Abstract
We found that erythropoietin (EPO) and stem cell factor (SCF) activated protein kinase B (PKB/Akt) in EPO-dependent HCD57 erythroid cells. To better understand signals controlling proliferation and viability, erythroid cells that resist apoptosis in the absence of EPO were subcloned and characterized (HCD57-SREI cells). Constitutive activations of PKB/Akt, STAT5a, and STAT5b were noted in these EPO-independent cells. PI3-kinase activity was an upstream activator of PKB/Akt because the PI3-kinase inhibitor LY294002 blocked both constitutive PKB/Akt and factor-dependent PKB/Akt activity. The LY294002 study showed that proliferation and viability of both HCD57-SREI and HCD57 cells correlated with the activity of PKB/Akt; however, PKB/Akt activity alone did not protect these cells from apoptosis. Treatment of HCD57 cells with SCF also activated PKB/Akt, but did not protect from apoptosis. This result suggested that PKB/PI3-kinase activity is necessary but not sufficient to promote viability and/or proliferation. Constitutive STAT5 activity, activated through an unknown pathway not including JAK2 or EPOR, may act in concert with the constitutive PI3-kinase/PKB/Akt pathway to protect the EPO-independent HCD57-SREI cells from apoptosis and promote limited proliferation.
ERYTHROPOIETIN (EPO) is the primary hormone which regulates the maturation of immature erythroid cells1-3 into erythrocytes. EPO is necessary for the proliferation, erythroid differentiation, and prevention of apoptosis of erythroid cells; it is not yet clear whether these three processes are regulated by distinct signaling pathways.1-3 Some studies suggest that EPO might act primarily to maintain viability by protecting mature erythroid cells from programmed cell death or apoptosis in the relatively narrow window of EPO-dependent differentiation.1 The “knock out” of EPO or EPO receptor (EPOR) results in EPO−/− or EPOR−/− null mice that allow EPO-independent proliferation and development to relatively mature erythroid cells (CFU-E) that then undergo apoptosis.4 This is consistent with the requirement of EPO only in the later (CFU-e) differentiation events as a viability factor. Whereas the anti-apoptotic model is supported in EPO-responsive erythroid cells (proerythroblasts and erythroblasts) generated from mice infected with the anemia strain of Friend virus1(where the Friend virus may potentially drive proliferation), a strictly anti-apoptotic signal from the EPOR may be unlikely in all erythroid cells. The finding that many cell lines require EPO for proliferation, but only a few erythroid cell lines partially differentiate in response to EPO and do not require EPO for proliferation, suggests that proliferation, differentiation, and anti-apoptotic pathways may be controlled separately by EPO. Cells do not proliferate without cell survival; therefore, establishing the existence of separate control of proliferation and protection from apoptosis is difficult.
EPO acts through cell-surface receptors on immature erythroid cells.1-3,5 The EPOR does not have a kinase domain and transmits an intracellular signal by interacting with cytoplasmic nonreceptor tyrosine protein kinases. Two of these kinases have been reported to become phosphorylated after EPO binding to cells: the Janus kinase, JAK2, and the c-fes kinase.6-8JAK2 can physically associate with the EPOR.6,9,10 This interaction is constitutive in HCD57 erythroid cells, but JAK2 appears to be selective in interacting with a subset of highly modified EPOR molecules.10 Known substrates of tyrosine protein kinases activated by EPO binding to its receptor include phosphatidylinositol-3 kinase (PI3-kinase), SHC proteins, c-raf, STAT1, STAT3, STAT5A, STAT5B, and other molecules.4 9-23
Recent studies have suggested that c-Kit, the receptor for stem cell factor, interacts with the EPOR and transduces an intracellular signal through the EPOR.24 Our laboratory has shown that stem cell factor (SCF) can stimulate proliferation of HCD57 cells but cannot protect them from apoptosis.5 EPO activates JAK2/STAT signaling and other signaling which protects the cells from apoptosis and stimulates proliferation. Because SCF does not activate JAK2/STAT5 signaling in this system, we have speculated that this pathway may protect erythroid cells from apoptosis.
Constitutive STAT activation has been observed in transformed cell lines and cancerous cells.25-35 In most cases, constitutively activated Janus kinases seem to be responsible for activating the STAT proteins; however, in other cells and cancers, other kinases may phosphorylate STAT molecules. Oncogenes such as v-abl,30bcr/abl,35 and v-src30 may directly phosphorylate and activate STAT proteins. These studies suggest a possible role of STAT proteins, independent of Janus kinases, in signaling the proliferation and protecting these transformed cells from apoptosis. However, the role of STAT5 signaling in erythroid cells is controversial. STAT5 activation may be linked to enhanced proliferation19,36; in contrast, a mutant EPOR incapable of activating STAT5 still promotes proliferation.37 Wakao et al38 and Iwatsuki et al39 have separately demonstrated that STAT5 is required for erythroid differentiation; in contrast, deletion of STAT5A and/or STAT5B results in STAT5 null mice that have no obvious reduction or defects in erythroid cells.40-42
The involvement of PI3-kinase in cell transformation has been implicated in several cell lines.43,44 PI3-kinase plays an important role in regulation of cell proliferation and apoptosis; in addition, constitutive PI3-kinase activity has been observed in cancerous cells and, therefore, may contribute to the malignant transformation of cells. The insulin receptor substrate proteins (IRS) are substrates of many tyrosine kinases, including the JAK2 kinase bound to the EPOR.45 46 Activated IRS-1, -2, -3 are known to be docking sites for PI3-kinase and are implicated in the activation of PI3-kinase either in complexes with receptors or independently of receptors.
An important downstream effector activated by the PI3- kinase signaling pathway is protein kinase B (PKB/Akt), also known as c-Akt and RAC-PK. PI3-kinase activity leads to accumulation of polyphosphatidylinositols lipids in the plasma membrane. PKB/Akt is apparently targeted to translocate to the polyphosphatidyinositol rich membrane via a pleckstrin homology domain and is activated at the membrane by phosphorylation at Thr308 and Ser474 from two distinct phosphatidylinositol dependent kinases.47 PKB/Akt activity is linked to the control of many diverse cellular functions that included insulin-dependent glucose transport, glycolysis, glycogen synthesis and protein translation, and viability of cells.47 Recent work has shown that PKB/Akt is able to phosphorylate the pro-apoptotic protein BAD. Phosphorylation of BAD correlates with the cell survival and it is suspected that phosphorylated BAD is ineffective in disrupting the protective action of Bcl anti-apoptotic proteins.
In the current study, we found that EPO and SCF activated PKB/Akt in the EPO-dependent erythroid cells but the PI3-kinase/PKB/Akt pathway was constitutively active in the apoptosis-resistant, EPO-independent HCD57-SREI cells. The finding of constitutive activity of STAT5 in this apoptosis-resistant subclone of HCD57 cells suggests that STAT5 activity which arises through a signal independent of Janus kinase activity and the EPOR may also act in concert with PKB/Akt activity to prevent cells from undergoing apoptosis induced by growth factor withdrawal.
MATERIALS AND METHODS
Cell culture.
HCD57 cells, obtained from Sandra Ruscetti (National Cancer Institute, Frederick, MD),6,31 were cultured in the presence of 1.0 U EPO/mL in Iscove’s modified Dulbecco’s medium and 25% fetal calf serum. In experiments to test the action of signaling by EPO, the cells were further cultured overnight in the same medium devoid of EPO. As previously reported,6 this deprivation of EPO increased cell-surface EPOR but was an insufficient time without EPO for apoptosis to begin. HCD57-SREI cells were subcloned by transferring HCD57 cells from medium containing EPO to medium free of EPO but containing 100 ng SCF/mL. A subclone of cells that survived and proliferated was then cultured in the same medium indicated above except that both EPO and SCF were omitted.
Apoptosis studies.
HCD57 and HCD57-SREI cells were cultured either in the absence of cytokines or in the presence of the indicated factors for 72 hours. The cells were then harvested and genomic DNA was isolated.5Ten micrograms of genomic DNA was resolved on a 2.25% agarose/1X TAE/300 ng EtBr/mL gel. DNA laddering indicative of apoptosis was visualized using UV light.
Western blot analysis.
Tyr(P)-containing proteins and STAT5 tyrosine phosphorylation and nuclear translocation were detected by either Western blotting alone or the immunoprecipitation and Western blotting method described previously.5 10 Polyclonal anti-Tyr(P) antibodies from Zymed (San Francisco, CA) were used to immunoprecipitate and concentrate the Tyr(P)-containing proteins before they were analyzed by Western blot. The blots were stripped of bound antibody and reprobed with anti-JAK2 (Upstate Biotechnology, Inc [UBI], Lake Placid, NY) antisera. The blot was stripped as described by the Amersham literature with the ECL kit (Amersham, Arlington Heights, IL). Briefly, the bound antibody was released in the presence of 2% sodium dodeyl sulfate (SDS) and reducing agents at 50°C for 30 minutes. STAT5 tyrosine phosphorylation and nuclear translocation were detected by Western blot analysis of 10 μg of nuclear extracts probed with the anti-Tyr(P) antisera described above or anti-STAT5 (Santa Cruz Biotechnology, Santa Cruz, CA) antisera. For the Bcl-Xl and Bcl-2 Western blots, 10 μg of total cellular lysate were probed with anti–Bcl-Xl and anti–Bcl-2 antisera (Santa Cruz Biotechnology). For PKB/Akt assay, 100,000 cells were lysed in SDS sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mmol/L dithiothreitol [DTT], and 0.1% bromphenol blue). The blots were probed with anti-phospho Akt (Ser473) antibody (New England Biolabs, Beverly, MA) followed by detection using the Phototope-HRP Western detection system (New England Biolabs). After stripping of phosphospecific antibody, the blot was reprobed with a general anti-PKB/Akt antibody (New England Biolabs) that recognizes both phosphorylated and unphosphorylated PKB/Akt.
Gel mobility shift assay of STAT5 binding.
Nuclear STAT5 binding to radiolabeled DNA (prolactin inducible element) (PIE) was done as described previously.11 For the supershift assays to verify the presence or absence of STAT protein in the DNA binding complex, 2 μg of anti-STAT1, anti-STAT3, anti-STAT5A or anti-STAT5B antibodies (Zymed) were preincubated with 20 μg of nuclear extract for 15 minutes on ice before addition of the radiolabeled DNA.
Detection of cell-surface c-Kit expression.
Surface c-Kit expression was detected using flow cytometry analysis. For each sample, 1 × 105 HCD57 or HCD57-SREI cells were washed in FACS buffer (Tris-buffered saline/4% fetal calf serum). After preincubation of the cells in a monoclonal antibody (MoAb) that blocks nonspecific binding of fluorescein isothiocyanate (FITC)-conjugated antibodies to IgG (rat MoAb 2.4G2; a generous gift from Dr William Paul, National Institutes of Health, Bethesda, MD), the cells were incubated with FITC anti-cKit or FITC anti-CD4 (Becton Dickinson, San Diego, CA) (to control for background antibody staining) at 10 mg/mL for 30 minutes at 4°C. The cells were then washed in fluorescence-activated cell sorter (FACS) buffer before analysis using a FACScan flow cytometer (Becton Dickinson).
Sequencing of the EPOR in HCD57 and HCD57-SREI cells.
cDNA encoding the EPOR was cloned using reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. Briefly, mRNA was isolated from HCD57 and HCD57-SREI cells using the Quick Prep Micro mRNA kit (Pharmacia, Piscataway, NJ). cDNA was generated from mRNA using the First Strand cDNA Synthesis kit (Pharmacia). The cDNA was subjected to PCR using Taq polymerase (GIBCO-BRL) and oligonucleotides corresponding to the 5′ and 3′ end of the murine EPOR gene. PCR fragments were isolated using the Geneclean procedure (BIO 101, Vista, CA) and cloned into the pCrII vector (Invitrogen, Carlsbad, CA). Multiple clones of EPOR cDNA from both HCD57 and HCD57-SREI cells were sequenced using the dideoxy method described by Sanger et al48 using the Sequenase version 2.0 kit (Amersham, Piscataway, NJ).
Immunoprecipitation, ERK-1, PI3-kinase, and PKB/Akt assay.
Immunoprecipitation of ERK-1 and quantitation of activity as determined by incorporation of 32P from [γ-32P] adenosine triphosphate (ATP) into myelin basic protein was performed as described previously.5 Immunoprecipitations and PI3-kinase assays were conducted as described previously49 with slight modifications. Briefly, 3 × 106 cells were washed twice with ice-cold phosphate-buffered saline (PBS) and disrupted in a standard lysis buffer (1% Nonidet P-40 [Pierce, Rockford, IL]; 20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 5 mmol/L EDTA, 100 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 10 mg/mL aprotinin). Lysates were centrifuged at 14,000g for 10 minutes at 4°C, and then were subjected to immunoprecipitation with specific antibodies to EPOR, p85 domain of PI3-kinase, IRS-2, JAK2, STAT5, and Vav by incubation at 4°C for 1 hour. Protein A-agarose beads (UBI, Lake Placid, NY) were added and incubated for another 1 hour at 4°C. The immune complexes were washed three times with lysis buffer, once with PBS, once with 0.5 mol/L LiCl in 0.1 mol/L Tris-HCl (pH 7.5), once with distilled water, and once with a buffer containing 100 mmol/L NaCl, 0.5 mmol/L EGTA, and 20 mmol/L Tris-HCl, pH 7.5. The beads were suspended in 40 μL of reaction buffer (100 mmol/L NaCl, 0.5 mmol/L EGTA, 20 mmol/L Tris-HCl, pH 7.5, and 0.2 mmol/L adenosine). L-a-phosphatidylinositol was added to reaction mixture at a final concentration of 0.3 mg/mL. The reaction was initiated by addition of 10 μCi [γ-32P]ATP, and cold ATP and MgCl2 to final concentrations of 135 mmol/L and 20 mmol/L, respectively. After 10 minutes at 25°C, the reaction was terminated by addition of 20 μL of 6N HCl. Phospholipids were extracted with 160 μL of CHC13:CH3OH (1:1), and separated by thin-layer chromatography (TLC). Spots corresponding to PI3-phosphate were visualized by a PhosphoImager (Molecular Dynamics). For PKB/Akt assay, cells were lysed in ice-cold buffer A (50 mmol/L Tris, pH 7.5, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 0.1% 2-mercaptoethanol, 0.1% Triton X-100 [Pierce], 50 mmol/L sodium fluoride, 5 mmol/L sodium pyrophosphate, 10 mmol/L sodium β-glycerophosphate). Lysates were centrifuged at 14,000g for 10 minutes at 4°C, and the supernatants were equalized for protein concentration (1 mg/mL) by addition of buffer A. PKB/Akt was immunoprecipitated from the supernatants containing 1 mg of protein with an anti-PKB/Akt antibody (New England Biolabs Inc) and incubated at 4°C for 1.5 hour. Protein A-agarose beads (UBI) were added and incubated for another 1 hour at 4°C. The beads were washed three times with buffer A containing 0.5 mol/L NaCl, twice with buffer B (50 mmol/L Tris-HCl, pH 7.5, 0.03% Brij-35, 0.1 mmol/L EGTA, and 0.1% 2-mercaptoethanol), and twice with assay dilution buffer (20 mmol/L MOPS, pH 7.2, 25 mmol/L b-glycerophosphate, 5 mmol/L EGTA, 1 mmol/L Na3VO4, and 1 mmol/L DTT). The beads were suspended in 30 μL of assay dilution buffer containing 10 mmol/L PKA inhibitor peptide (Sigma, St Louis, MO). PKB/Akt-specific substrate peptide (UBI) was added to reaction mixture at a final concentration of 100 mmol/L. The reaction was initiated by addition of 10 mCi [γ-32P] ATP. After 10 minutes at 25°C, the supernatants were mixed with 40% trichloroacetic acid (TCA) and spotted onto P81 phosphocellulose paper. The paper was washed three times with 0.75% phosphoric acid, once with acetone, and counted by a scintillation counter.
RESULTS
Proliferation and apoptosis of EPO-independent HCD57-SREI cells.
Earlier studies in this laboratory5 showed that the treatment of HCD57 cells with SCF in the absence of EPO increased proliferation for 3 days but the effect was limited because the cells underwent apoptosis. While SCF clearly acted as a mitogen, EPO was also required to prevent apoptosis. The proliferation of HCD57 cells in either EPO or SCF is also shown in Fig 1A. In contrast, other workers have shown that introduction of the EPOR into nonerythroid cells will allow SCF to promote proliferation for infinite generations, possibly through an interaction of c-kit with the EPOR.24 We subcloned the HCD57 cells, hoping to select for an exclusively SCF-dependent cell line which had enhanced signaling of SCF through the EPOR. To accomplish this, the EPO-dependent HCD57 cells were cultured in the absence of EPO but in the presence of 100 ng SCF/mL.
Proliferation and survival of HCD57 and HCD57-SREI cells. (A) The cells were cultured in the indicated factor, 1 U EPO/mL, 100 ng SCF/mL, a combination of 100 ng SCF and 1 U EPO/mL, or no added factors in medium containing 25% fetal calf serum. At the indicated day after culture, viable cells were counted in the presence of trypan blue. Error bars indicate the standard deviation from triplicate measurements. (B) HCD57-SREI cells have escaped apoptosis in the absence of EPO. HCD57 cells and HCD57-SREI cells were cultured in the presence of 1 U EPO/mL (+) absence of EPO (−) for 72 hours. The genomic DNA was isolated and analyzed for fragmentation of DNA characteristic of apoptosis as described previously.5
Proliferation and survival of HCD57 and HCD57-SREI cells. (A) The cells were cultured in the indicated factor, 1 U EPO/mL, 100 ng SCF/mL, a combination of 100 ng SCF and 1 U EPO/mL, or no added factors in medium containing 25% fetal calf serum. At the indicated day after culture, viable cells were counted in the presence of trypan blue. Error bars indicate the standard deviation from triplicate measurements. (B) HCD57-SREI cells have escaped apoptosis in the absence of EPO. HCD57 cells and HCD57-SREI cells were cultured in the presence of 1 U EPO/mL (+) absence of EPO (−) for 72 hours. The genomic DNA was isolated and analyzed for fragmentation of DNA characteristic of apoptosis as described previously.5
After 5 days in medium without EPO but containing SCF, virtually all the HCD57 cells had undergone apoptosis such that viable cells were less than 5% of the cell population (Fig 1A, left panel). During the next 2 weeks, the number of viable cells dropped below 1% of total cells. However, after 2 weeks in culture, significant numbers of healthy cells appeared in the culture. These cells were cultured for 10 weeks without EPO, at which point greater than 95% of the cells were viable as determined by trypan blue exclusion. From these pooled cells, two single-cell clones were isolated by limiting dilution. However, the subcloned cell lines appeared to be identical to the three separate isolations of pooled cells, suggesting a single population of cells was selected by these conditions.
We have been able to select these EPO-independent cells only from high-passage HCD57 cells and have tried in five different experiments to select EPO-independent cells from low-passage HCD57 cells without success. Therefore, it is probable that the selection in the EPO minus culture (with or without SCF) repeatedly finds the same subpopulation of EPO-independent cells that are present in the high-passage population of HCD57 cells through a spontaneous mutation.
Whereas SCF had only a transitory effect of increased proliferation but could not prevent apoptosis in the parental HCD57 cells, SCF stimulated proliferation of these surviving cells (HCD57-SREI) over at least 5 days of culture (Fig 1A, right panel). EPO also stimulated proliferation. Surprisingly, these cells required neither EPO nor SCF for limited proliferation and total protection from apoptosis. As shown in Fig 1B, these surviving cells did not undergo apoptosis in the absence of EPO or SCF (Fig 1B, SREI-EPO) whereas the parental HCD57 cells degraded DNA to fragments that are characteristic of apoptosis (Fig 1B, HCD57-EPO). Therefore, the cells were named HCD57-SREI (for SCF-Responsive but EPO-Independent for protection from apoptosis). HCD57-SREI cells have escaped apoptosis in the absence of EPO but proliferation may be stimulated by either EPO or SCF.
HCD57-SREI do not produce EPO.
Some erythroleukemic cell lines may produce autocrine EPO. To investigate whether the HCD57-SREI cells were secreting EPO into the medium, we tested whether the parental HCD57 cells could survive in medium conditioned by the HCD57-SREI cells. HCD57 cells did not proliferate and underwent apoptosis in this conditioned medium even when the HCD57-SREI medium was concentrated (data not shown). In addition, the proliferation of HCD57-SREI cells was not dependent on a high concentration of cells in culture and was not affected by the addition of an anti-EPO antibody that blocked the proliferation of HCD57 cells in EPO-containing medium (data not shown). To investigate whether HCD57-SREI cells might produce EPO that is not secreted, we probed a Northern blot of RNA prepared from HCD57-SREI cells with a probe to EPO. No EPO mRNA was detected (data not shown). Thus, it appeared that the EPO-independent phenotype of the HCD57-SREI cells did not result from either autocrine EPO production or secretion of another factor that maintains the viability of erythroid cells.
EPOR in HCD57-SREI is not activated through an auto-activating mutation.
We tested whether the EPO-independent phenotype resulted from a mutation of the EPOR gene. The arginine 129 to cysteine mutation in the EPOR is an autoactivating mutation50 that allows sulfhydryl bonding to form covalent dimers of EPOR in the absence of EPO. Therefore, we isolated 10 cDNA clones of the EPOR from these HCD57-SREI cells for sequencing. None of the 10 clones were found to have the arginine 129 mutation. Full-length sequencing of two HCD57-SREI cDNA clones showed four identical mutations compared with a previously published sequence51 (amino acid 5, A to G mutation in the second base of codon; amino acid 273, T to G mutation of the first base of the codon; amino acid 334, mutation of A to G in the third base of the codon; amino acid 350 mutation of T to G in the third base of the codon). However, these mutations are conservative such that the amino acid sequence of the protein is not altered. Thus, the EPOR peptide in HCD57-SREI cells is wild type and has not undergone an autoactivating mutation.
Processing and expression of EPOR and c-Kit in HCD57-SREI cells.
In the parental HCD57 cells and other primary erythroid cells, most of the cell-surface EPOR molecules are posttranslationally processed by extensive N-linked glycosylation and phosphorylation of nontyrosine residues into a 78-kD form.6 10 We examined the posttranslational processing and metabolism of the EPOR in HCD57-SREI cells to test whether overexpression of EPOR peptide, alternative posttranslational processing, or failure to downregulate in the presence of EPO might contribute to EPO independence (or apparent independence as a result of hypersensitivity to trace levels of EPO). Figure 2A shows that the EPOR in HCD57-SREI cells appears to be identically processed from the 62-kD peptide into the 78-kD EPOR as the parental HCD57 cells; however, the level of expression of the EPOR was reduced in the HCD57-SREI cells. This reduced level of EPOR expression correlated with the determination that HCD57 cells (deprived of EPO overnight to upregulate receptors) had 3,800 binding sites for 125I-EPO whereas the HCD57-SREI cells only expressed 500 binding sites for 125I-EPO. The binding affinity for 125I-EPO was similar in both cell lines (kd = 810 nmol/L and 780 nmol/L). In the presence of EPO, the high-molecular-weight 68- to 78-kD forms of EPOR in both HCD57 and HCD57-SREI cells were similarly downregulated within 40 minutes (Fig 2A, lanes D and H). Thus, there appeared to be no alteration of the EPOR protein in HCD57-SREI cells that would explain the EPO-independent phenotype.
Processing and expression of c-Kit and EPOR in HCD57 and HCD57-SREI cells. (A) HCD57 cells deprived of EPO overnight (lanes A through D) or HCD57-SREI cells cultured without EPO (lanes D through G) were left untreated (lanes A and E), or treated with 10 U EPO/mL for the indicated times at 37°C. The cells were rapidly cooled, and the forms of EPOR present were determined by immunoprecipitation of the EPOR and detection of the receptor proteins using the same affinity-purified anti-COOH terminal, anti-EPOR IgG. The form of EPOR between 62 and 78 kD are marked (upper arrows). The IgG on the gel from the heavy chain of anti-EPOR present in the immunoprecipitates is also indicated (lower arrow). (B) HCD57-SREI cells exhibit upregulated cell-surface c-Kit expression. HCD57 and HCD57-SREI cells were incubated with an FITC-conjugated c-Kit antibody and detected using flow cytometry. HCD57-SREI cells exhibit 81.4% more cell surface c-Kit (peak C) than HCD57 (peak B). Peak A represents background staining (CD4).
Processing and expression of c-Kit and EPOR in HCD57 and HCD57-SREI cells. (A) HCD57 cells deprived of EPO overnight (lanes A through D) or HCD57-SREI cells cultured without EPO (lanes D through G) were left untreated (lanes A and E), or treated with 10 U EPO/mL for the indicated times at 37°C. The cells were rapidly cooled, and the forms of EPOR present were determined by immunoprecipitation of the EPOR and detection of the receptor proteins using the same affinity-purified anti-COOH terminal, anti-EPOR IgG. The form of EPOR between 62 and 78 kD are marked (upper arrows). The IgG on the gel from the heavy chain of anti-EPOR present in the immunoprecipitates is also indicated (lower arrow). (B) HCD57-SREI cells exhibit upregulated cell-surface c-Kit expression. HCD57 and HCD57-SREI cells were incubated with an FITC-conjugated c-Kit antibody and detected using flow cytometry. HCD57-SREI cells exhibit 81.4% more cell surface c-Kit (peak C) than HCD57 (peak B). Peak A represents background staining (CD4).
We next examined cell-surface expression of c-Kit by incubation of HCD57 and HCD57-SREI cells with a c-Kit antibody and detection of surface-bound antibody by flow cytometry. Detection of surface c-Kit expression showed an 81.4% increase in c-Kit surface expression in HCD57-SREI cells (Fig 2B, peak C) as compared with HCD57 cells (Fig 2B, peak B).
Expression of Bcl-XL and Bcl-2 in HCD57 and HCD57-SREI cells.
The above experiments indicated a nonreceptor explanation for EPO-independence in HCD57-SREI cells. Overexpression of the Bcl family of apoptosis modulating proteins can result in resistance to apoptosis. Previous work had implicated Bcl-Xl but not Bcl-2 as a critical control point in protecting from apoptosis in HCD57 cells.52Therefore, we examined the expression of Bcl-Xl and Bcl-2 in the HCD57-SREI cells. There was a similar level of expression of these two apoptosis suppressing proteins in the HCD57-SREI cells (Fig3, lanes D and H) as the parental HCD57 cells (Fig 3, lanes B and F). Thus, the protection from apoptosis in the HCD57-SREI cells did not result from overexpression of the Bcl gene products. However, we found that whereas the withdrawal of EPO suppressed the expression of both Bcl-Xl and Bcl-2 proteins in HCD57 cells, Bcl-Xl levels were maintained in the presence or absence of EPO in HCD57-SREI cells (Fig 3, lanes C and G). The EPO-independent phenotype of HCD57-SREI cells, therefore, may be caused by an alteration in signaling upstream of the regulation of Bcl-Xl gene that maintains the expression of the Bcl-Xl protein even in the absence of EPO.
Bcl-Xl and Bcl-2 are maintained in HCD57-SREI cells when EPO is withdrawn. HCD57 cells (lanes A, B, E, and F) and HCD57-SREI cells (lanes C, D, G, and H) were cultured for 72 hours in either medium containing 1 U EPO/mL (lanes B, D, F, H) or no EPO (lanes A, C, E, G). Ten micrograms of cellular protein was analyzed by Western blotting using anti-Bcl-Xl (lanes A through D) or anti–Bcl-2 (lanes E through H) antisera. The proteins of interest were visualized with ECL and are marked by an arrow.
Bcl-Xl and Bcl-2 are maintained in HCD57-SREI cells when EPO is withdrawn. HCD57 cells (lanes A, B, E, and F) and HCD57-SREI cells (lanes C, D, G, and H) were cultured for 72 hours in either medium containing 1 U EPO/mL (lanes B, D, F, H) or no EPO (lanes A, C, E, G). Ten micrograms of cellular protein was analyzed by Western blotting using anti-Bcl-Xl (lanes A through D) or anti–Bcl-2 (lanes E through H) antisera. The proteins of interest were visualized with ECL and are marked by an arrow.
Tyrosine protein phosphorylation in HCD57 and HCD57-SREI cells.
Alterations in cellular signaling in HCD57-SREI cells might explain why Bcl-Xl levels were maintained in the absence of EPO. Therefore, we examined the phosphotyrosine-containing proteins in either untreated cells or cells treated with SCF or EPO in HCD57-SREI and parental HCD57 cells. If an autoactivating mutation in the EPOR or a mutation resulting in expression of EPO were present, we would expect to see constitutive phosphorylation of the EPOR and JAK2 kinase as previously reported.53 In addition, the constitutive secretion of SCF would be expected to lead to constitutive tyrosine protein phosphorylation of c-Kit. However, as shown in Fig4A (top panel), there was no apparent constitutive phosphorylation of bands corresponding to EPOR, JAK2, c-Kit, or other major phosphotyrosine [Tyr(P)] containing protein in HCD57-SREI cells, virtually ruling out the possibility of an autoactivating mutation in either EPOR or JAK2 or autocrine production of EPO or SCF.
Effect of EPO and SCF on tyrosine protein phosphorylation in HCD57 cell and HCD57-SREI cells. (A) HCD57 cells deprived of EPO overnight (lanes A, B, C) or HCD57-SREI cells cultured without EPO (lanes D, E, F) were left untreated (A, D) or treated with 10 U EPO/mL for 10 minutes (B, E) or 100 ng SCF/mL for 10 minutes (C, F). The cells were then rapidly chilled in an ice bath, a detergent lysate was prepared, and phosphotyrosine containing proteins were immunoprecipitated with a polyclonal anti-Tyr(P) antibody. The immunoprecipitated proteins were then run on SDS-polyacrylamide gel electrophoresis, Western blotted, and probed with a monoclonal anti-Tyr(P) antibody (top panel) and then stripped of bound antibody and reprobed with an anti-JAK2 antiserum (bottom panel). Bands were visualized with ECL. (B) SHC protein tyrosine phosphorylation in HCD57-SREI cells (lanes 1 through 3) and HCD57 cells (lanes 4 through 6). The cells were treated with nothing (lanes 1 and 4), SCF (lanes 2 and 5), and EPO (lanes 3 and 6) as described above and SHC proteins were immunoprecipitated. The Western blot was probed with anti-Tyr(P) (top panel) and then stripped of bound antibody and reprobed with anti-SHC antiserum (bottom panel). (C) MAP kinase (ERK-1) activity in HCD57 and HCD57-SREI cells. HCD57 cells were deprived of EPO overnight in IMDM containing 25% serum. Aliquots of HCD57 cells that had been deprived of EPO (lanes 1 through 4) and HCD57-SREI cell continually cultured without EPO (lanes 5 through 8) were deprived of serum for 1 hour. After this 1-hour incubation in serumless medium, either nothing (lanes 1 and 5), 1.0 U EPO/mL (lanes 2 and 6), 25% fetal calf serum (lanes 3 and 7), or a combination of 25% serum plus 1.0 U EPO/mL was added for 10 minutes at 37°C. After the 10-minute incubation, the cells were rapidly chilled in cold medium, solubilized, and the ERK-1, MAP kinase activity determined by immunoprecipitation and phosphorylation of myelin basic protein as described in Materials and Methods. (D) Constitutive STAT5 phosphorylation in HCD57-SREI cells. HCD57-SREI cells (lanes A through C, G through I) and HCD57 cells (lanes E through F, J through L) were treated with nothing (lanes A, D, G, J) or 10 U EPO/mL for either 5 minutes (lanes B, E, H, K) or 10 minutes (lanes C, F, I, L) at 37°C. Ten micrograms of total cellular extract (lanes A through F) or STAT5 immunoprecipitated from 200 μg of cellular extract (lanes G through L) were analyzed by Western blotting with anti-Tyr(P) antiserum. Molecular-weight markers and migration of proteins of interest are indicated.
Effect of EPO and SCF on tyrosine protein phosphorylation in HCD57 cell and HCD57-SREI cells. (A) HCD57 cells deprived of EPO overnight (lanes A, B, C) or HCD57-SREI cells cultured without EPO (lanes D, E, F) were left untreated (A, D) or treated with 10 U EPO/mL for 10 minutes (B, E) or 100 ng SCF/mL for 10 minutes (C, F). The cells were then rapidly chilled in an ice bath, a detergent lysate was prepared, and phosphotyrosine containing proteins were immunoprecipitated with a polyclonal anti-Tyr(P) antibody. The immunoprecipitated proteins were then run on SDS-polyacrylamide gel electrophoresis, Western blotted, and probed with a monoclonal anti-Tyr(P) antibody (top panel) and then stripped of bound antibody and reprobed with an anti-JAK2 antiserum (bottom panel). Bands were visualized with ECL. (B) SHC protein tyrosine phosphorylation in HCD57-SREI cells (lanes 1 through 3) and HCD57 cells (lanes 4 through 6). The cells were treated with nothing (lanes 1 and 4), SCF (lanes 2 and 5), and EPO (lanes 3 and 6) as described above and SHC proteins were immunoprecipitated. The Western blot was probed with anti-Tyr(P) (top panel) and then stripped of bound antibody and reprobed with anti-SHC antiserum (bottom panel). (C) MAP kinase (ERK-1) activity in HCD57 and HCD57-SREI cells. HCD57 cells were deprived of EPO overnight in IMDM containing 25% serum. Aliquots of HCD57 cells that had been deprived of EPO (lanes 1 through 4) and HCD57-SREI cell continually cultured without EPO (lanes 5 through 8) were deprived of serum for 1 hour. After this 1-hour incubation in serumless medium, either nothing (lanes 1 and 5), 1.0 U EPO/mL (lanes 2 and 6), 25% fetal calf serum (lanes 3 and 7), or a combination of 25% serum plus 1.0 U EPO/mL was added for 10 minutes at 37°C. After the 10-minute incubation, the cells were rapidly chilled in cold medium, solubilized, and the ERK-1, MAP kinase activity determined by immunoprecipitation and phosphorylation of myelin basic protein as described in Materials and Methods. (D) Constitutive STAT5 phosphorylation in HCD57-SREI cells. HCD57-SREI cells (lanes A through C, G through I) and HCD57 cells (lanes E through F, J through L) were treated with nothing (lanes A, D, G, J) or 10 U EPO/mL for either 5 minutes (lanes B, E, H, K) or 10 minutes (lanes C, F, I, L) at 37°C. Ten micrograms of total cellular extract (lanes A through F) or STAT5 immunoprecipitated from 200 μg of cellular extract (lanes G through L) were analyzed by Western blotting with anti-Tyr(P) antiserum. Molecular-weight markers and migration of proteins of interest are indicated.
The Tyr(P)-containing proteins were very similar in the HCD57 and HCD57-SREI cells after either EPO or SCF treatment with the exception that the tyrosine-phosphorylated 78-kD band corresponding to EPOR was more prominent in EPO-treated HCD57 cells, correlating with the increased expression of the 78-kD EPOR. As shown by very careful examination of Fig 4A, there was only a trace of Tyr(P) in the EPOR in either HCD57 or HCD57-SREI cells treated with SCF. In data not shown, we observed that there was twofold to threefold more tyrosine phosphorylation of the 78-kD EPOR immunoprecipitated from HCD57-SREI cells treated with SCF than the EPOR in comparably treated HCD57 cells. This level of EPOR phosphorylation was only about 10% of the phosphorylation achieved with EPO stimulation. A 150-kD band which apparently corresponds to the SCF receptor was more strongly phosphorylated in SCF-treated HCD57-SREI than HCD57 cells, correlating with increased expression of c-Kit on HCD57-SREI cells. Another approximately 30-kD protein of unknown identity was highly phosphorylated in SCF-treated HCD57-SREI cells compared with HCD57 cells. Stripping this blot and reprobing with anti-JAK2 antiserum showed that JAK2 was neither autophosphorylated in HCD57-SREI cells nor tyrosine phosphorylated after SCF treatment (Fig 4A, bottom panel, lane F).
We also studied the tyrosine phosphorylation of the SHC proteins in both EPO-treated and SCF-treated HCD57 and HCD57-SREI cells (Fig 4B). As suggested by the antiphosphotyrosine Western blots in Fig 4A, immunoprecipitated SHC was not constitutively phosphorylated in either cell type (Fig 4B, lanes 1 and 4). The lesser effect of EPO on SHC phosphorylation in HCD57-SREI cells reflected the reduced expression and phosphorylation of the EPOR.
In HCD57 cells, neither the EPO-dependent nor SCF-dependent SHC tyrosine phosphorylation appeared to correlate with induced ERK-1 activity.5 However, recent information shows that ERK may be activated by SHC-independent mechanisms.54 Therefore, we tested MAP kinase (ERK-1) activity by immunoprecipitating ERK-1 proteins and measuring the activity that phosphorylated myelin basic protein. In data not shown, a low level of constitutive activity of ERK-1 was found in both HCD57 and HCD57-SREI cells in the absence of growth factors but in the presence of 25% fetal calf serum. This observation seems to eliminate the SHC/ERK pathway from a role in the resistance to apoptosis upon EPO withdrawal seen in HCD57-SREI cells.
We then removed the serum for 1 hour and measured the basal levels of ERK-1 in the absence of serum and the ERK activity after the addition of 25% serum, 10 U EPO/mL, or a combination of serum and EPO. As shown in Fig 4C, there was no detectable ERK-1 activity in either the HCD57 cells or HCD57-SREI cells in the absence of serum and EPO. It is interesting to note that this experiment showed that MAP kinase activity is poorly activated by either EPO alone or serum alone and that there is a strong synergistic effect of serum and EPO in both HCD57 and HCD57-SREI cells. However, the magnitude of the synergistic effect of combining EPO and serum was much greater in the HCD57-SREI cells than observed in the HCD57 cells. We found this surprising but three separate experiments showed almost identical results as that shown in Fig 4C.
STAT5A/B is constitutively phosphorylated, translocated to the nucleus, and bound to DNA in the HCD57-SREI cells.
Although not evident in the above experiments, a band of about 100 kD constitutively tyrosine phosphorylated in the SREI subclone but not the HCD57 cells, was seen in some experiments. To test whether this approximately 100-kD band might be an activated STAT protein, we immunoprecipitated STAT5A/B from HCD57 cells and HCD57-SREI cells and analyzed the tyrosine phosphorylation state of STAT5 in the presence and absence of EPO (Fig 4D). This experiment confirmed that STAT5 was constitutively phosphorylated on tyrosine residues in the HCD57-SREI cells (Fig 4D, lane G). Additional experiments with STAT5A- and STAT5B-specific antisera show that both forms of STAT5 were phosphorylated, but we did not find other STATs to be constitutively active. Interestingly, the major tyrosine phosphorylated band at 95 kD seen in EPO-treated HCD57 and HCD57-SREI cells was not the STAT5 protein as many have assumed. STAT5 was a minor component of this 95-kD band (STAT5 migrates at the very top of this broad band). Although STAT1 is also activated by EPO in HCD57 cells, the level of phosphorylation of STAT1 is an order of magnitude less than STAT5 and, therefore, does not contribute significantly to this band, leaving the identity of the band a mystery.
We did not see constitutively phosphorylated STAT5 in the Tyr(P) immunoprecipitate of HCD57-SREI cells (Fig 4A); however, this was due to the inability of the polyclonal anti-Tyr(P) antibody used to immunoprecipitate the Tyr(P)-containing proteins (Zymed) to recognize phosphorylated STAT5. Figure 4D shows that when the total cellular extract was examined by blotting with anti-Tyr(P) [without immunoprecipitation to concentrate Tyr(P) proteins] the STAT5A/B constitutive tyrosine phosphorylation was not apparent. We were able to detect constitutive phosphorylation of STAT5 only when 20-fold more HCD57-SREI cells were used to immunoprecipitate STAT5 A/B compared with the number of cells used in the total cellular extract. However, parallel experiments with very concentrated cell extracts did not show constitutively phosphorylated JAK kinases or EPOR.
We also tested whether there was constitutive activation of STAT proteins in the nuclear fraction of SREI cells. As shown in Fig5, HCD57-SREI cells showed constitutive nuclear localization of STAT5 proteins (Fig 5A, lane 5), tyrosine phosphorylation of a 95-kD band corresponding to STAT5 (Fig 5B, lane 5), and activation of DNA binding activity characteristic of STAT5 (Fig5C, lane 5). The addition of EPO to HCD57-SREI cells led to additional activation of STAT5 phosphorylation, translocation, and DNA binding activity above the constitutive level (Fig 5C, lane 6). In contrast, the HCD57 cells did not have any detectable constitutive activation of STAT5 activity in the absence of EPO treatment (Fig 5C, lane 1). SCF treatment of HCD57 cells did not activate STAT5 nor did SCF treatment of HCD57-SREI cells increase the activity of STAT5 above the constitutive activation (Fig 5C, lane 7). To confirm that the DNA binding activity to the PIE DNA element constitutively activated in HCD57-SREI cells was STAT5 and to further discriminate whether that activity was due to STAT5A or STAT5B, we used super-shift analysis with various anti-STAT antisera. Antibodies to STAT1, STAT3, STAT5A, and STAT5B were incubated with nuclear extracts to test the identity of the proteins binding to the PIE DNA. As shown in Fig 5D, no super-shift was detected when anti-STAT1 or anti-STAT3 antibodies were present, indicating the likely absence or low abundance of these factors in the DNA binding complex. However, both anti-STAT5A and anti-STAT5B antibodies super-shifted the complex. This result suggests that heterodimers of STAT5A and STAT5B are the predominant members of the PIE DNA binding complex constitutively bound in HCD57-SREI cells in the absence of factors and in EPO-treated HCD57 and HCD57-SREI cells. Interestingly, two minor bands present in the complex indicated that both STAT5A homodimers and STAT5B homodimers also bind to the DNA. These homodimers could be discriminated from the heterodimers of STAT5A and STAT5B by faster migration on the polyacrylamide gel, with STAT5A:STAT5A homodimers migrating faster than the STAT5B:STAT5B homodimers.
Characterization of nuclear extracts made from EPO- and SCF-treated HCD57 (lanes 1 through 4) and HCD57-SREI cells (lanes 5 through 7). Cells (2 × 107) were treated with nothing (lanes 1 and 5), EPO (10 U/mL) (lanes 2 and 6), interferon-γ (5 ng/mL) (lane 4), or SCF (100 ng/mL) (lanes 3 and 6) for 10 minutes at 37°C. The immunoblot was probed with MoAb to STAT5 (A) and reprobed with MoAbs to PY (B). The 95K arrows mark the molecular weight and position where the STAT5 proteins migrate on the gel. (C) Mobility-shift assays of the PIE binding nuclear proteins from EPO and SCF treatment. Nuclear protein preparation and gel shift analyses with the PIE sequence were performed as previously described.11 Nuclear protein (20 μg) from control (C) lanes 1 and 5, and EPO-treated extracts (lanes 2 and 6) and SCF-treated cells (lanes 3 and 7) were incubated with the radiolabeled oligonucleotide, and shifted bands were visualized by autoradiography. Only upon a very dark exposure can a minor band below the position of the major band be seen that arises from STAT1 binding in either EPO- or interferon-treated HCD57 cells. The HCD57 cells seem to be very unresponsive to interferon-γ compared with primary erythroid cells. (D) Super-shift analysis shows that STAT5A/B heterodimers are the constitutively active DNA-binding proteins. The cells were treated as before, or left untreated and the gel mobility shift assay was performed as above except that either anti-STAT1, anti-STAT3, anti-STAT5A, or anti-STAT5B were preincubated for 15 minutes on ice with the nuclear extracts before the addition of the radiolabeled PIE DNA. The bar indicates the supershift of DNA binding that occurred with either anti-STAT5A or anti-STAT5B antisera. The deduced positions of apparent shifts resulting from either STAT5A:STAT5B heterodimers (STAT5A:B), STAT5A homodimers (STAT5A:A), or STAT5B homodimers (STAT5B:B) are indicated by arrows.
Characterization of nuclear extracts made from EPO- and SCF-treated HCD57 (lanes 1 through 4) and HCD57-SREI cells (lanes 5 through 7). Cells (2 × 107) were treated with nothing (lanes 1 and 5), EPO (10 U/mL) (lanes 2 and 6), interferon-γ (5 ng/mL) (lane 4), or SCF (100 ng/mL) (lanes 3 and 6) for 10 minutes at 37°C. The immunoblot was probed with MoAb to STAT5 (A) and reprobed with MoAbs to PY (B). The 95K arrows mark the molecular weight and position where the STAT5 proteins migrate on the gel. (C) Mobility-shift assays of the PIE binding nuclear proteins from EPO and SCF treatment. Nuclear protein preparation and gel shift analyses with the PIE sequence were performed as previously described.11 Nuclear protein (20 μg) from control (C) lanes 1 and 5, and EPO-treated extracts (lanes 2 and 6) and SCF-treated cells (lanes 3 and 7) were incubated with the radiolabeled oligonucleotide, and shifted bands were visualized by autoradiography. Only upon a very dark exposure can a minor band below the position of the major band be seen that arises from STAT1 binding in either EPO- or interferon-treated HCD57 cells. The HCD57 cells seem to be very unresponsive to interferon-γ compared with primary erythroid cells. (D) Super-shift analysis shows that STAT5A/B heterodimers are the constitutively active DNA-binding proteins. The cells were treated as before, or left untreated and the gel mobility shift assay was performed as above except that either anti-STAT1, anti-STAT3, anti-STAT5A, or anti-STAT5B were preincubated for 15 minutes on ice with the nuclear extracts before the addition of the radiolabeled PIE DNA. The bar indicates the supershift of DNA binding that occurred with either anti-STAT5A or anti-STAT5B antisera. The deduced positions of apparent shifts resulting from either STAT5A:STAT5B heterodimers (STAT5A:B), STAT5A homodimers (STAT5A:A), or STAT5B homodimers (STAT5B:B) are indicated by arrows.
The finding of constitutive STAT5 activity without a corresponding constitutive activity of JAK2 led us to further investigate if there might be constitutive activation of other members of the Janus kinases. Therefore, we immunoprecipitated JAK1, JAK2, JAK3, and TYK2 from HCD57-SREI cells either untreated or treated with EPO. When the immunoprecipitate was analyzed by Western blotting with anti-Tyr(P) antiserum, only JAK2 from EPO-treated cells was observed to be phosphorylated (data not shown).
Does a phosphatase defect explain the HCD57-SREI resistance to apoptosis?
The observation showing that sodium vanadate (Na3VO4), an inhibitor of tyrosine protein phosphatase activity, can activate STAT protein independently of Janus kinases suggested that constitutive activation of STAT5 might be due to the loss of phosphatase activity in HCD57-SREI cells.55,56The SHP-1 tyrosine protein phosphatase has been strongly implicated in the modulation of both JAK2/EPOR and STAT signaling.22,57-60 We and others have previously reported that HCD57 cells are hypersensitive to EPO.10 61 Therefore, we tested the hypothesis that the hypersensitivity of HCD57 cells to EPO might be caused by a reduction of the SHP-1, and that the loss of EPO-dependence in the HCD57-SREI cells could be the result of the further reduction or complete loss of this phosphatase. We compared the levels of SHP-1 in these two cell lines with a population of CFU-E and proerythroblasts purified from normal human blood. Figure6 shows that identical levels of SHP-1 were present in normal immature human erythroid cells, HCD57 cells, and the apoptosis-resistant HCD57-SREI cells.
The tyrosine protein phosphatase, SHP-1, is equally expressed in HCD57, HCD57-SREI cells and primary human erythroid cells. Frozen HCD57 and HCD57-SREI cells were sent to Dr Amittha Wickrema who compared 10 μg of total cellular protein from these cell lines with an equal amount of primary erythroid cells at the CFU-E stage of development that develop in culture from more immature erythroid cells purified from human blood. The Western blot was probed with anti–SHP-1 antiserum and visualized by ECL.
The tyrosine protein phosphatase, SHP-1, is equally expressed in HCD57, HCD57-SREI cells and primary human erythroid cells. Frozen HCD57 and HCD57-SREI cells were sent to Dr Amittha Wickrema who compared 10 μg of total cellular protein from these cell lines with an equal amount of primary erythroid cells at the CFU-E stage of development that develop in culture from more immature erythroid cells purified from human blood. The Western blot was probed with anti–SHP-1 antiserum and visualized by ECL.
Constitutive activation of PI3-kinase in HCD57 and HCD57-SREI cells.
PI3 kinase has been reported to be activated as part of the EPO signaling pathway. Because PI3-kinase has been implicated in several cell types as critical to the regulation of cell proliferation, differentiation, and survival,62-64 we measured PI3-kinase activity and tested for interaction of PI3-kinase with proteins known to associate with PI3-kinase. This study compared these parameters in the EPO-dependent HCD57 cells and the EPO-independent HCD57-SREI cells. Figure 7A shows that the PI3-kinase activity measured by incorporation of 32P from gamma-labeled ATP into phosphatidylinositol by the immunoprecipitated PI3-kinase (anti-p85 subunit of PI3-kinase) appeared constitutive in both HCD57 and HCD57-SREI cells and was not additionally increased by treatment of EPO. The bottom panel of Fig 7A shows the combined results from five experiments that confirm the lack of EPO effect. To verify that this unexpected constitutive activity which phosphorylated phosphatidylinositol was truly PI3-kinase activity, we added the PI3-kinase inhibitor, LY294002, to the reaction mixture and showed that the activity was inhibited in a dose-responsive fashion at concentrations of inhibitor known to specifically block PI3-kinase activity (shown in Fig 7B). For a further control, we examined the PI3-kinase activity in immunoprecipitated STAT5, JAK2, Vav, as well as PI3-kinase. PI3-kinase activity was only detected in the immunoprecipitated PI3-kinase (anti-p85 immunoprecipitate), as shown in Fig 7C.
PI 3-kinase was constitutively activated in HCD57 and HCD57-SREI cells. (A) Analysis of PI 3-kinase activity in HCD57 and HCD57-SREI cells. (Top panel, A) 3 × 106 HCD57 (lanes 1 and 2) and HCD57-SREI (lanes 3 and 4) cells were deprived of EPO overnight and treated with 10 U/mL EPO (lanes 2 and 4) for 5 minutes or left untreated (lanes 1 and 3). Cells were lysed and immunoprecipitated by anti-p85 subunit of PI 3-kinase antibody, and the precipitates were assayed for PI3-kinase activity as described in Materials and Methods. The positions of the PI 3-phosphate product (PI3-P) and the origin are indicated. (B) Inhibition of in vitro PI3-kinase activity by LY294002. (Top panel, B) HCD57 cells were deprived of EPO overnight and treated with EPO for 5 minutes. Immunoprecipitates were prepared from HCD-57 cells as described previously. PI 3-kinase assay was performed in the presence of various concentrations of LY294002 (lanes 5 through 8) as indicated. (Bottom panels, A and B) PI 3-kinase activities were quantified and the results were expressed as fold activity compared to the activity of HCD57 cells not treated with EPO. Data are means ± SD from three independent experiments. (C) Analysis of PI 3-kinase activity in different immunoprecipitates. Cell lysates were prepared from HCD-57 cells cultured in normal medium and immunoprecipitated by anti-p85 subunit of PI 3-kinase (lane 9), anti-JAK2 (lane 10), anti-STAT5 (lane 11), or anti-Vav (lane 12) antibodies. The precipitates were assayed for PI 3-kinase activity as described in Materials and Methods. The positions of the PI3-phosphate product (PI3-P) and the origin are indicated.
PI 3-kinase was constitutively activated in HCD57 and HCD57-SREI cells. (A) Analysis of PI 3-kinase activity in HCD57 and HCD57-SREI cells. (Top panel, A) 3 × 106 HCD57 (lanes 1 and 2) and HCD57-SREI (lanes 3 and 4) cells were deprived of EPO overnight and treated with 10 U/mL EPO (lanes 2 and 4) for 5 minutes or left untreated (lanes 1 and 3). Cells were lysed and immunoprecipitated by anti-p85 subunit of PI 3-kinase antibody, and the precipitates were assayed for PI3-kinase activity as described in Materials and Methods. The positions of the PI 3-phosphate product (PI3-P) and the origin are indicated. (B) Inhibition of in vitro PI3-kinase activity by LY294002. (Top panel, B) HCD57 cells were deprived of EPO overnight and treated with EPO for 5 minutes. Immunoprecipitates were prepared from HCD-57 cells as described previously. PI 3-kinase assay was performed in the presence of various concentrations of LY294002 (lanes 5 through 8) as indicated. (Bottom panels, A and B) PI 3-kinase activities were quantified and the results were expressed as fold activity compared to the activity of HCD57 cells not treated with EPO. Data are means ± SD from three independent experiments. (C) Analysis of PI 3-kinase activity in different immunoprecipitates. Cell lysates were prepared from HCD-57 cells cultured in normal medium and immunoprecipitated by anti-p85 subunit of PI 3-kinase (lane 9), anti-JAK2 (lane 10), anti-STAT5 (lane 11), or anti-Vav (lane 12) antibodies. The precipitates were assayed for PI 3-kinase activity as described in Materials and Methods. The positions of the PI3-phosphate product (PI3-P) and the origin are indicated.
As a further control that the EPO signaling pathway was not activated in the EPO-independent HCD57-SREI cells, we also measured the PI3-kinase activity that associated with the EPOR in either untreated or EPO treated HCD57 and HCD57-SREI cells. As shown in Fig8A, in either cell, there was no PI3-kinase activity in untreated cells, but EPO treatment led to a large increase in PI3-kinase activity bound to the EPOR.
PI 3-kinase activity associates with the EPOR and IRS-2. (A) HCD57 (lanes 1 and 2) and HCD57-SREI (lanes 3 and 4) cells were deprived of EPO overnight and treated with 10 U/mL EPO (lanes 2 and 4) for 5 minutes or left untreated (lanes 1 and 3). Cells were lysed and immunoprecipitated by anti-EPOR antiserum, and the precipitates were assayed for PI3-kinase activity as described in Materials and Methods. The positions of the PI3-phosphate product (PI3-P) and the origin are indicated. (B) HCD57 (lanes 5 and 6) and HCD57-SREI (lanes 7 and 8) cells were deprived of EPO overnight and treated with 10 U/mL EPO (lanes 6 and 8) for 5 minutes or left untreated (lanes 5 and 7). Cells were lysed and immunoprecipitated by anti–IRS-2 antibody, and the precipitates were assayed for PI3-kinase activity as described in Materials and Methods. The positions of the PI3-phosphate product (PI3-P) and the origin are indicated.
PI 3-kinase activity associates with the EPOR and IRS-2. (A) HCD57 (lanes 1 and 2) and HCD57-SREI (lanes 3 and 4) cells were deprived of EPO overnight and treated with 10 U/mL EPO (lanes 2 and 4) for 5 minutes or left untreated (lanes 1 and 3). Cells were lysed and immunoprecipitated by anti-EPOR antiserum, and the precipitates were assayed for PI3-kinase activity as described in Materials and Methods. The positions of the PI3-phosphate product (PI3-P) and the origin are indicated. (B) HCD57 (lanes 5 and 6) and HCD57-SREI (lanes 7 and 8) cells were deprived of EPO overnight and treated with 10 U/mL EPO (lanes 6 and 8) for 5 minutes or left untreated (lanes 5 and 7). Cells were lysed and immunoprecipitated by anti–IRS-2 antibody, and the precipitates were assayed for PI3-kinase activity as described in Materials and Methods. The positions of the PI3-phosphate product (PI3-P) and the origin are indicated.
During this study we noticed that there was a constitutively tyrosine-phosphorylated protein of 185-kD that coprecipitated with PI3-kinase in both HCD57 and HCD57-SREI cells. In unpublished work (H.B. and S.T.S., 1998), we found that this protein was insulin receptor substrate-2 (IRS-2). To verify that the PI3-kinase that was associated with the IRS-2 protein was active, we measured the PI3-kinase activity in the IRS-2 immunoprecipitates. As shown in Fig8B, PI3-kinase activity was constitutive and unaffected by EPO treatment in either HCD57 or HCD57-SREI cells, strongly suggesting that IRS-2 is responsible for the constitutive activity of PI3-kinase in the in vitro assay. It is possible that the PI3-kinase is activated and becomes associated with the tyrosine-phosphorylated IRS only after the cells are disrupted and does not reflect the in vivo activity of PI3-kinase.
To test the physiological role of either the constitutive or factor-stimulated PI3-kinase in the EPO-dependent HCD57 cells and the EPO-independent HCD57-SREI cells, the effect of inhibiting PI3-kinase activity by the PI3-kinase selective inhibitor, LY294002, was tested by measuring the number of viable cells and DNA fragmentation as indicators of proliferation and apoptosis, respectively. Figure 9A shows the effect of the indicated concentrations of the LY294002 on viable cell number of either HCD57 cells in EPO or the EPO-independent HCD57-SREI cells in the presence or absence of EPO. The HCD57-SREI cells in the absence of EPO are more sensitive to the inhibitor compared with either HCD57 or HCD57-SREI cells exposed to EPO. At 10 μmol/L LY294002, there are only half as many viable HCD57-SREI cells in the absence of EPO surviving compared with HCD57 or HCD57-SREI cells in the presence of EPO. At 25 μmol/L LY294002, HCD57-SREI cells minus EPO have fallen to 20% of control while either the HCD57 or HCD57-SREI cells in EPO were only moderately diminished (50% to 70% the number of cells that have proliferated in the absence of inhibitor). To test whether the effect of the PI3-kinase inhibitor could be determined to be an effect only on proliferation versus an effect that might be the summation of eliminating cells by inducing apoptosis or programmed cell death, we isolated DNA from cells treated with various concentration of LY294002 for 24 hours and analyzed whether this DNA was intact or degraded into the ladder pattern that distinguished apoptosis. As shown in Fig 9B, no DNA fragmentation was observed in HCD57 until the highest concentration of inhibitor was used. However, in the EPO-independent HCD57-SREI cells (no EPO), DNA fragments characteristic of the intranucleosomal cleavage induced in apoptosis were observed in the lowest concentration of LY294002 used. Therefore, the reduction in viable HCD57-SREI cells that results from inhibition of PI3-kinase certainly is in part caused by apoptosis.
Effect of inhibition of PI3-kinase activity on the proliferation and apoptosis of HCD57 and HCD-SREI cells. (A) Cells cultured in the presence of 1 U EPO/mL (HCD57, ◊; HCD57-SREI, ⊖) or absence of EPO (HCD57-SREI, x) were treated with LY294002 at the indicated concentrations for 72 hours, and the viable cells were counted in the presence of trypan blue. Values are expressed as a percentage of the corresponding control (treated with vehicle dimethylsulfoxide [DMSO]). Data are means ± SE of at least triplicate measurements. (B) Cells were cultured in the presence of 1 U EPO/mL (HCD57) or absence of EPO (HCD57-SREI). HCD-57 (lanes 2 through 6) and HCD57-SREI (lanes 7 through 11) cells were treated with vehicle DMSO (lanes 3 and 8), LY294002 at indicated concentrations (lanes 4 through 6 and 9 through 11), or untreated (lanes 2 and 7) for 24 hours. The genomic DNA was isolated and analyzed for fragmentation of DNA characteristic of apoptosis as described in Materials and Methods.
Effect of inhibition of PI3-kinase activity on the proliferation and apoptosis of HCD57 and HCD-SREI cells. (A) Cells cultured in the presence of 1 U EPO/mL (HCD57, ◊; HCD57-SREI, ⊖) or absence of EPO (HCD57-SREI, x) were treated with LY294002 at the indicated concentrations for 72 hours, and the viable cells were counted in the presence of trypan blue. Values are expressed as a percentage of the corresponding control (treated with vehicle dimethylsulfoxide [DMSO]). Data are means ± SE of at least triplicate measurements. (B) Cells were cultured in the presence of 1 U EPO/mL (HCD57) or absence of EPO (HCD57-SREI). HCD-57 (lanes 2 through 6) and HCD57-SREI (lanes 7 through 11) cells were treated with vehicle DMSO (lanes 3 and 8), LY294002 at indicated concentrations (lanes 4 through 6 and 9 through 11), or untreated (lanes 2 and 7) for 24 hours. The genomic DNA was isolated and analyzed for fragmentation of DNA characteristic of apoptosis as described in Materials and Methods.
Factor-dependent and constitutive activation of PKB/Akt.
The observation of both EPO-dependent and constitutive PI3-kinase activity led us to test if the downstream effectors of PI3-kinase correlated with observed PI3-kinase activity. Activity of PKB/Akt was assessed two ways, by activity (phosphorylation)-specific antibodies and the ability of immunoprecipitated PKB/Akt to phosphorylate a peptide substrate. In sharp contrast to the finding of constitutive activity of PI3-kinase in the anti-p85 PI3-kinase immunoprecipitate in both EPO-dependent HCD57 cells and EPO-independent HCD57-SREI cells (in Fig 7), there was constitutive activity of PKB/Akt activity only in the EPO-independent HCD57-SREI. There was no detectable phosphorylation of PKB/Akt in the HCD57 cells in the absence of added factors (Fig10). The constitutive activation of PKB/Akt in HCD57-SREI cells and EPO-dependent activation in HCD57 cells was confirmed by the in vitro assay of PKB/Akt. The in vitro assay of PKB/Akt gave the following result when PKB/AKt activity was determined (assayed as described in Materials and Methods and expressed as the average of three assays to determine the cpm of 32P from ATP incorporated into a substrate peptide ± standard deviation): HCD57 − EPO = 132 ± 94 cpm; HCD57 + EPO = 6,725 ± 163 cpm; and HCD57-SREI cells − EPO = 933 ± 328 cpm. Moreover, treatment of the HCD57 cells with either EPO or SCF led to a rapid activation of PKB/Akt (Fig 10A). Comparison of the EPO-dependent and SCF-dependent time course of PKB/Akt phosphorylation showed that the EPO effect was greater in magnitude than the SCF effect. PKB/Akt phosphorylation was maximal after 5 minutes of treatment with either EPO or SCF, it then declined by 1 hour and was virtually undetectable after 6 hours (Fig10B).
PKB/Akt activity is constitutive in HCD57-SREI cells but activity is induced by EPO and SCF in HCD57 cells. (Top, A) HCD57 cells deprived of EPO overnight (lanes 1, 2, 5, 6) or HCD57-SREI cells cultured without EPO (lanes 3, 4, 7, 8) were left untreated (1, 3, 5, 7) or treated with 10 U EPO/mL for 5 minutes (lanes 6, 8) or 100 ng SCF/mL for 5 minutes (lanes 2, 4). Cell lysates were prepared and subjected to Western blot analysis as described in Materials and Methods. The blot was probed with anti-phospho-PKB/Akt antibody (top panel). After being stripped, the blot was reprobed with a general anti-PKB/Akt antibody that recognizes all forms of the protein (bottom panel). (B) Time course of EPO and SCF-induced PKB/Akt activation. HCD57 cells deprived of EPO overnight were left untreated (lanes 1, 5) or treated with 10 U EPO/mL (lanes 2 through 4) or 100 ng SCF/mL (lanes 6 through 8) for indicated time periods. Cell lysates were prepared and subjected to Western blot analysis as described in Materials and Methods. The blot was probed with anti-phospho-PKB/Akt antibody (top panel). After being stripped, the blot was reprobed with the general anti-PKB/Akt antibody used above (bottom panel). (C) PI3-kinase inhibitor blocked EPO-induced PKB/Akt phosphorylation in HCD57-SREI cells. HCD57-SREI cells cultured without EPO were left untreated (lanes 1, 7), pretreated with DMSO (lanes 2, 8), or pretreated with LY294002 (lanes 3 through 6, 9 through 12) at the indicated concentration for 4 hours. Then cells were left untreated (lanes 1 through 6) or treated with 10 U EPO/mL for 5 minutes (lanes 7 through 12). Cell lysates were prepared and subjected to Western blot analysis as described in Materials and Methods. The blot was probed with anti-phospho-PKB/Akt antibody (top panel). The blot was stripped of the phospho-specific antibody and reprobed with an antibody that recognizes both phosphorylated and nonphosphophorylated PKB/Akt to verify equal loading (bottom panel).
PKB/Akt activity is constitutive in HCD57-SREI cells but activity is induced by EPO and SCF in HCD57 cells. (Top, A) HCD57 cells deprived of EPO overnight (lanes 1, 2, 5, 6) or HCD57-SREI cells cultured without EPO (lanes 3, 4, 7, 8) were left untreated (1, 3, 5, 7) or treated with 10 U EPO/mL for 5 minutes (lanes 6, 8) or 100 ng SCF/mL for 5 minutes (lanes 2, 4). Cell lysates were prepared and subjected to Western blot analysis as described in Materials and Methods. The blot was probed with anti-phospho-PKB/Akt antibody (top panel). After being stripped, the blot was reprobed with a general anti-PKB/Akt antibody that recognizes all forms of the protein (bottom panel). (B) Time course of EPO and SCF-induced PKB/Akt activation. HCD57 cells deprived of EPO overnight were left untreated (lanes 1, 5) or treated with 10 U EPO/mL (lanes 2 through 4) or 100 ng SCF/mL (lanes 6 through 8) for indicated time periods. Cell lysates were prepared and subjected to Western blot analysis as described in Materials and Methods. The blot was probed with anti-phospho-PKB/Akt antibody (top panel). After being stripped, the blot was reprobed with the general anti-PKB/Akt antibody used above (bottom panel). (C) PI3-kinase inhibitor blocked EPO-induced PKB/Akt phosphorylation in HCD57-SREI cells. HCD57-SREI cells cultured without EPO were left untreated (lanes 1, 7), pretreated with DMSO (lanes 2, 8), or pretreated with LY294002 (lanes 3 through 6, 9 through 12) at the indicated concentration for 4 hours. Then cells were left untreated (lanes 1 through 6) or treated with 10 U EPO/mL for 5 minutes (lanes 7 through 12). Cell lysates were prepared and subjected to Western blot analysis as described in Materials and Methods. The blot was probed with anti-phospho-PKB/Akt antibody (top panel). The blot was stripped of the phospho-specific antibody and reprobed with an antibody that recognizes both phosphorylated and nonphosphophorylated PKB/Akt to verify equal loading (bottom panel).
Because of the possibility that the PKB/Akt might be activated by upstream activators not in the PI3-kinase pathway (due to lack of correlation of PKB/Akt activity with the constitutive PI3-kinase activity in HCD57 cells), the activity of PKB/Akt detected with the activation (phospho)-specific antibody was tested after inhibition of PI3-kinase activity with LY294002. As shown in Fig 10C, treatment of the HCD57-SREI cells in the presence or absence of EPO resulted in inhibition of PKB/Akt, establishing that PI3-kinase was the upstream activator. The effect of LY294002 on PKB/Akt activation was also tested in HCD57 cells in the presence of EPO and PKB/Akt inhibition was very similar to inhibition observed in HCD57-SREI cells in the presence of EPO (data not shown); however, in the EPO-independent, HCD57-SREI cells in the absence of EPO, PKB/Akt phosphorylation was more sensitive to LY294002. PKB/Akt activity in HCD57-SREI cells without EPO was greatly reduced by treatment with 5 μmol/L LY294002 whereas treatment with 25 μmol/L LY294002 was required for the same magnitude of inhibition of PKB/Akt phosphorylation in either HCD57 cells or HCD57-SREI cells in EPO. This result established a correlation between the level of PKB/Akt activity and cell proliferation and/or viability. HCD57-SREI cells in the absence of EPO proliferated less (Fig 9A), had a higher percentage of cells in apoptosis (Fig 9B), and had less PKB/Akt activity when exposed to LY294002 (Fig 10C) than either the HCD57-SREI cells exposed to EPO or HCD57 cells in EPO treated in parallel with LY294002.
DISCUSSION
Experiments reported here strongly suggest a major role of the PI3-kinase/PKB(Akt) signaling pathway in maintaining the viability and/or proliferation of erythroid cells. Interestingly, whether PI3-kinase/PKB activity is stimulated by EPO treatment or is constitutively activated through an unknown mechanism in EPO-independent, apoptosis-resistant cells, blocking the activity of this pathway results in apoptosis of either cell type. This strongly implicates PI3-kinase/PKB activity as a necessary signal for survival. The observation that SCF treatment also activates PI3-kinase/PKB in erythroid cells but does not protect these cells from apoptosis5 (and Fig 1A) indicates that PI3-kinase/PKB signals may be necessary, but PI3-kinase/PKB activity are not sufficient for the protection from apoptosis. Therefore, another signaling pathway in addition to PI3-kinase/PKB pathway must also be active to maintain the viability of erythroid cells. The above-described study suggests that constitutive STAT5 activity in combination with PI3-kinase/PKB signals might be sufficient to protect the EPO-independent cells from apoptosis; however, signaling pathways yet to be discovered or known signaling pathways not tested could also be active and required for survival and proliferation.
We undertook the subcloning of the HCD57 cells in the presence of SCF to test if SCF was capable of activating the complete EPO signaling pathway through the interaction of the EPOR and c-Kit molecules.5,24 Although we established the HCD57-SREI cell line and found long-term responsiveness to SCF, no subclones depended on SCF for survival. This strongly suggests that SCF (either through c-Kit or the EPOR) cannot generate the same anti-apoptotic signal in the HCD57 erythroid cell that is triggered through the EPOR by EPO. The current study is consistent with our past work indicating a role of JAK2/STAT5 upstream of the EPO-dependent prevention of apoptosis.5 Whereas EPO activated the JAK2/STAT5 pathway and prevented apoptosis, SCF failed to protect from apoptosis and did not activate JAK2/STAT5. However, we cannot determine that STAT5 activity is required for protection from apoptosis in the current study. The effect of antisense RNA or a dominant negative STAT5 mutant may shed some further light on the role of STAT5 in the regulation of EPO-independent proliferation and survival.
The SCF effect on preventing apoptosis may be dependent on the cell line selected for study. SCF can prevent apoptosis in the nonerythroid, hematopoietic cell line FDC-2.65 Wu et al24found that SCF could completely replace the requirement for other hematopoietic factors only through the cooperation of the c-Kit and EPOR. The study by Joneja et al65 contradicts Wu et al and confirms that SCF does not activate JAK2, but the differences in these published results may simply reflect that some cells are inherently more resistant to apoptosis after growth factor withdrawal. We report here that HCD57-SREI cells have escaped the apoptosis normally triggered by EPO withdrawal. However, these cells will readily undergo apoptosis when kinases are inhibited by a wide spectrum inhibitor, staurosporine (not shown), or the inhibition of PI3-kinase activity by LY294002 (Fig 9B), proving that the machinery for carrying out the final stages of apoptosis is intact in HCD57-SREI cells.
HCD57-SREI cells have evolved a mechanism of resisting apoptosis that includes the EPO-independent expression of the Bcl family of proteins. We previously showed the induction of JunB and AP1 DNA binding activity after EPO withdrawal in HCD57 cells and that a dominant negative form of AP1 prevented both the apoptosis and the loss of expression of Bcl-Xl after EPO withdrawal.66 In contrast to the EPO-dependent cells, the apoptosis-resistant HCD57-SREI cells did not show any changes in AP1 binding activity or express junB when the cells were deprived of EPO.66 Because the apoptosis-inducing activity of AP1 is suppressed in HCD57-SREI cells, control of expression of Bcl-X1 through an AP1-dependent mechanism is consistent with the hypothesis we have suggested previously.66 The observation of a correlation of Bcl-Xl expression with resistance to apoptosis extends earlier work of Silva et al,52 who showed that unregulated expression of human Bcl-Xl could prevent the murine HCD57 cells from undergoing apoptosis.
The LY294002 study showed that PI3-kinase activity was necessary for erythroid cell survival and proliferation, even if other signaling pathways were active. PKB/Akt activity correlated inversely with susceptibility to apoptosis; PKB activity was inhibited by fivefold lower concentrations of LY294002 and more apoptosis was induced in HCD57-SREI cells in the absence of EPO than either cell line treated with EPO. IRS-2 and PI3-kinase have both been reported to be activated by EPO binding to the EPOR.20,21,47 67-69 However, the constitutive activation of these signals in either the HCD57 cells or the HCD57-SREI cells does not involve JAK2 or the EPOR. The HCD57-SREI cells neither produced nor secreted EPO, did not appear to secrete another factor that allowed HCD57 cells to survive in the absence of EPO, and did not have a mutated EPOR activated in the absence of EPO. The EPOR also did not become tyrosine phosphorylated without the binding of EPO. In addition, there was no detectable association of IRS-2 or PI3-kinase until EPO interacted with the EPOR.
We report here that IRS-2 and PI3-kinase were constitutively active in both HCD57 and HCD57-SREI cells; however, only constitutive PKB/Akt activity appeared to be a signal that protected the HCD57-SREI cells from apoptosis in the absence of EPO. It is puzzling that the apparent constitutive activity of PI3-kinase in the EPO-dependent HCD57 cells did not result in the downstream constitutive activity of the PKB/Akt activity. There are at least two explanations for this paradox: the first is that the PI3-kinase detected in the in vitro activity is an artifact of disrupting the cells and immunoprecipitating PI3-kinase or IRS-2. The second possibility is that the PI3-kinase may truly be activated by IRS-2 in HCD57 cells but that this activity is not translocated to the plasma membrane until either EPO or SCF is added to the cells.
There are reports that link constitutive activity of PI-3 kinase to carcinogenesis43,44; however, the apparent constitutive activation of IRS-2/PI3-kinase in the HCD57 cells does not allow these cells to proliferate or survive in the absence of EPO. This study suggests that the activity of the downstream modulator of PI3-kinase, PKB/Akt, is a better marker of transformed cells. BCR/ABL is known to activate the PI3-kinase/PKB signaling pathway, implicating this signal in the pathology of chronic myelogenous leukemia.47,70 PKB was initially identified as the cellular homologue of the v-aktoncogene and PKB/c-Akt is overexpressed in tumors.47Preliminary evidence suggests that overexpressed PKB/Akt better correlates with tumor aggressiveness rather than tumor induction. This possibility is consistent with the finding here of an erythroleukemic cell line progressing from a hormone-dependent cell line to a hormone-independent cell line as a result of acquiring constitutively active PKB/Akt.
Klingmüller et al71 have recently suggested that PI3-kinase acts in the EPO signaling pathway as an intermediate in the activation of the MAP kinase cascade. The current study does not support this hypothesis. Constitutive activity of the PI3-kinase/PKB pathway (even in the absence of serum) compared with the lack of detectable ERK-1 activity in unstimulated cells strongly suggests that PI3-kinase activity alone is insufficient to trigger MAP kinase activation. It is interesting to note that the synergistic effect of serum and EPO on MAP kinase activation is magnified in the HCD57-SREI cells compared with the parental HCD57 cells (Fig 4C). It is possible that the constitutive PI3-kinase/PKB/Akt activity in the HCD57-SREI cells may act upstream to magnify the synergistic activation of the MAP kinase cascade by serum and EPO, even though the PI3-kinase/PKB pathway does not activate either SHC or MAP kinase.
The HCD57-SREI cells expressed constitutively activated STAT5a and STAT5b, yet this signaling pathway was completely dependent on EPO in the parental, EPO-dependent HCD57 cells. Therefore, STAT5 may act upstream of an intracellular signal that protects erythroid cells from apoptosis (but this study shows that signals such as PKB/Akt are also required). Recent studies showing that “knock out” of STAT5a/b does not have significant effects on erythroid cell development do not rule out the role of constitutive STAT5 activation acting to prevent apoptosis.40-42 Indeed, even if STAT5 may not be required for erythroid cell proliferation and protection from apoptosis, constitutive activation of STAT5 may, nevertheless, have consequences in erythroid cell signaling. Our experiments failed to show any constitutive activation of JAK2 or other known member of the Janus kinase family. Therefore, the mechanism by which STAT5 becomes phosphorylated and activated to bind to DNA in HCD57-SREI cells is not apparent.
It has been reported that sodium vanadate activates STAT proteins independently of JAK kinase.55,56 This work suggests a low level of STAT activation by non-Janus kinase(s) that only becomes apparent when tyrosine protein phosphatase activity is inhibited. This observation and our own finding that sodium vanadate can activate STAT5 in FVA cells11 suggests that a possible reduction or loss of tyrosine phosphatase activity in HCD57-SREI cells. The tyrosine protein phosphatase SHP-1 has been implicated in regulating both the activity of the EPOR/JAK222 and has been reported to dephosphorylate STAT proteins in the nucleus.58 We tested the hypothesis that SHP-1 might be reduced or absent in the EPO-independent HCD57-SREI cells by measuring the expression of SHP-1 by Western blotting (Fig 6). We found that the levels of SHP-1 proteins in both HCD57 and HCD57-SREI cell lines were identical to that of primary human erythroid cells. Although this result suggests that the defect in HCD57-SREI cells responsible for the escape from apoptosis in the absence of EPO is not due to SHP-1, this experiment does not rule out the loss of other phosphatase activity in these cells.
Constitutive activity of the JAK/STAT pathway is linked to leukemogenesis and the transformation of other cancerous cells.26-35 Recent reports have identified the constitutive activation of STAT5 and STAT1 in peripheral cells from acute lymphoblastic leukemia and constitutive STAT1, STAT3, and STAT5 activity in peripheral cells from patients with acute myeloid leukemia.25 Constitutive activation of STAT5 is found in a variety of primary lymphoid and myeloid leukemia cells and constitutive STAT1 and STAT3 Epstein-Barr virus–related lymphoma cells.26 Constitutive STAT3 has been associated with breast cancer cells.27,28 The observation that oncogenes like v-Src, v-Abl, and Bcr/abl may activate the JAK/STAT pathway independently of growth factors30,33 35 further suggests that constitutive STAT activity may contribute to the transformation of cells from factor-dependent to factor-independent proliferation. This study suggests a role for constitutive STA5a/b activity in a supporting role to constitutive PKB/Akt activity in the progression of an erythroleukemic cell line to factor-independent viability and proliferation.
ACKNOWLEDGMENT
The authors thank Dr John Ryan (Virginia Commonwealth University, Richmond, VA) for his assistance with the flow cytometry analysis.
Supported by National Institutes of Health Grant No. RO1DK39781 to S.T.S. and an American Heart Association Fellowship Grant to S.M.J.-H.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Stephen T. Sawyer, PhD, Department of Pharmacology & Toxicology, Medical College of Virginia of Virginia Commonwealth University, PO Box 980613, Richmond, VA 23298-0613. e-mail: ssawyer@hsc.vcu.edu.

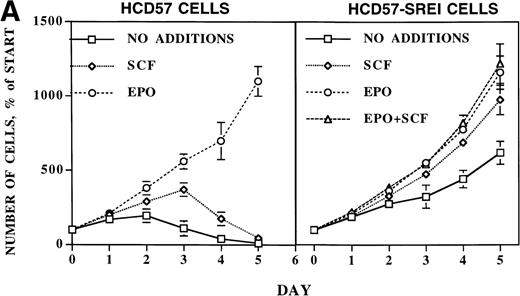
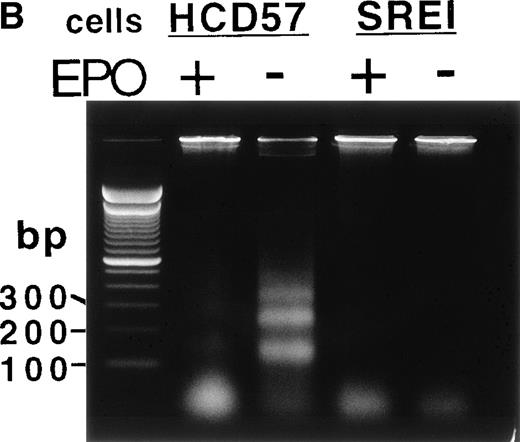
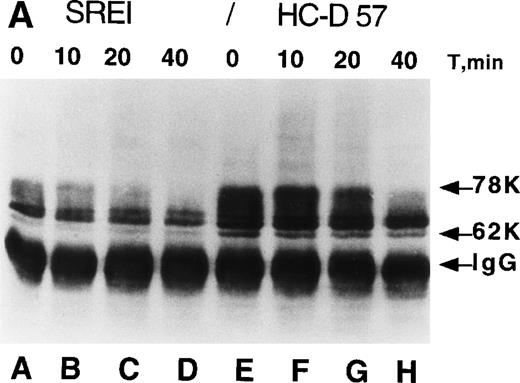
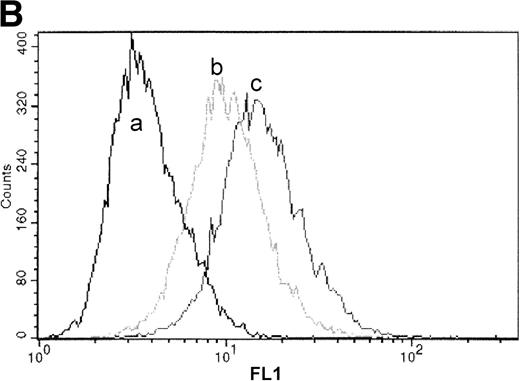
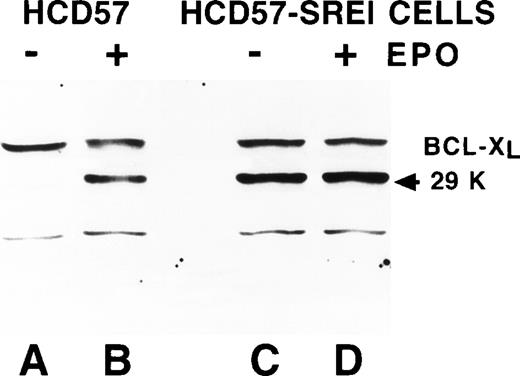
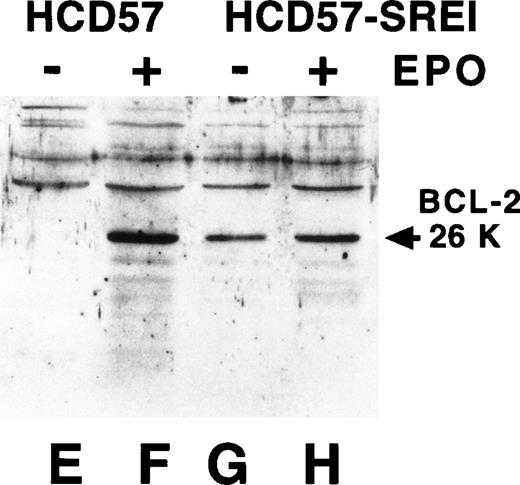
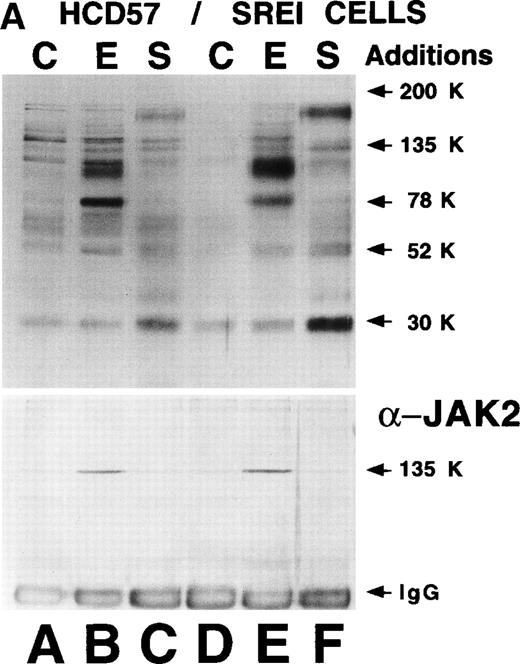
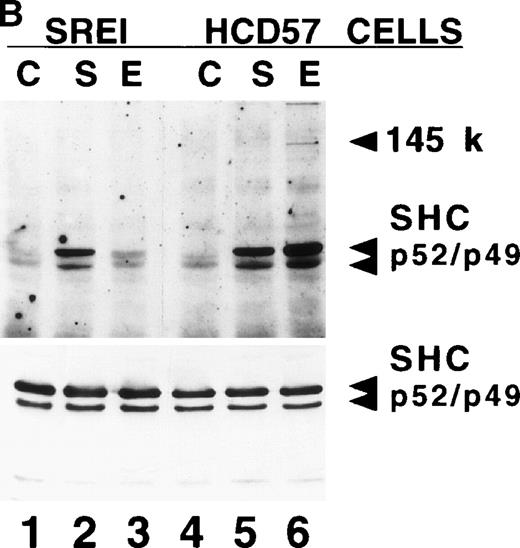
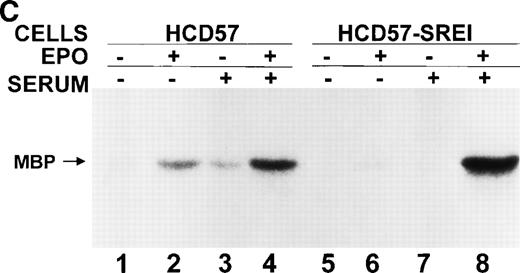
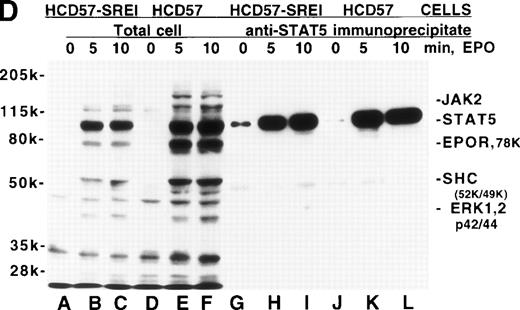
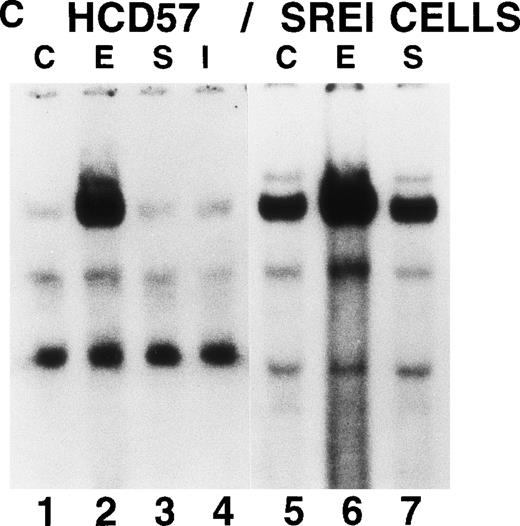
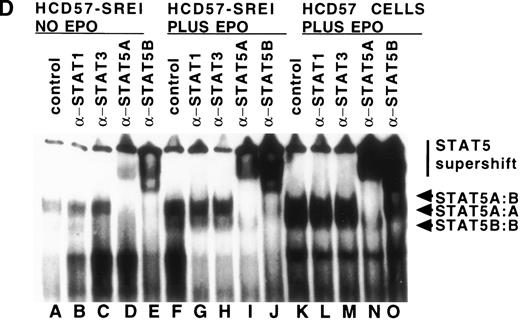
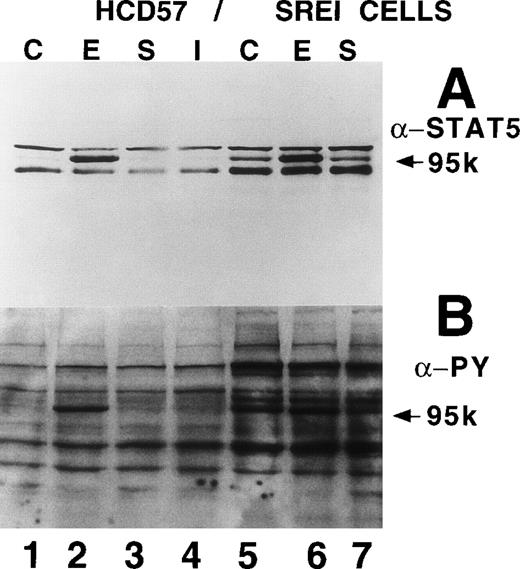
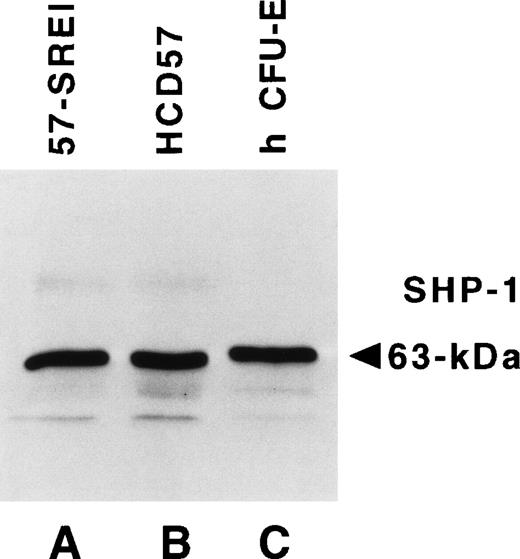
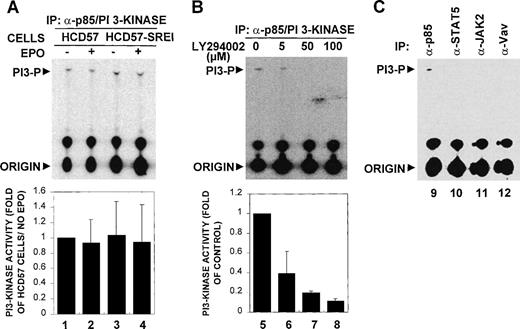
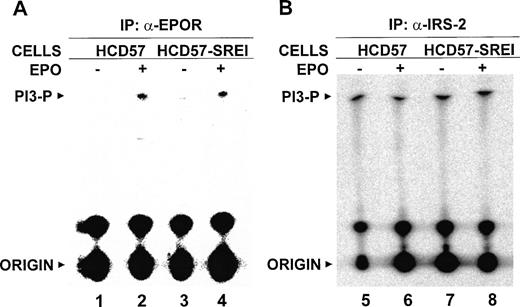
![Fig. 9. Effect of inhibition of PI3-kinase activity on the proliferation and apoptosis of HCD57 and HCD-SREI cells. (A) Cells cultured in the presence of 1 U EPO/mL (HCD57, ◊; HCD57-SREI, ⊖) or absence of EPO (HCD57-SREI, x) were treated with LY294002 at the indicated concentrations for 72 hours, and the viable cells were counted in the presence of trypan blue. Values are expressed as a percentage of the corresponding control (treated with vehicle dimethylsulfoxide [DMSO]). Data are means ± SE of at least triplicate measurements. (B) Cells were cultured in the presence of 1 U EPO/mL (HCD57) or absence of EPO (HCD57-SREI). HCD-57 (lanes 2 through 6) and HCD57-SREI (lanes 7 through 11) cells were treated with vehicle DMSO (lanes 3 and 8), LY294002 at indicated concentrations (lanes 4 through 6 and 9 through 11), or untreated (lanes 2 and 7) for 24 hours. The genomic DNA was isolated and analyzed for fragmentation of DNA characteristic of apoptosis as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3757/4/m_blod41134009ax.jpeg?Expires=1769322650&Signature=fvtH0OPcJBB-pRzP1KJwdkTZ3IGbQVjLhm8utMCkjGkjKCsLoITt2RWV~lhS5Zzgz33XjTA0RBXtVsR84~AG5tUMmhhWUgGPVXjk8GxyNR5CA91-lFqDnPN0wQrMMA1NLiSm5AaX-6S9q8aAIjkrVruDS8bxQXu64kQU6ucfKMvaQyZClW9lrcQWxcgCZz1tU6dWc1PABJ45z7hkU6tH40hXfVqQ~488VmwO7nnJcQFztlMtaTjBE5IYBu-11TcrqKyB-mkKWPo3ODwc2yotz0a9BYdTMyytYWyVsi7GGNfPXPauedkLUJIf3BpLYk7tCYI~0zNW~7SLC-jfTLjXdg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Effect of inhibition of PI3-kinase activity on the proliferation and apoptosis of HCD57 and HCD-SREI cells. (A) Cells cultured in the presence of 1 U EPO/mL (HCD57, ◊; HCD57-SREI, ⊖) or absence of EPO (HCD57-SREI, x) were treated with LY294002 at the indicated concentrations for 72 hours, and the viable cells were counted in the presence of trypan blue. Values are expressed as a percentage of the corresponding control (treated with vehicle dimethylsulfoxide [DMSO]). Data are means ± SE of at least triplicate measurements. (B) Cells were cultured in the presence of 1 U EPO/mL (HCD57) or absence of EPO (HCD57-SREI). HCD-57 (lanes 2 through 6) and HCD57-SREI (lanes 7 through 11) cells were treated with vehicle DMSO (lanes 3 and 8), LY294002 at indicated concentrations (lanes 4 through 6 and 9 through 11), or untreated (lanes 2 and 7) for 24 hours. The genomic DNA was isolated and analyzed for fragmentation of DNA characteristic of apoptosis as described in Materials and Methods.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3757/4/m_blod41134009bw.jpeg?Expires=1769322650&Signature=KW-RQX3SEKQCqgCTAahquermrC4lnk7wiEh3GWAjgSjHA4ktapmFHE9KPVvvh-07s1eNOd~p0XFgyS2Ry-DZ-KklT5RshdnZOS~19EPjSCPO2mVEv4fA5rfA4HOE0yVGs7AjWfm0K8FfP3vCVffHk971jiGjHTeE2BVpyvO-9DC8Q3HP-ZVMbEgS10a37pGwBOR-j2iLnvmKTatWzKxdkdPFXEYNCqPmhXh7nxmEoMUgFMmbMqKMHl1KwwunzVZGf72umTwPjLIM4etE6MzD3D1GMm5bAtOWKO1mEYZhapKgEBDKYjdTEQMvj8ij-Qpc4iHqgmDTt0t6EN4hH~TO0w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
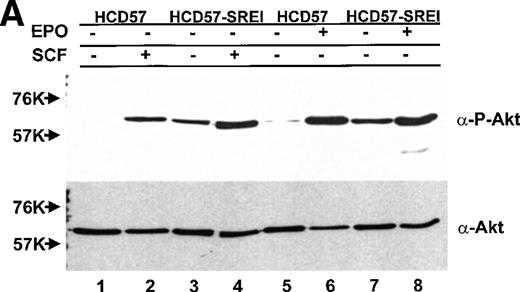
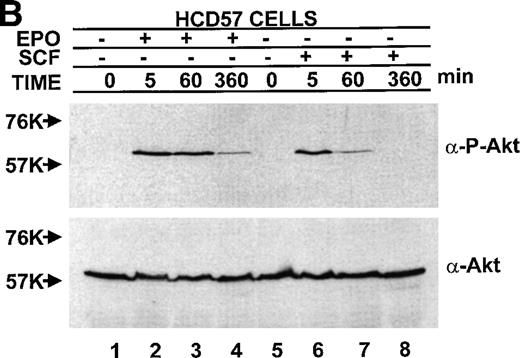
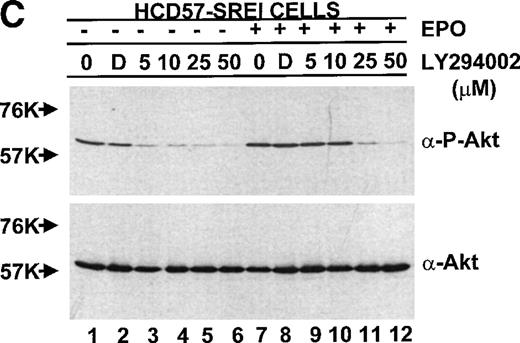
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal