleukin-1–receptor indicating that its expression was not due to endogenous production of either cytokine. In addition, E-selectin was increased in cells stimulated with basic fibroblast growth factor, a well-known mitogen for endothelial cells. E-selectin in proliferating endothelial cells is functional, as shown by E-selectin–dependent adhesion of the promyelocytic leukemia cell line HL-60 to subconfluent human microvascular endothelial cells. In summary, these studies indicate that E-selectin can be regulated by a non-inflammatory pathway that is related to the proliferative state of the endothelium.
THE THREE MEMBERS of the selectin family of cell adhesion molecules, E-, P-, and L-selectin, initiate rolling of leukocytes along endothelium at sites of inflammation.1 These carbohydrate-binding proteins share a common structural domain organization and all three bind sialylated fucosylated glycans such as sialyl Lewis-X (NeuAcα2,3Galβ1,4[Fucα1,3]GlcNAc) and sialyl Lewis-A (NeuAcα2,3Galβ1,3[Fucα1,4]GlcNAc), as well as related structures.2 The selectins promote leukocyte rolling by binding to sialyl LewisX-containing counter receptors, the best characterized being P-Selectin Glycoprotein Ligand-1 or PSGL-1.3 P-, E-, and L-selectin–deficient mice have been generated to decipher the function of each selectin in different inflammatory settings.4-6 In endothelium, both E- and P-selectin participate in leukocyte adhesion and have overlapping functions.5 Analysis of E-selectin–deficient mice has shown functional roles for E-selectin in granulocyte rolling7 and stable adhesion of leukocytes to microvascular endothelium.8 Mice deficient in both P- and E-selectin exhibit dramatic leukocytosis, elevated levels of endogenous cytokines, decreased leukocyte rolling, and increased susceptibility to infections.9 10
Several studies indicate that E-selectin may also function in angiogenesis. In vitro studies show that E-selectin is associated with endothelial proliferation,11 migration,12 and tube formation,13 all essential components of angiogenesis. Furthermore, E-selectin is expressed in angiogenic tissues in vivo. For example, E-selectin is present in proliferating endothelial cells in vivo in noninflamed human hemangioma, a tumor of endothelium that occurs in infants.14 The level of E-selectin–positive blood vessels correlates with the proliferative phase of hemangioma, indicating that E-selectin is expressed at the right time and place to function in angiogenesis.14E-selectin was also found to be significantly increased in human breast carcinoma, with highest levels found in the most aggressive estrogen-receptor–negative tumors.15 E-selectin has been detected in microvessels of many other human tumors16-19and in normal noninflamed microvessels in human decidua,20placenta, neonatal foreskin,14 and gingival tissue.21 Its expression in noninflamed tissues suggests that, in addition to its role in leukocyte adhesion, E-selectin may participate in other important processes in the microvasculature.
In vitro analysis of E-selectin has been performed primarily using human umbilical vein endothelial cells (HUVECs), which have served as a model for endothelial activation and function.22-24E-selectin is typically not detected in nonstimulated confluent HUVECs but can be induced by treatment of the cells with cytokines such as tumor necrosis factor-α and interleukin-1β or lipopolysaccharide (LPS). E-selectin mRNA and polypeptide expression peaks 4 to 6 hours after induction and then decreases over 24 hours to undetectable levels even with continuous exposure to cytokine.23,24 This pattern of inducible E-selectin expression is consistent with its role in leukocyte adhesion and its original identification as an endothelial cell activation antigen. However, expression of E-selectin mRNA and polypeptide has been observed in many types of endothelial cells without addition of cytokines or LPS. For example, E-selectin mRNA has been detected in bovine capillary endothelial (BCE) cells by Northern blot analysis,13,25 in murine lung-derived microvascular endothelial cells by ribonuclease protection analysis,26and in human bone marrow–derived endothelial cells by reverse transcription-polymerase chain reaction analysis.27Constitutive E-selectin polypeptide expression has been reported in human brain-derived endothelial cells28 and in BCE cells.11 Cytokine-independent expression has also been detected in low passage HUVECs by ribonuclease protection analysis and enzyme-linked immunosorbent assay (ELISA).29 In each of these studies, the level of E-selectin was upregulated by cytokine treatment indicating that the pathway for cytokine stimulation was functional in the endothelial cell cultures.
We and others have begun to examine the noninflammatory expression of E-selectin. We showed that E-selectin polypeptide is expressed in proliferating bovine endothelial cells and that E-selectin–positive cells are enriched in G2/M phases of the cell cycle.11 Litwin et al30 investigated the role of matrix and cell-cell contacts on cytokine-independent expression of E-selectin in HUVECs and made several notable observations. First, increased E-selectin in subconfluent endothelial cells was unaffected by blocking antibodies against either tumor necrosis factor-α or interleukin-1β, indicating that the increased E-selectin was not due to autocrine stimulation by these cytokines. Second, cell-matrix and cell-cell interactions were important regulators of the cytokine-independent expression of E-selectin in that (1) cell attachment to integrin ligands was required and (2) disruption of cell contacts in confluent monolayers with anti–PECAM-1 antibody resulted in increased E-selectin. They concluded that cytokine-independent expression of E-selectin is regulated by endothelial cell density and formation of endothelial cell junctions. Unpublished data were cited in the report30 suggesting that the cytokine-independent expression of human E-selectin was not related to cell proliferation. In the present study, we investigated the noninflammatory expression of E-selectin by examining the distribution of E-selectin–positive cells in G0/G1, S, and G2/M phases of the cell cycle in LPS-stimulated versus nonstimulated proliferating endothelial cells. Our results indicate that noninflammatory expression of E-selectin is regulated at least in part by cell proliferation.
MATERIALS AND METHODS
Isolation and culture of human dermal microvascular endothelial cells (HDMEC).
HDMEC were isolated from newborn foreskin using Ulex europaeus I–coated Dynabeads (Dynal, Inc, Oslo, Norway)31 with some modifications.32 The HDMECs were grown in EBM 131 (Clonetics, San Diego, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), 1 × glutamine, penicillin, streptomycin (GPS) (Irvine Scientific, Irvine, CA), 2 ng/mL human recombinant basic fibroblast growth factor (bFGF) on 1% gelatin-coated dishes in a 5% CO2 incubator. For abbreviation, we designate this growth medium EBM-B. Cells were passaged 1:3 every 4 to 6 days, and used at passages 3 to 12.32 The FBS used for these studies contained not more than 0.06 EU/mL endotoxin, as determined by the manufacturer. Human recombinant bFGF was kindly provided by Scios Inc (Mountain View, CA). For comparison with other studies on HDMECs, cells were also grown in EBM 131 supplemented with 20% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, 0.25 μg/mL amphotericin B (ie, fungizone) (GIBCO-BRL, Gaithersburg, MD), 2 mmol/L glutamine (Irvine Scientific), 0.5 mmol/L N6, 2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate (Sigma Chemical Co, St Louis, MO), 1.0 μg/mL hydrocortisone (Sigma). For abbreviation, we designate this medium EBM-A.
Other cells used.
The human promyelocytic leukemia cell line HL-60 (ATCC cat. no. CLL-240) was grown in suspension in RPMI medium-1640 (GIBCO-BRL) supplemented with 10% FBS, 1 × GPS and maintained at a density of less than 1 × 106 cells/mL. BCE cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% calf serum, GPS, and 3 ng/mL bFGF as described.11
FACS and cell-cycle analysis.
A gentle fixation and permeabilization method for simultaneous analysis of cell surface antigens and quantification of DNA33 was used. HDMECs were gently scraped from the dishes in phosphate-buffered saline (PBS) with 5 mmol/L EDTA, sedimented, resuspended in Hanks’ balanced salt solution with calcium and magnesium (HBSS) and counted. The cells were centrifuged again and resuspended in HBSS, 2% calf serum, 0.02% NaN3 (Ab solution). The cells were incubated with 5 μg/mL anti-human E-selectin monoclonal antibody MoAb14 or an isotype-matched murine immunoglobulin G (IgG) (Becton Dickinson, Mountain View, CA) for 30 minutes at 4°C on a rotating platform. The cells were washed twice with Ab solution and incubated with 1/200 dilution of horse anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate as above. The cells were washed, resuspended in 0.25% paraformaldehyde in PBS for 1 hour at 4°C, and permeabilized with 0.2% Tween in HBSS for 15 minutes at 37°C. The cells were sedimented again, resuspended in Ab solution at 1 × 106 cells/mL, and incubated with 200 μg/mL RNase A (11.25 Kunitz units/mL) for 90 minutes at room temperature. Propidium iodide was added to 10 μg/mL and the cells were stored at 4°C until analyzed on a Becton Dickinson FACScan flow cytometer for FITC (E-selectin) and propidium iodide (DNA). FITC-negative was defined as the level of fluorescence observed in 99% of HDMECs incubated with isotype-matched control IgG1. Cells stained with anti–E-selectin with fluorescence intensity higher than this were designated FITC+ or E-selectin–positive. The relative mean fluorescence intensity (Δ MFI) was calculated by subtracting the MFI of 10,000 HDMECs incubated with isotype-matched control antibody from the MFI of 10,000 HDMECs incubated with anti-human E-selectin MoAb. For the analysis of bFGF-stimulated cells, immunostaining was performed as above, but after fixation in paraformaldehyde cells were washed once with HBSS and resuspended in 0.5 mL of Krishan’s reagent (1% sodium citrate, 20 μg/mL RNAse A, 0.3% Nonidet-P 40, 50 μg/mL propidium iodide).
ELISA.
HDMEC were plated at a density of 3,000 cells per well in a 96-well plate in EBM-B in the absence or presence of soluble TNF-α-receptor (1 μg/mL) or soluble interleukin (IL)-1β–receptor (1 μg/mL) and incubated for 48 hours. After 48 hours, untreated parallel wells were stimulated with 2 ng/mL TNF-α in the presence or absence soluble tumor necrosis factor (TNF)-α–receptor (1 μg/mL) or with 2 U/mL IL-1β in the presence or absence of soluble IL-1β-receptor (1 μg/mL) for 4 hours. The soluble recombinant dimeric human TNF receptor p80/IgG1 Fc fusion protein and soluble recombinant human IL-1 receptor type I were kindly provided by Immunex Corp (Seattle, WA). The cells were washed twice with PBS and fixed in −20°C methanol for 10 minutes on ice. E-selectin was detected as described previously.32 Absorbance at 410 nm obtained with isotype-matched control IgG1 was subtracted as background. Each assay point was performed in triplicate. Bar graphs represent mean ± standard deviation.
HL-60 adhesion assay.
HDMECs were plated on gelatin-coated 35-mm dishes and assayed 3 days after plating (proliferating) or 7 days after plating (confluent). Cell monolayers were fed with fresh medium with or without 0.2 μg/mL LPS 5 hours before the adhesion assay. For antibody blocking, the monolayers were incubated in 1 mL of EBM-B with 20 μg/mL of anti-human E-selectin MoAb H18/7 (Becton Dickinson) or with 20 μg/mL isotype-matched control IgG2a (Becton Dickinson) for 30 minutes at room temperature on a rocking platform. The media was removed and cell monolayers washed once with RPMI media with or without 2.5 mmol/L EGTA. HL-60 cells were washed once with RPMI media alone, resuspended in the presence or absence of 2.5 mmol/L EGTA, and added to the cell monolayers at 2 × 106 cells/0.6 mL. Cells were incubated at 4°C on a rocking platform for 45 minutes, washed 5 times with 2 mL of RPMI with or without 2.5 mmol/L EGTA, and fixed with 2.5% glutaraldehyde. Bound HL-60 cells were counted in 10 randomly selected fields. The number of bound cells is expressed as mean ± standard error of the mean (SEM).
RESULTS AND DISCUSSION
Cell-cycle distribution of E-selectin-positive cells in LPS-stimulated versus nonstimulated HDMEC.
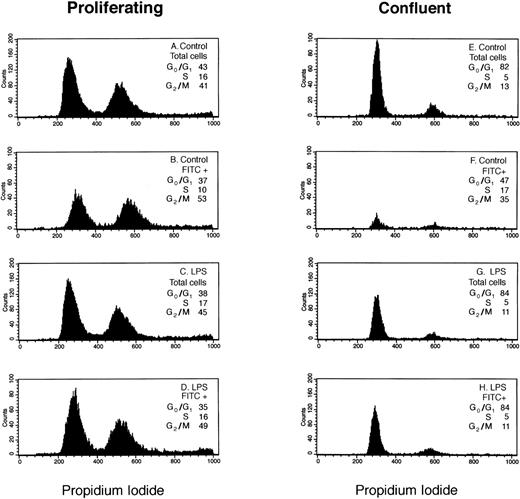
We analyzed LPS-stimulated versus nonstimulated HDMEC to determine if in response to an inflammatory stimulus the distribution of E-selectin–positive cells in G0/G1, S, and G2/M phases of the cell cycle differs from that observed in the total cell population. Figure 1 shows results from a representative experiment in which the distributions of total and E-selectin–positive (FITC+) endothelial cells in G0/G1, S, and G2/M phases of the cell cycle were determined by flow cytometry using propidium iodide.
Cell-cycle analysis of E-selectin-positive HDMECs. Proliferating (A through D) and confluent (E through H) HDMECs were treated without (Control; A, B, E, and F) or with 0.2 μg/mL LPS (LPS; C, D, G, and H) for 4 hours. Cells were analyzed for E-selectin expression and distribution in G0/G1 and S to G2/M as described in Materials and Methods. (A, C, E, and G) Histograms of DNA content (ie, propidium iodide staining) for the total cell population. (B, D, F, and H) Histograms of DNA content in E-selectin–positive cells (FITC+). The percent distribution of cells in different phases of the cell cycle (G0/G1, S, G2/M) is shown for each panel.
Cell-cycle analysis of E-selectin-positive HDMECs. Proliferating (A through D) and confluent (E through H) HDMECs were treated without (Control; A, B, E, and F) or with 0.2 μg/mL LPS (LPS; C, D, G, and H) for 4 hours. Cells were analyzed for E-selectin expression and distribution in G0/G1 and S to G2/M as described in Materials and Methods. (A, C, E, and G) Histograms of DNA content (ie, propidium iodide staining) for the total cell population. (B, D, F, and H) Histograms of DNA content in E-selectin–positive cells (FITC+). The percent distribution of cells in different phases of the cell cycle (G0/G1, S, G2/M) is shown for each panel.
In LPS-treated HDMECs (Fig 1C, D, G, and H), the cell-cycle distribution of E-selectin–positive cells (FITC+) was similar or identical to that of the cell population as a whole (total cells) indicating that in response to LPS, E-selectin was increased uniformly in cells at each phase of the cell cycle. This occurred in both proliferating (Fig 1C and D) and confluent (Fig 1G and H) HDMECs. In contrast, the cell-cycle distribution of FITC+ cells differed from that of the total cells in proliferating nonstimulated HDMECs in that there was a 12% shift in cells from G0/G1 and S to G2/M (Fig 1Av B). The enrichment of E-selectin–positive cells in G2/M was more pronounced in confluent HDMECs wherein the percentage of E-selectin–positive cells in G2/M was increased 22% compared with the total cell population (Fig 1Ev F). As expected, fewer cells express E-selectin (ie, FITC+) in confluent cells. The shape of the histogram curves in Fig 1B and 1F are clearly different from the other 6 panels and show the relative shift of E-selectin–positive cells into G2/M in nonstimulated cells.
Table 1 presents the cell-cycle distribution of the total and E-selectin–positive cell populations, as determined by flow cytometry, determined in three additional experiments. These data show that results presented in Fig 1 (A and B) are reproducible and confirms the relative shift of E-selectin–positive cells into G2/M in nonstimulated cells.
Cell-Cycle Analysis of E-Selectin–Positive Cells Under Noninflammatory Conditions
| . | Experiment 1 . | Experiment 2 . | Experiment 3 . | |||
|---|---|---|---|---|---|---|
| Total Cells (%) . | FITC+ (%) . | Total Cells (%) . | FITC+ (%) . | Total Cells (%) . | FITC+ (%) . | |
| G0/G1 | 53 | 46 | 52 | 47 | 71 | 64 |
| S | 15 | 10 | 9 | 4 | 9 | 5 |
| G2/M | 32 | 44 | 39 | 49 | 20 | 31 |
| . | Experiment 1 . | Experiment 2 . | Experiment 3 . | |||
|---|---|---|---|---|---|---|
| Total Cells (%) . | FITC+ (%) . | Total Cells (%) . | FITC+ (%) . | Total Cells (%) . | FITC+ (%) . | |
| G0/G1 | 53 | 46 | 52 | 47 | 71 | 64 |
| S | 15 | 10 | 9 | 4 | 9 | 5 |
| G2/M | 32 | 44 | 39 | 49 | 20 | 31 |
Endothelial cell culture conditions and analyses by flow cytometry were performed as described for the experiment presented in Fig 1.
Table 2 summarizes the levels of E-selectin expression for the experiment presented in Fig 1. In absence of LPS stimulation, E-selectin was well-expressed in proliferating cells (33% FITC+), but very low in confluent cells (7% FITC+). In the presence of LPS, E-selectin was increased in both growing and confluent cells. The ΔMFI under all four culture conditions shows several important points (see Table 2). First, the level of non-inflammatory E-selectin expression was 20-fold higher in proliferating HDMECs compared with confluent HDMECs. Second, because of the high basal expression of E-selectin in proliferating HDMECs, LPS caused only a twofold increase in E-selectin in proliferating HDMEC compared with the 20-fold increase in confluent HDMEC. Third, the net increase in E-selectin in LPS-treated cells, as measured by flow cytometry, was similar in both proliferating and confluent endothelial cells (ie, ΔMFI ∼ 190). In summary, our results indicate that LPS increased E-selectin uniformly in cells in different phases of the cell cycle while in nonstimulated cells, E-selectin–positive cells were enriched in G2/M.
E-Selectin Expression in Proliferating Versus Confluent HDMECs
| . | Proliferating Cells . | Confluent Cells . | ||
|---|---|---|---|---|
| Control . | +LPS . | Control . | +LPS . | |
| % FITC+ | 33 | 60 | 7 | 69 |
| Δ MFI | 211 | 402 | 9 | 193 |
| Panel in Fig 1 | B | D | F | H |
| . | Proliferating Cells . | Confluent Cells . | ||
|---|---|---|---|---|
| Control . | +LPS . | Control . | +LPS . | |
| % FITC+ | 33 | 60 | 7 | 69 |
| Δ MFI | 211 | 402 | 9 | 193 |
| Panel in Fig 1 | B | D | F | H |
The analyses were performed as described for Fig 1 and in Materials and Methods.
E-selectin expression in proliferating endothelial cells is not due to endogenous TNF-α or IL-1β.
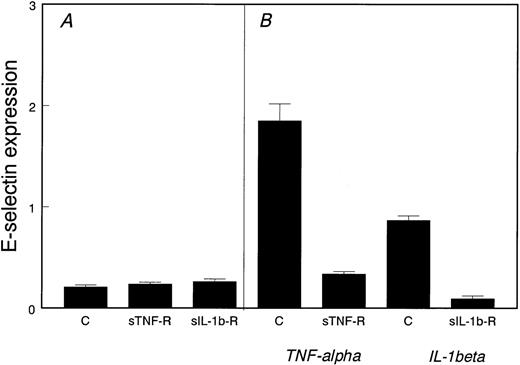
To determine if proliferating HDMECs produce endogenous TNF-α or IL-1β that could in turn induce E-selectin expression, we tested the effect of neutralizing soluble receptor antagonists for these two cytokines. As shown in Fig 2, excess soluble TNF-α–receptor or soluble IL-1β–receptor did not reduce E-selectin in proliferating cells (Fig 2A). As a positive control, Fig2B shows that the soluble receptor antagonists were effective in blocking both TNF-α– and IL-1β–induced E-selectin expression.
Noninflammatory expression of E-selectin in proliferating HDMECs. (A) E-selectin was measured by ELISA in proliferating HDMECs incubated for 2 days in the absence (C) or presence of soluble TNF-–receptor (sTNF-R) or soluble IL-1β–receptor (sIL-1b-R). (B) As a positive control, HDMECs were stimulated with either TNF- or IL-1β for 4 hours in the absence (C) or presence of the corresponding soluble receptor antagonist.
Noninflammatory expression of E-selectin in proliferating HDMECs. (A) E-selectin was measured by ELISA in proliferating HDMECs incubated for 2 days in the absence (C) or presence of soluble TNF-–receptor (sTNF-R) or soluble IL-1β–receptor (sIL-1b-R). (B) As a positive control, HDMECs were stimulated with either TNF- or IL-1β for 4 hours in the absence (C) or presence of the corresponding soluble receptor antagonist.
Increased E-selectin in bFGF-treated HDMECs.
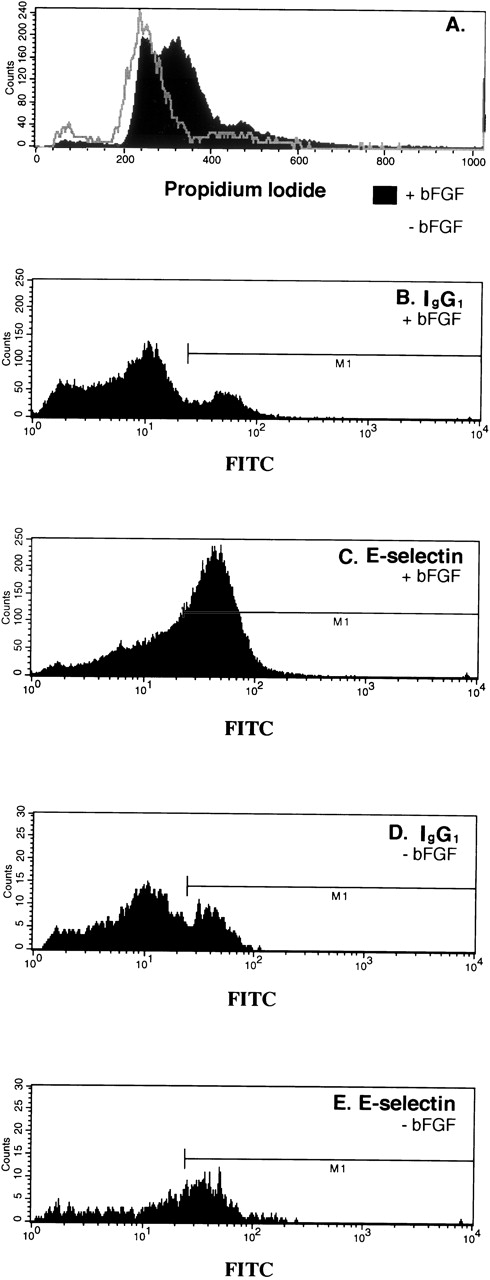
Our data presented thus far suggest that mitogens that stimulate endothelial cell proliferation should stimulate E-selectin expression. To test this directly, we examined the effect of bFGF on E-selectin expression. To do this experiment, confluent HDMECs were serum-starved and bFGF-starved by incubating in EBM 131, 0.5% FCS without bFGF for 24 hours. The cells were then trypsinized and replated in the EBM 131, 5% FCS at subconfluent density with or without 2 ng/mL bFGF for 46 hours. As seen in Fig 3A, bFGF caused a dramatic shift of the cells out of G0/G1. Coincident with this shift, E-selectin–positive cells increased from 27% (Fig 3E) to 46% (Fig3C). The percent FITC-positive cells (M1) detected with isotype-matched control IgG (Fig 3B and D) was substracted from percent detected with anti-human E-selectin (Fig 3C and E). In summary, the mitogenic effect of bFGF on HDMECs corresponded with an increase in E-selectin as measured by flow cytometry.
Increased E-selectin in bFGF-stimulated HDMECs. Cells were serum-starved and bFGF-starved for 24 hours, trypsinized, and replated at subconfluent density in the absence (gray line) or presence of 2 ng/mL bFGF (solid black region) for 46 hours, and then analyzed by flow cytometry for cell-cycle distribution (A) and E-selectin expression (C and E). Nonspecific antibody binding was measured using isotype-matched control IgG1 (B and D). Forty-six percent of the cells were E-selectin–positive (C) and 27% were positive (E).
Increased E-selectin in bFGF-stimulated HDMECs. Cells were serum-starved and bFGF-starved for 24 hours, trypsinized, and replated at subconfluent density in the absence (gray line) or presence of 2 ng/mL bFGF (solid black region) for 46 hours, and then analyzed by flow cytometry for cell-cycle distribution (A) and E-selectin expression (C and E). Nonspecific antibody binding was measured using isotype-matched control IgG1 (B and D). Forty-six percent of the cells were E-selectin–positive (C) and 27% were positive (E).
Linear correlation of E-selectin expression with percentage of cells in G2/M.
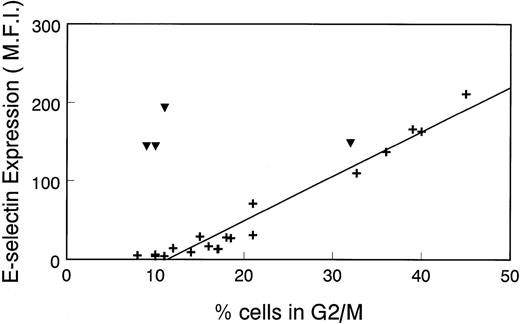
To further illustrate the relationship between E-selectin expression and the cell cycle, we plotted the Δ MFI for E-selectin versus percentage of cells in G2/M that was determined in several different experiments. As seen in Fig 4, there is a linear correlation between Δ MFI and percentage of cells in G2/M in nonstimulated HDMECs (+). Consistent with results in Fig 1, there is no relationship between Δ MFI and percentage of cells in G2/M in LPS-treated cells (▾). This analysis confirms the results in Fig 1 and, importantly, shows that in the absence of inflammatory stimuli, E-selectin expression can be used to assess the proliferative state of endothelial cells.
Linear relationship between ▵ MFI of cells incubated with anti–E-selectin MoAb and percentage of cell population in G2/M. ▵MFI versus the percentage of HDMEC in G2/M was plotted in a scatter graph format. Cells were nonstimulated (+) or treated with 0.2 μg/mL LPS for 16 hours (▾). Data from 17 experiments, including those shown in Fig 1 and Table 1, were used for this analysis.
Linear relationship between ▵ MFI of cells incubated with anti–E-selectin MoAb and percentage of cell population in G2/M. ▵MFI versus the percentage of HDMEC in G2/M was plotted in a scatter graph format. Cells were nonstimulated (+) or treated with 0.2 μg/mL LPS for 16 hours (▾). Data from 17 experiments, including those shown in Fig 1 and Table 1, were used for this analysis.
Effect of growth medium on E-selectin expression.
For these studies, the HDMECs were grown and maintained in a relatively simple growth medium (EBM-B). We have shown that cells grown in EBM-B (EBM 131, 10% FBS, GPS, 2 ng/mL bFGF) maintain continued responsiveness to growth factors and cytokines for at least 3 months and up to 12 trypsin passages.32 More typically, human microvascular endothelial cells are grown in media such as EBM-A, which contains 20% FBS, fungizone, dibutryl cAMP, and hydrocortisone.34-36 To compare the effect of growth media on levels of E-selectin polypeptide, we analyzed E-selectin expression and the distribution of cells in G0/G1, S, and G2/M in cells grown in EBM-B versus EBM-A. In three separate experiments, the Δ MFI was decreased 39% in cells grown in EBM-A compared to cells grown in EBM-B with a concommitant decrease in percentage of cells in G2/M (data not shown). This indicates that growth media can affect the expression of E-selectin in cultured cells.
E-selectin expressed in proliferating cells mediates adhesion of HL-60 cells.
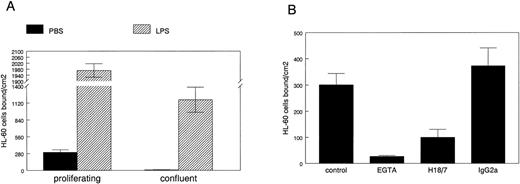
To determine if E-selectin expressed in proliferating HDMECs can mediate leukocyte adhesion, we used a well-established cell-cell adhesion assay in which HL-60 cells are allowed to adhere to endothelial cells grown on gelatin-coated culture dishes under normal growth conditions. HL-60 is a human promyelocytic leukemia cell line that was used in the functional identification of E-selectin as an endothelial-leukocyte adhesion molecule.23 24 HL-60 cell adhesion to proliferating versus confluent HDMECs was measured as described in Materials and Methods (Fig5A). HL-60 cells bound to proliferating but not to confluent HDMECs (solid bars). As expected, LPS-stimulation resulted in a dramatic increase in HL-60 cell adhesion to both cultures (hatched bars). HL-60 cells did not adhere to gelatin-coated dishes that did not have endothelial cells plated on them (data not shown).
HL-60 cell adhesion to HDMEC. HL-60 cell adhesion to endothelial cells treated for 5 hours with PBS (solid bars) or with 0.2 μg/mL LPS (hatched bars) was measured in HDMEC 3 days (proliferating) or 7 days (confluent) after trypsin passage (A). (B) HL-60 adhesion to proliferating HDMEC was measured in presence of 2.5 mmol/L EGTA, 20 μg/mL anti–E-selectin MoAb H18/7, or an isotype-matched IgG2a control MoAb. Cells bound ± SEM was calculated from 10 randomly selected fields.
HL-60 cell adhesion to HDMEC. HL-60 cell adhesion to endothelial cells treated for 5 hours with PBS (solid bars) or with 0.2 μg/mL LPS (hatched bars) was measured in HDMEC 3 days (proliferating) or 7 days (confluent) after trypsin passage (A). (B) HL-60 adhesion to proliferating HDMEC was measured in presence of 2.5 mmol/L EGTA, 20 μg/mL anti–E-selectin MoAb H18/7, or an isotype-matched IgG2a control MoAb. Cells bound ± SEM was calculated from 10 randomly selected fields.
The HL-60 cell adhesion to nonactivated proliferating HDMECs was analyzed in more detail in Fig 5B. HL-60 cell adhesion to proliferating HDMECs was inhibited by 2.5 mmol/L EGTA, showing that the adhesion was calcium-dependent, a feature expected for selectin-mediated binding. HL-60 cell adhesion to proliferating HDMECs was inhibited by a function blocking anti-human E-selectin MoAb designated H18/7,24 but not by an isotype-matched control Ig. This shows that a significant portion of the HL-60 cell adhesion to HDMECs is mediated by E-selectin. This experiment also verifies that E-selectin expressed in proliferating human microvascular endothelial cells is on the cell surface and functional. If the same is true for E-selectin expressed in proliferating endothelial cells in vivo,14 E-selectin–mediated activities might have functional significance in angiogenic tissues or diseases.
In summary, we show directly that noninflammatory expression of E-selectin is correlated with cellular proliferation in human microvascular endothelial cells. Therefore, E-selectin expression may be a useful parameter for monitoring the proliferative state of endothelial cells. E-selectin polypeptide is enriched, but not restricted to cells in G2/M. Its presence in other phases of the cell cycle may be related to the time required for turnover of E-selectin or perhaps to other regulatory factors such as cell-cell and cell-matrix interactions as suggested by Litwin et al.30Our results are consistent with a previous study in which transforming growth factor-β, a protein that inhibits endothelial cell progression through the cell cycle,37 was shown to downregulate E-selectin in low passage HUVECs.29 The functional significance of E-selectin expression in proliferating endothelial cells is under investigation in our laboratory.
ACKNOWLEDGMENT
We thank Dr Debra Chao for help with flow cytometry analyses. We thank Alan Flint and Marc Alande of the FACS Core Facility at Children’s Hospital and members of the FACS Core Facility at the Dana Farber Cancer Institute for their technical assistance.
Supported by grants from the National Institutes of Health (No. F32 HL09390 to J.L., No. P01 CA 45548 and R29 GM46757 to J.B.), and a grant from the Charlotte Geyer Foundation to J.B.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Joyce Bischoff, PhD, Surgical Research Laboratory, Children’s Hospital, 300 Longwood Ave, Boston, MA 02115; e-mail: bischoff@a1.tch.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal