Abstract
This report shows that interleukin-4 (IL-4), which plays a key role in regulating immune responses, fails to support cellular growth. We investigated whether this failure of IL-4 to promote growth was because of its unique inability to activate the Ras/Raf/Erk pathway. Consistent with other reports, expression in Ba/F3, a factor-dependent hematopoietic cell line, of either activated Q61KN-Ras or a hormone-inducible activated Raf-1, resulted in suppression of apoptosis but not in long-term growth. However, in the presence of IL-4, Ba/F3 cells that expressed either Q61KN-Ras or activated Raf-1 grew continuously at a rate comparable with that stimulated by IL-3. Investigation of the biochemical events associated with the stimulation of long-term growth showed that, as expected, the presence of activated Raf-1 resulted in an increased activity of extracellular signal regulated kinase (ERK) mitogen-activated protein kinase (MAPK) but not of c-jun N-terminal kinase/stress-activated protein kinase (JNK). However, surprisingly, if IL-4 was present, cells expressing active Raf-1 exhibited increases in JNK activity. These observations point to a novel mechanism for JNK activation involving synergy between Raf-1 and pathways activated by IL-4 and suggest that in hematopoietic cells proliferation is correlated not only with “mitogen activated” ERK activity, but also with JNK activity.
INTERLEUKIN-4 (IL-4) belongs to a molecular family, which includes hematopoietic growth factors, growth hormone, and leptin. IL-4 is produced by T cells and mast cells and acts on immune cells to promote an antibody-mediated immune response to infection. IL-4 was originally termed B-cell growth factor by virtue of its ability to enhance the proliferation of B cells, as well as their secretion of IgG1 and IgE.1 Subsequently, IL-4 was shown to also have growth-promoting effects on T cells and mast cells.2,3Although IL-4 is frequently classified as a growth factor, there is, in fact, little evidence that it can itself act as a mitogen. IL-4 was unable to induce resting B cells to leave G0 and enter the G1 phase of the cell cycle.4,5 Moreover, the reported enhancing effects on growth of primary T cells or mast cells were detected in short-term assays, which measured [3H]-thymidine incorporation, mitochondrial activity, or cell numbers at 24 to 48 hours.3 6
The lack of clear biological evidence that IL-4 is a growth factor correlates with the failure of IL-4 to activate Ras family members7,8 or the downstream kinase cascades. Although all three of the receptor subunits for IL-4 are members of the cytokine receptor superfamily, IL-4 (and the highly related IL-13) are atypical because they fail to activate the extracellular signal regulated kinase (ERK) cascade,9 the c-jun N-terminal kinase/stress-activated protein kinase (JNK) cascade,10 and the p38 mitogen-activated protein (MAP) kinase cascade.11 Activation of Ras and the MAP-family cascades can have varying effects on the cell cycle depending on the strength of the signal. In general, activation of Raf and the downstream kinases correlates with progression through the cell cycle.12Activation of ERK is an absolute requirement for both hematopoietic cells and fibroblasts to progress from G0 to S-phase,13 and overexpression of a constitutively active mutant of the upstream activator of ERK, MAP/ERK Kinase 1 (MEK1), promotes cell-cycle entry.14
IL-4 stimulates the phosphatidlyinositol-3′-kinase (PI3′kinase) pathway,15 perhaps through stimulation of phosphorylation of the insulin receptor substrates (IRS-1 and IRS-2), which can then associate with the p85 subunit of PI3′kinase.16,17 Activity of the PI3′-kinase pathway is required for IL-4 to protect cells from apoptosis18 and to upregulate levels of c-myc mRNA (Wieler and Schrader, submitted). Stimulation of cells with IL-4 also leads to activation of JAK-1 and JAK-3 or Tyk-2 and STAT-6.19 STAT-6 mediates the effects of IL-4 on the development of TH2 cells and the IgE class of antibodies20 and some of its anti-inflammatory effects of IL-4.21
We have confirmed the failure of IL-4 to promote cell-cycle entry and have hypothesized that this relates to its inability to activate the Ras/Raf/ERK pathway. We show here that the inability of IL-4 to promote cell-cycle progression is complemented by expression of activated mutants of N-Ras or conditionally active mutants of Raf-1. Surprisingly, we find that IL-4 and Raf not only synergize to promote proliferation, but also to increase JNK activity.
MATERIALS AND METHODS
PKH26 cell labeling.
Ba/F3 cell membranes were labeled with PKH26 according to the manufacturer’s directions (Sigma, Mississauga, Ontario, Canada). An aliquot of cells was analyzed by flow cytometry at time 0, and the remaining cells were incubated at 37°C in media supplemented with 2% (vol/vol) of a 10× concentrate of media conditioned by WEHI-3B (W3) or 3% (vol/vol) media conditioned by AgXO63 cells that had been transfected with the murine IL-4 cDNA (IL-4 CM).22 Aliquots of cells were analyzed by flow cytometry at 24, 48, and 72 hours after the addition of cytokine.
Recombinant plasmids.
The cDNA for Q61KN-Ras was a gift from Rob Kay (Terry Fox Labs, Vancouver, Canada). Polymerase chain reaction mutagenesis was used to replace the N-Ras ATG with a BamHI site to allow for in-frame fusion to a sequence encoding the hemagglutinin (HA) tag downstream of the cytomegalovirus promoter in pcHA (Giulio Superti-Furga, EMBL, Heidelberg, Germany). The fidelity of the cDNA was confirmed by sequencing. The cDNAs encoding ΔRaf-1:ER or ΔRaf-1:ER K70W in pBABEpuro23 were a gift from Martin McMahon (UCSF Cancer Center, San Francisco, CA). The ΔRaf-1:ER protein product consists of the enhanced green fluorescent protein (EGFP) at the N-terminus, which is fused to the kinase domain of human c-Raf-1 and, in turn, fused to the hormone binding domain of the human-estrogen receptor. The ΔRaf-1:ER K70W protein consists of the kinase domain of human c-Raf-1, which lacks the lysine residue critical for enzymatic activity, and is fused to the hormone binding domain of the estrogen receptor. The glutathione-S-transferase (GST)-JNK1 cDNA in pEFBOS was a gift from Leonard Zon.24
Cell culture and transfections.
Ba/F3 cells and transfectants were grown at 37°C in humidified incubators gassed with 5% CO2. Cells were routinely passaged in RPMI 1640 (GIBCO-BRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) (Intergen, Purchase, NY), 50 μmol/L 2-ME, and 2% (vol/vol) W3. Cells expressing ΔRaf-1:ER or ΔRaf-1:ER K70W were maintained as above, but in phenol-red free RPMI 1640 (GIBCO-BRL). For each transfection, cells were mixed with 15 μg of linearized vector and subjected to electroporation by using a Bio-Rad gene pulser at 960 μF and 270 V. Individual clones of neomycin-resistant (for Q61KN-Ras and GST-JNK1) or puromycin-resistant (for ΔRaf-1:ER or ΔRaf-1:ER K70W) cells were tested for expression of the exogenous cDNA of interest. Expression of Q61KN-Ras was evaluated by immunoblotting cell lysates with antibodies against the HA tag (12CA5; Boehringer Mannheim, Laval, Quebec, Canada). Expression of ΔRaf-1:ER was determined by flow-cytometric quantitation of EGFP levels. Expression of ΔRaf-1:ER K70W was determined by immunoprecipitation with antiestrogen receptor antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; no. sc543) and immunoblotting with an anti-Raf-1 antibody (Pharmingen, San Diego, CA). Several positive clones with similar levels of expression of the exogenous cDNA were derived in each case.
Cell viability and proliferation assays.
Proliferation and viability of cells was assessed by cell counting or [3H]-thymidine incorporation into de novo synthesized DNA. For cell counting experiments, the cells were incubated at 1 × 105 cells/mL at 37°C in complete media supplemented as indicated with 2% (vol/vol) W3, 2 μg/mL synthetic IL-4 (Ian Clark-Lewis, The Biomedical Research Centre, Vancover, British Columbia, Canada), or 3% (vol/vol) IL-4 CM, and/or 100 nmol/L 4-hydroxy-tamoxifen (4HT), (Research Biochemicals, Natick, MA). The 4HT was stored as a 100 μmol/L stock in 100% ethanol at −20°C. An equivalent volume of ethanol was added to one set of cultures to rule out solvent effects. Cells that excluded trypan blue were counted at the indicated times, and cultures were diluted as appropriate to maintain a consistent density. For [3H]-thymidine uptake assays, cells were plated at 250 cells per well in a Terasaki microtitre plate, incubated for 40 hours at 37°C in the indicated conditions, and then pulsed with 15 μCi/mL for a further 8 hours. Cells were harvested and incorporation of [3H]-thymidine assessed in a scintillation counter. Chemically synthesized IL-3 (Ian Clark-Lewis, The Biomedical Research Centre) was used at a concentration of 1 μg/mL shown to stimulate maximal proliferation of Ba/F3 cells. Chemically synthesized IL-4 was used as indicated at a saturating dose (2 μg/mL).
Immunoprecipitations, kinase assays, and immunoblotting.
To assess biochemical parameters under conditions in which cells were normally grown, cells were incubated at 37°C for 16 hours at an initial density of 2 × 105 cells/mL in a 40-mL volume of complete medium supplemented as indicated with IL-3 (2% W3), IL-4 (2 μg/mL synthetic IL-4 or 3% IL-4 CM), and/or 4HT (100 nmol/L 4HT). Cells were lysed in lysis buffer10 and the total amount of protein in each lysate was determined by the BCA protein assay (Pierce, Rockford, IL). Normalized amounts of protein were subjected to immunoprecipitation and kinase activity was determined in vitro as previously described.10 ΔRaf-1:ER was immunoprecipitated with an antiestrogen receptor antibody (Santa Cruz no. sc543) coupled to protein A-Sepharose. For assay of Raf activity, the kinase assay buffer (KAB) consisted of 25 mmol/L HEPES, 10 mmol/L MgCl2, 1 mmol/L MnCl2, and 1 mmol/L DTT. The reaction was initiated by the addition of 0.5 μg GST-MEK1 (UBI, Lake Placid, NY), 1 μM cold ATP and 10 μCi of [γ-32P]ATP, and incubated for 30 minutes at 30°C. ERK-1 and ERK-2 were immunoprecipitated with agarose-conjugated antibodies (Santa Cruz no. sc154). To assay ERK activity, the KAB consisted of 20 mmol/L HEPES, 5 mmol/L MgCl2, 5 mmol/L EGTA, 50 mmol/L β-glycerol phosphate, 2 mmol/L sodium vanadate, and 5 mmol/L 2-ME. The reaction was initiated by the addition of 15 μg Myelin Basic Protein (MBP) (Sigma) and 10 μCi of [γ-32P]ATP and incubated for 10 minutes at 30°C. JNK-1 was immunoprecipitated with agarose-conjugated antibodies (Santa Cruz Biotechnology, catalogue no. sc474). To assay JNK activity, the KAB consisted of 25 mmol/L HEPES, 25 mmol/L MgCl2, 2 mmol/L DTT, 50 mmol/L β-glycerol phosphate, and 0.5 mmol/L sodium vanadate. The reaction was initiated by addition of 1 μg GST-cJun and 10 μCi of [γ-32P]ATP and incubated for 20 minutes at 30°C. Reactions were stopped by addition of sodium dodecyl sulfate sample buffer. The eluate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and phosphorylated proteins were detected by autoradiography. To assess equivalency of loading, membranes were immunoblotted with either an anti-ERK-2 antibody (Santa Cruz no. sc94), an anti-JNK-1 antibody (Santa Cruz no. sc1648), an anti-GST antibody (Molecular Probes) or an anti-GFP antibody (Clontech, Palo Alto, CA).
RESULTS
IL-4 is not a true growth factor in Ba/F3 cells.
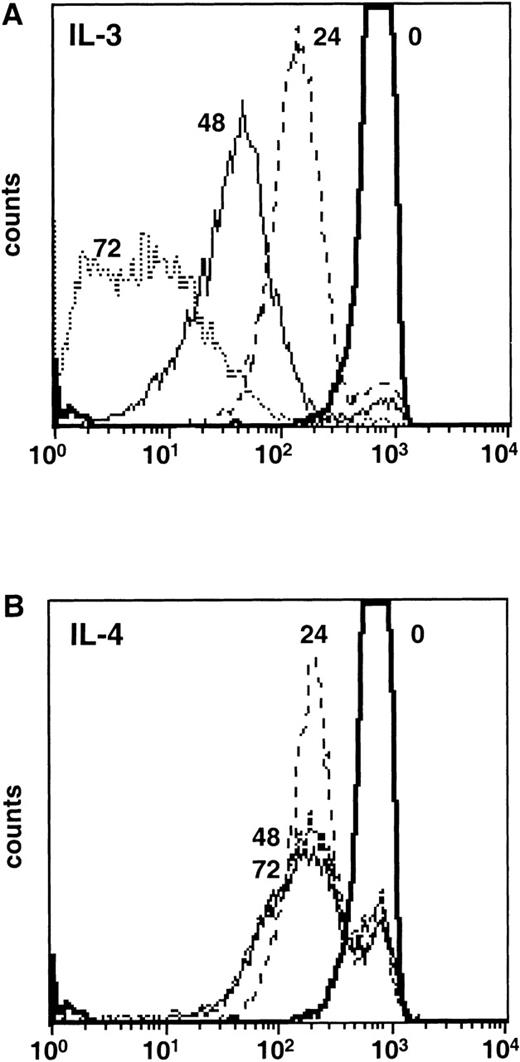
The addition of IL-4 to cultures of primary mast cells, T cells, B cells, or factor-dependent hematopoietic cell lines over a period of 24 to 48 hours results in increased survival and incorporation of [3H]-thymidine.3,6 However, there is no sustained increase in cell numbers and the cells eventually die. These observations are compatible with the notions that either IL-4 promoted short-term survival and cells that had previously entered the cell cycle were permitted to complete DNA synthesis, or alternatively, that IL-4 was indeed able to stimulate cell-cycle progression and growth in a fraction of cells, but failed to support their long-term survival. To distinguish between these possibilities, we exploited a technique based on labeling the membranes of cells with a fluorescent dye (PKH26) that cannot be transferred to neighboring cells but is shared between daughter cells.25 Flow-cytometric analysis of labeled Ba/F3 cells that were growing in IL-3 for increasing periods of time showed the expected series of peaks corresponding to dilution of the dye by cell division (Fig 1A). In contrast, analysis of labeled Ba/F3 cells that were cultured for 3 days in IL-4 showed a large peak (66%; Fig 1B) of cells that corresponded to cells that had completed one cell division with no peaks corresponding to two or more cell divisions. These data show that IL-4 is unable to stimulate the repeated entry of cells into cycle.
IL-4 is not a growth factor in Ba/F3 cells. Ba/F3 cells were labeled with the fluorescent dye PKH26. Immediately after labeling, incorporation of the dye was analyzed by flow cytometry (time 0). Cells were cultured in complete media supplemented with (A) IL-3 or (B) IL-4 and aliquots of cells were analyzed by flow cytometry at 24, 48, and 72 hours to determine the amount of dye dilution.
IL-4 is not a growth factor in Ba/F3 cells. Ba/F3 cells were labeled with the fluorescent dye PKH26. Immediately after labeling, incorporation of the dye was analyzed by flow cytometry (time 0). Cells were cultured in complete media supplemented with (A) IL-3 or (B) IL-4 and aliquots of cells were analyzed by flow cytometry at 24, 48, and 72 hours to determine the amount of dye dilution.
IL-4 synergizes with Ras to stimulate long-term proliferation.
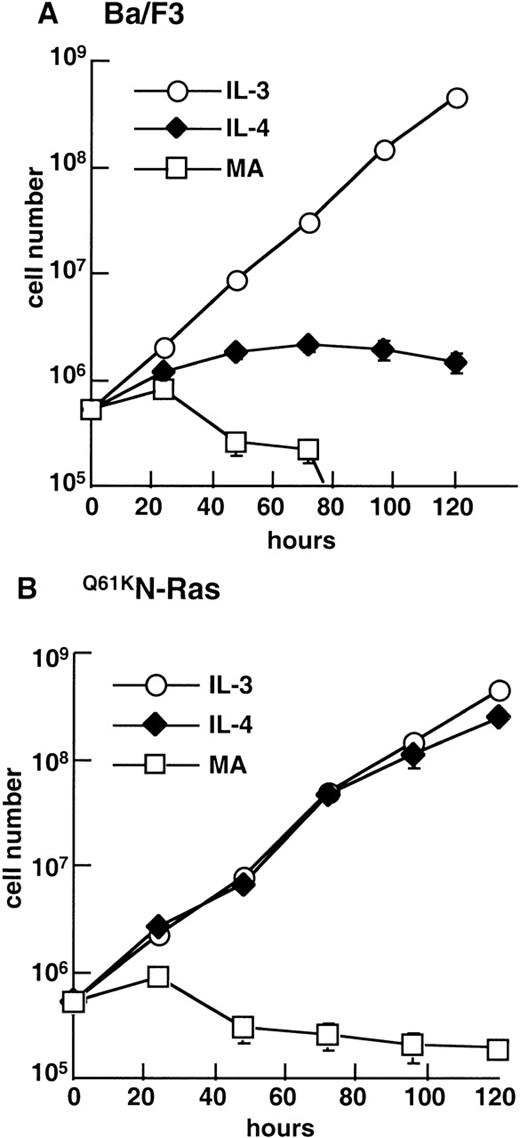
To test the hypothesis that the failure of IL-4 to promote the long-term growth of hematopoietic cells rests solely in its inability to stimulate Ras activity, we generated stable clones of Ba/F3 cells that expressed an activated mutant of N-Ras (Q61KN-Ras), fused to an N-terminal HA epitope-tag. We compared the ability of parental Ba/F3 cells (Fig 2A) and Ba/F3 cells expressing Q61KN-Ras (Q61KN-Ras cells) to proliferate in the presence or absence of IL-4 by counting cells over a period of 5 days. Q61KN-Ras cells failed to grow in the absence of IL-3 (Fig 2B), although, unlike parental Ba/F3 cells, which were all dead by 96 hours, they did not undergo apoptosis in the absence of factor over the 5-day duration of the experiment. In striking contrast to parental Ba/F3, in the presence of IL-4,Q61KN-Ras cells grew exponentially at a rate comparable with those of parental or Q61KN-Ras cells in IL-3 (Fig 2B).
Activated Q61KN-Ras in combination with IL-4 stimulates the long-term growth of Ba/F3 cells. (A) Ba/F3 or (B)Q61KN-Ras cells were washed free of IL-3 and incubated in IL-3, IL-4, or media alone with no factor (MA) at a density of 1 × 105 cells/mL. Cells were counted in triplicate at the indicated times and diluted as appropriate to maintain a consistent density of cells. The results are representative of two independent experiments, with two independent clones. Error bars represent the SEM of triplicate samples.
Activated Q61KN-Ras in combination with IL-4 stimulates the long-term growth of Ba/F3 cells. (A) Ba/F3 or (B)Q61KN-Ras cells were washed free of IL-3 and incubated in IL-3, IL-4, or media alone with no factor (MA) at a density of 1 × 105 cells/mL. Cells were counted in triplicate at the indicated times and diluted as appropriate to maintain a consistent density of cells. The results are representative of two independent experiments, with two independent clones. Error bars represent the SEM of triplicate samples.
IL-4 synergizes with Raf-1 to promote long-term proliferation.
There is evidence that Ras is upstream of the three MAP-kinase family cascades,26 as well as the PI3′-kinase pathway.27 Because IL-4 activates the PI3′-kinase pathway,15 but fails to activate Raf-1 or any of the three MAP-family kinases, ERK, JNK or p38 MAP-kinase,9-11 we focused on these. As activation of the Raf/ERK pathway has been strongly correlated with growth, we first asked whether the synergistic effect of an activated Ras on signals provided by IL-4 could be replaced by expression of activated Raf-1 protein. We expressed a fusion protein consisting of an active fragment of human Raf-1 fused to an enhanced EGFP and the hormone-binding domain of the estrogen receptor, so that kinase activity could be induced by addition of estradiol or an analog, 4HT. When expressed in NIH-3T3 cells and activated by 4HT this protein results in transformation and rapid activation of MEK1 and ERK.28 We expressed the cDNA in Ba/F3 cells and individual clones (hereafter referred to as ΔRaf-1:ER cells) were obtained and screened for EGFP by flow cytometry. Consistent with observations in NIH-3T3 cells,29 expression of ΔRaf-1:ER increased 3- to 5-fold after overnight incubation in 100 nmol/L 4HT (data not shown). As documented below, enhanced expression of ΔRaf1:ER was also accompanied by elevated Raf-1 activity.
We assayed [3H]-thymidine uptake to assess the effects of activation of ΔRaf-1:ER by increasing concentrations of 4HT (Fig3A). Even at high concentrations, 4HT had only a small effect on DNA synthesis. In contrast, in the presence of IL-4, the addition of 4HT resulted in a dose dependent, synergistic stimulation of DNA synthesis. The maximal biological response to 4HT in the presence of saturating levels of IL-4 was observed at 100 nmol/L, and this concentration was used for all subsequent experiments. Parental Ba/F3 cells failed to respond to 4HT alone or in combination with IL-4 (Fig 3A).
Activation of ▵Raf-1:ER in combination with IL-4 stimulates DNA synthesis and the long-term growth of Ba/F3 cells. (A) Ba/F3 or ▵Raf-1:ER cells were washed free of IL-3 and plated at 250 cells/well in a Terasaki microtitre plate in medium alone or IL-4, with increasing concentrations of 4HT. After 40 hours, the cells were pulsed with [3H]-thymidine for an additional 8 hours, harvested, and counted in a scintillation counter. (B) ▵Raf-1:ER or (C) ▵Raf-1:ER K70W cells were washed free of IL-3 and incubated in IL-3, IL-4, 100 nmol/L 4HT, IL-4 plus 100 nmol/L 4HT or without factor (MA) at a density of 1 × 105 cells/mL. Cells were counted in triplicate at the indicated times and diluted as appropriate to maintain a consistent density. The results are representative of several independent experiments, with two independent clones. Error bars represent the SEM of triplicate samples.
Activation of ▵Raf-1:ER in combination with IL-4 stimulates DNA synthesis and the long-term growth of Ba/F3 cells. (A) Ba/F3 or ▵Raf-1:ER cells were washed free of IL-3 and plated at 250 cells/well in a Terasaki microtitre plate in medium alone or IL-4, with increasing concentrations of 4HT. After 40 hours, the cells were pulsed with [3H]-thymidine for an additional 8 hours, harvested, and counted in a scintillation counter. (B) ▵Raf-1:ER or (C) ▵Raf-1:ER K70W cells were washed free of IL-3 and incubated in IL-3, IL-4, 100 nmol/L 4HT, IL-4 plus 100 nmol/L 4HT or without factor (MA) at a density of 1 × 105 cells/mL. Cells were counted in triplicate at the indicated times and diluted as appropriate to maintain a consistent density. The results are representative of several independent experiments, with two independent clones. Error bars represent the SEM of triplicate samples.
Next, we investigated whether Raf-1 activity and IL-4 synergized to support long-term proliferation of ΔRaf-1:ER cells by counting cells over a period of 4 days. As shown in Fig 3B, ΔRaf-1:ER cells cultured in media alone rapidly declined in number, and this decline could be delayed by the presence of IL-4. The induction of Raf-1 activity by the presence of 4HT also prevented the decline in cell numbers seen in media alone, and the number of cells increased slowly. In contrast, the combination of 4HT and IL-4 resulted in a dramatic, synergistic stimulation of cell growth that closely resembled that seen with the combination of IL-4 and activated Ras (Fig 2B). To confirm that the synergistic proliferation in the presence of active Raf-1 and IL-4 was dependent on Raf-1 kinase activity, we repeated the experiment with stable clones of Ba/F3 cells that expressed a kinase inactive form of ΔRaf-1:ER (ΔRaf-1:ER K70W cells). ΔRaf-1:ER K70W cells in the absence of factor or in IL-4 died at the same rate as parental Ba/F3 cells whether or not 4HT was present (Fig 3C). Thus, the synergistic proliferation induced by active Raf-1 and IL-4 was dependent on Raf-1 kinase activity. Furthermore, as described below, this synergistic proliferation was dependent on the activity of kinases downstream of Raf-1 as addition of the MEK1/2 inhibitor PD90859 inhibited the proliferation of ΔRaf-1:ER cultured in IL-4 and 4HT.
Stimulation of ΔRaf-1:ER activity leads to ERK activation.
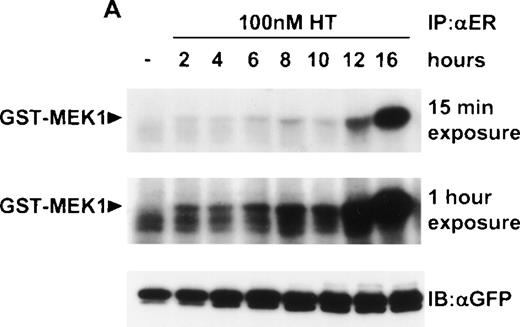
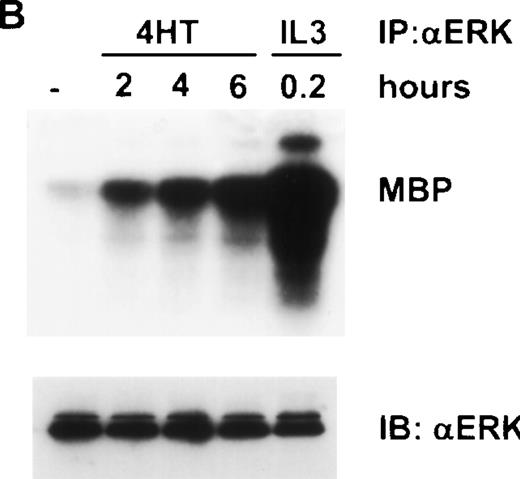
These results suggested that the relevant pathway downstream of Ras that synergized with IL-4 was indeed the Raf/MEK/ERK pathway. To confirm this notion at a biochemical level, we examined the activity of Raf and ERK in ΔRaf-1:ER cells after the addition of 4HT. We observed an increase in activity of ΔRaf-1:ER at 2 hours after the addition of 4HT (Fig 4A). However, enzymatic activity of ΔRaf-1:ER continued to increase, peaking 12 to 16 hours after the addition of 4HT (Fig 4A). Similarly, we detected a significant increase in ERK activity 2 hours after the addition of 4HT, and this activity continued to increase with time (Fig 4B). We performed all subsequent experiments on cells incubated with 4HT for 16 hours to ensure maximal Raf kinase activity. To allow comparison with parental Ba/F3 cells, which in the absence of IL-3 were only viable over a 16-hour period if IL-4 were present, we normally assessed the effect of 4HT in the presence of IL-4. Stimulation of ERK activity in ΔRaf-1:ER cells by addition of 4HT was dose dependent (Fig5) and occurred at concentrations of 4HT above 25 nmol/L. Parental Ba/F3 or ΔRaf-1:ER K70W cells did not show any increase in ERK activity after stimulation with 4HT (data not shown). Ba/F3 or ΔRaf-1:ER cells that had been stimulated with IL-3 or IL-4 for 16 hours showed no detectable levels of ERK activity (Fig5; data not shown).
Addition of 4HT activates Raf-1 and ERK kinase activity. ▵Raf-1:ER cells were stimulated at 37°C in RPMI, 10% FCS and 100 nmol/L 4HT for the indicated time. The cells were lysed and analyzed for (A) Raf-1 activity by immunoprecipitation with an antiestrogen receptor antibody followed by an in vitro kinase assay by using GST-MEK1 as a substrate or (B) ERK activity by immunoprecipitation with an anti-ERK antibody, followed by an in vitro kinase assay by using MBP as a substrate. Positive control cells were stimulated with IL-3 for 10 minutes. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. A 15 minute and a 1-hour exposure are shown to clearly demonstrate the activity of ▵Raf-1:ER at both the 16 and 2 hour time points. The quantity of immunoprecipitated (IP) protein in each lane was assessed by immunoblotting (IB) with antibodies against GFP (GFP) or ERK (ERK).
Addition of 4HT activates Raf-1 and ERK kinase activity. ▵Raf-1:ER cells were stimulated at 37°C in RPMI, 10% FCS and 100 nmol/L 4HT for the indicated time. The cells were lysed and analyzed for (A) Raf-1 activity by immunoprecipitation with an antiestrogen receptor antibody followed by an in vitro kinase assay by using GST-MEK1 as a substrate or (B) ERK activity by immunoprecipitation with an anti-ERK antibody, followed by an in vitro kinase assay by using MBP as a substrate. Positive control cells were stimulated with IL-3 for 10 minutes. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. A 15 minute and a 1-hour exposure are shown to clearly demonstrate the activity of ▵Raf-1:ER at both the 16 and 2 hour time points. The quantity of immunoprecipitated (IP) protein in each lane was assessed by immunoblotting (IB) with antibodies against GFP (GFP) or ERK (ERK).
Dose-dependent activation of ERK in ▵Raf-1:ER cells after stimulation with 4HT for 16 hours. ▵Raf-1:ER cells were stimulated at 37°C for 16 hours at 2 × 105 cells/mL in RPMI, 10% FCS plus IL-3, IL-4, or IL-4 plus increasing concentrations of 4HT. ERK was IP from the cell lysate and kinase activity was determined in vitro by using MBP as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The quantity of IP protein in each lane was assessed by IB with antibodies against ERK (ERK).
Dose-dependent activation of ERK in ▵Raf-1:ER cells after stimulation with 4HT for 16 hours. ▵Raf-1:ER cells were stimulated at 37°C for 16 hours at 2 × 105 cells/mL in RPMI, 10% FCS plus IL-3, IL-4, or IL-4 plus increasing concentrations of 4HT. ERK was IP from the cell lysate and kinase activity was determined in vitro by using MBP as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The quantity of IP protein in each lane was assessed by IB with antibodies against ERK (ERK).
To determine whether the activation of ERK that we observed in ΔRaf-1:ER cells stimulated with 4HT was critical for the proliferative activity seen in the presence of IL-4, we used an inhibitor of MEK1/2, PD90859. ΔRaf-1:ER cells were incubated in 4HT and IL-4 for 16 hours and PD90859 (30 μmol/L) was added 1 hour before lysis of cell. This resulted in complete inhibition of the activation of ERK (data not shown). Importantly, PD90859 also induced a dose-dependent inhibition of the proliferation of ΔRaf-1:ER cells stimulated by 4HT and IL-4 (data not shown).
Stimulation of ΔRaf-1:ER activity leads to JNK activation.
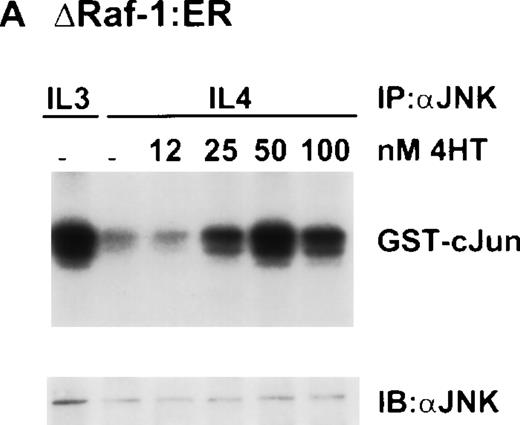
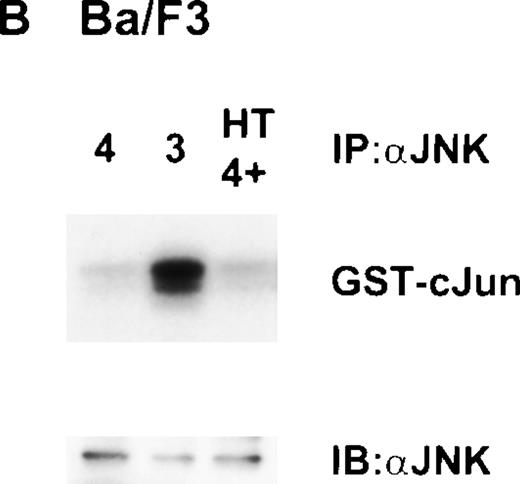
Other work from our laboratory has shown that growth stimuli like IL-3 or Steel Locus factor induced activation of JNK, although IL-4 did not,10 thus correlating the ability of a growth factor to stimulate JNK with its ability to stimulate growth. Therefore, we investigated the activity of JNK in ΔRaf-1:ER cells. Surprisingly, stimulation of ΔRaf-1:ER cells with 4HT for 16 hours in the presence of IL-4, resulted in dose-dependent activation of JNK (Fig6A). Our observation that expression of active Raf-1 in these conditions resulted in activation of JNK was unexpected as many investigators have shown JNK to be downstream of MEK-kinase 1 (MEKK1), but not of Raf-1,30 and IL-4 does not activate JNK.10 JNK was originally identified as a stress-activated kinase,31 and we needed to rule out the possibility that activation of JNK resulted from stress induced by the addition of 4HT. Therefore, we evaluated the response to 4HT in parental Ba/F3 cells incubated for 16 hours in the presence of IL-4. In three independent experiments, we failed to see increased JNK activity (Fig 6B).
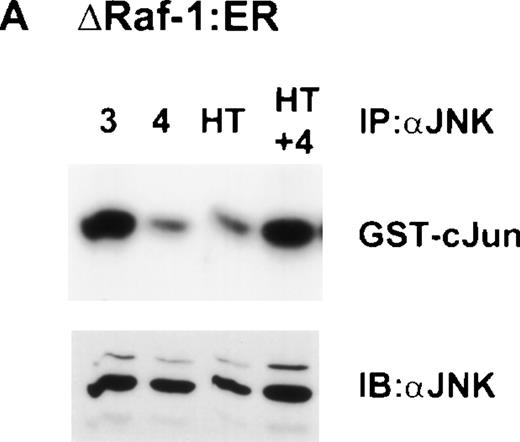
Dose-dependent activation of JNK in ▵Raf-1:ER but not Ba/F3 cells after stimulation with 4HT for 16 hours. (A) ▵Raf-1:ER cells were stimulated at 37°C for 16 hours at 2 × 105cells/mL in RPMI, 10% FCS plus IL-3, IL-4, or IL-4 plus increasing concentrations of 4HT. (B) Ba/F3 cells were stimulated for 16 hours at 37°C in RPMI supplemented with 10% FCS and IL-3 (3), IL-4 (4), or IL-4 plus 100 nmol/L 4HT (4 + HT). JNK was IP from the cell lysate, and kinase activity was determined in vitro by using GST-cJun as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The quantity of immunoprecipitated protein in each lane was assessed by immunoblotting (IB) with antibodies against JNK (JNK).
Dose-dependent activation of JNK in ▵Raf-1:ER but not Ba/F3 cells after stimulation with 4HT for 16 hours. (A) ▵Raf-1:ER cells were stimulated at 37°C for 16 hours at 2 × 105cells/mL in RPMI, 10% FCS plus IL-3, IL-4, or IL-4 plus increasing concentrations of 4HT. (B) Ba/F3 cells were stimulated for 16 hours at 37°C in RPMI supplemented with 10% FCS and IL-3 (3), IL-4 (4), or IL-4 plus 100 nmol/L 4HT (4 + HT). JNK was IP from the cell lysate, and kinase activity was determined in vitro by using GST-cJun as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The quantity of immunoprecipitated protein in each lane was assessed by immunoblotting (IB) with antibodies against JNK (JNK).
We were interested to know if MEK1/2 activity was required for this unexpected activation of JNK. However, in contrast to our observations on the activation of ERK, the effect of PD90859 on JNK activation in ΔRaf-1:ER cells cultured overnight in 4HT and IL-4 was inconsistent. In some experiments PD90859 had no effect on JNK activation, whereas in others the presence of PD90859 inhibited the increase JNK activity stimulated by 4HT in the presence of IL-4 (data not shown). It is possible that the presence of PD90859 over a 16-hour period had itself a tendency to stress the cells and that this was a factor in the experiments in which addition of PD98059 did not block activation of JNK.
Activation of JNK is not due to the production of an autocrine factor.
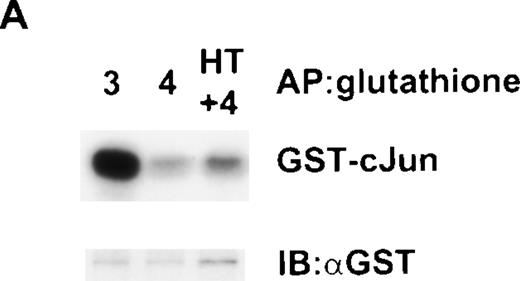
Activation of JNK has been reported in NIH-3T3 cells expressing activated ΔRaf-1:ER and was due to an autocrine production of heparin-binding epidermal growth factor.30,32,33 Ba/F3 cells do not express receptors for EGF,34 but we were interested to know whether the activation of JNK we observed was caused by another autocrine pathway. To address this question, we collected medium from ΔRaf-1:ER cells that had been stimulated with 4HT for 48 hours and concentrated it 10-fold. We incubated cells with this medium, starting with a concentration of 50% and observed that it had no ability to suppress apoptosis or promote growth of Ba/F3 cells, either alone or in combination with IL-4 (data not shown). Moreover, incubation of Ba/F3 cells for 16 hours in this conditioned medium failed to result in activation of JNK (data not shown). However, this approach does not exclude the possibility of an autocrine factor that was cell-surface bound or extremely labile. To address this possibility, we first made stable clones of Ba/F3 cells that expressed JNK-1 that was tagged with GST at the N-terminus so that it was distinguishable from the endogenous JNK in Ba/F3 cells. We mixed 1 × 106 of these Ba/F3 GST-JNK-1 cells together with 1 × 107 ΔRaf-1:ER cells and cultured the mixture of cells for 16 hours with IL-3, IL-4, or IL-4 plus 4HT. We used a 10:1 ratio of ΔRaf-1:ER cells to Ba/F3 GST-JNK-1 cells to maximize the possibility of detecting an autocrine factor. We then lysed the mixture of cells and, using glutathione Sepharose beads, specifically precipitated GST-JNK-1 from the Ba/F3 GST-JNK-1 cells. As shown in Fig7A, this GST-JNK-1 from the Ba/F3 GST-JNK-1 cells that had been cultured together with a majority of ΔRaf-1:ER cells stimulated with IL-4 plus 4HT was not activated. In contrast, analysis of GST-JNK-1 from control mixed cultures that had been stimulated with IL-3 showed the expected activation of GST-JNK-1. After depletion of GST-JNK-1 from the lysates, we were able to determine the activity of the total endogenous JNK-1 in the mixed lysate by immunoprecipitation with an anti–JNK-1 antibody. These immunoprecipitates contained JNK from both ΔRaf-1:ER and Ba/F3 GST-JNK-1 cells, but because there was a great excess of ΔRaf-1:ER cells, the signal from ΔRaf-1:ER cells predominated. As shown in Fig7B, endogenous JNK was activated in the mixed cultures that were stimulated with IL4 and 4HT, reflecting the activation of JNK-1 in ΔRaf-1:ER cells we had previously observed (Fig 6A). The data presented in Fig 7, thus, provide strong evidence that the activation of JNK induced in ΔRaf-1:ER cells cultured with IL-4 and 4HT was not secondary to the production of an autocrine factor.
Failure to detect an autocrine factor that can activate JNK in a cell-mixing experiment. Cells (1 × 106 Ba/F3) expressing GST-JNK1 were mixed together with 1 × 107▵Raf-1:ER cells and stimulated for 16 hours at 37°C in RMPI, 10% FCS with either IL-3 (3), IL-4 (4), or IL-4 plus 100 nmol/L 4HT (4 + HT). (A) GST-JNK1 was affinity purified (AP) from the mixed cell lysate with glutathione Sepharose beads, and the activity was determined in vitro assay by using GST-cJun as a substrate. After SDS-PAGE and autoradiography, the membrane was immunoblotted with antibodies against GST to assess equivalency of loading (GST). (B) After the lysate had been cleared with glutathione Sepharose, endogenous JNK was IP with anti-JNK1 antibodies, and the kinase activity was determined in vitro by using GST-cJun as a substrate. After SDS-PAGE and autoradiography, the membrane was immunoblotted with antibodies against JNK1 (JNK) to assess equivalency of loading.
Failure to detect an autocrine factor that can activate JNK in a cell-mixing experiment. Cells (1 × 106 Ba/F3) expressing GST-JNK1 were mixed together with 1 × 107▵Raf-1:ER cells and stimulated for 16 hours at 37°C in RMPI, 10% FCS with either IL-3 (3), IL-4 (4), or IL-4 plus 100 nmol/L 4HT (4 + HT). (A) GST-JNK1 was affinity purified (AP) from the mixed cell lysate with glutathione Sepharose beads, and the activity was determined in vitro assay by using GST-cJun as a substrate. After SDS-PAGE and autoradiography, the membrane was immunoblotted with antibodies against GST to assess equivalency of loading (GST). (B) After the lysate had been cleared with glutathione Sepharose, endogenous JNK was IP with anti-JNK1 antibodies, and the kinase activity was determined in vitro by using GST-cJun as a substrate. After SDS-PAGE and autoradiography, the membrane was immunoblotted with antibodies against JNK1 (JNK) to assess equivalency of loading.
IL-4 and Raf-1 synergize to activate JNK.
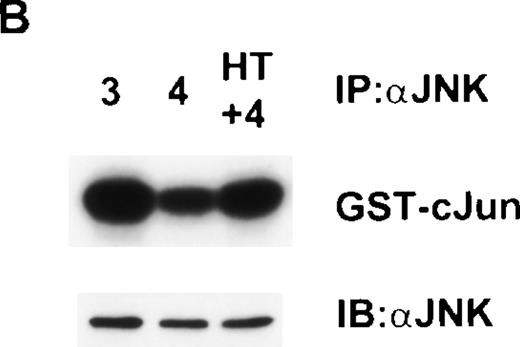
In the experiments described above, IL-4 was always included on the basis that in its absence the control, parental Ba/F3 cells died over the 16-hour period of the experiment. However, as shown in Fig 3B, ΔRaf-1:ER cells were viable when cultured in 4HT alone. Therefore, we were able to ask whether 4HT-induced Raf-1 activity alone was sufficient to induce JNK activity or whether addition of IL-4 was also necessary. To our surprise, although incubation of ΔRaf-1:ER cells for 16 hours with 4HT alone induced maximal activity of Raf-1 and Erk (Fig 4), JNK was only activated when both IL-4 and 4HT were present (Fig 8A). To confirm that the stimulation of JNK activity was dependent on Raf-1 kinase activity, we repeated the experiment in ΔRaf-1:ER K70W cells. The results showed that like the synergistic stimulation of growth, the synergistic activation of JNK by Raf-1 and IL-4 was dependent on kinase activity of the ΔRaf-1:ER protein (Fig 8B). To investigate the kinetics of this synergy, we stimulated ΔRaf-1:ER cells with 4HT for 16 hours to ensure maximal levels of ΔRaf-1:ER protein and activity and then acutely stimulated the cells for 10 minutes with IL-3 or IL-4 (Fig9A). Stimulation of these cells with IL-4 for 10 minutes failed to induce JNK activity, whereas control cells stimulated with IL-3 for 10 minutes exhibited activation of JNK (Fig9B).
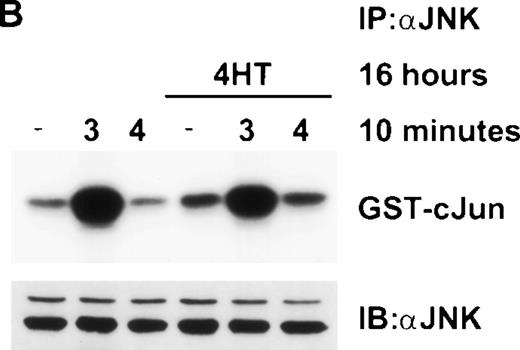
Raf-1 and IL-4 synergize to activate JNK. (A) ▵Raf-1:ER or (B) ▵Raf-1:ER K70W cells were stimulated for 16 hours at 37°C in RPMI, 10% FCS supplemented with IL-3 (3), IL-4 (4), 100 nmol/L 4HT (4HT), or both IL-4 and 100 nmol/L 4HT (4 + HT). JNK-1 was IP and JNK activity was determined in vitro by using GST-cJun as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The membranes were immunoblotted with anti-JNK antibodies to assess equivalency of loading (JNK).
Raf-1 and IL-4 synergize to activate JNK. (A) ▵Raf-1:ER or (B) ▵Raf-1:ER K70W cells were stimulated for 16 hours at 37°C in RPMI, 10% FCS supplemented with IL-3 (3), IL-4 (4), 100 nmol/L 4HT (4HT), or both IL-4 and 100 nmol/L 4HT (4 + HT). JNK-1 was IP and JNK activity was determined in vitro by using GST-cJun as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The membranes were immunoblotted with anti-JNK antibodies to assess equivalency of loading (JNK).
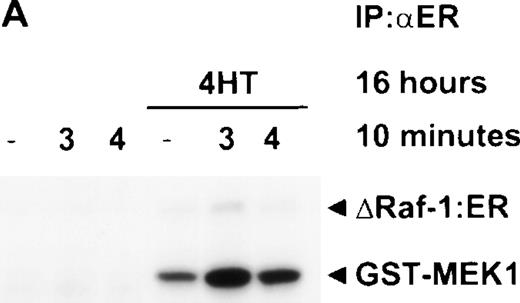
Stimulation with IL-4 for 10 minutes in the presence of activated Raf-1 is not sufficient to activate JNK. ▵Raf-1:ER cells were stimulated for 16 hours at 37°C in RPMI, 10% FCS with or without 100 nmol/L 4HT. The cells were then stimulated for 10 minutes with IL-3 (3) or IL-4 (4) or left untreated as a control (−). (A) ▵Raf-1:ER was IP with antiestrogen receptor antibodies and kinase activity was determined in vitro by using GST-MEK1 as a substrate. (B) JNK was IP with anti-JNK1 antibodies and kinase activity was determined in vitro by using GST-cJun as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The membrane was subsequently immunoblotted with an anti-JNK1 antibody to determine equivalency of loading.
Stimulation with IL-4 for 10 minutes in the presence of activated Raf-1 is not sufficient to activate JNK. ▵Raf-1:ER cells were stimulated for 16 hours at 37°C in RPMI, 10% FCS with or without 100 nmol/L 4HT. The cells were then stimulated for 10 minutes with IL-3 (3) or IL-4 (4) or left untreated as a control (−). (A) ▵Raf-1:ER was IP with antiestrogen receptor antibodies and kinase activity was determined in vitro by using GST-MEK1 as a substrate. (B) JNK was IP with anti-JNK1 antibodies and kinase activity was determined in vitro by using GST-cJun as a substrate. Phosphorylated proteins were visualized after SDS-PAGE and autoradiography. The membrane was subsequently immunoblotted with an anti-JNK1 antibody to determine equivalency of loading.
DISCUSSION
Here we show that IL-4 is not a true growth factor in Ba/F3 cells (Fig1) and present evidence that this is due to its inability to activate the Ras pathway, and, in particular, the Raf-1 kinase. We have demonstrated that, whereas neither activated Ras (Fig 2B) nor Raf-1 (Fig 3A and B) alone could stimulate proliferation, either could synergize with IL-4 and support long-term growth (Figs 2B and 3). Our data support the notion that activation of Ras and Raf are necessary, but not sufficient, for cell-cycle progression13 and are consistent with other reports that expression of activated Ras or Raf did not lead to factor independent growth of hematopoietic cells, but were able to inhibit apoptosis induced by withdrawal of IL-3.35 36
The Raf/MEK/ERK pathway is a key regulator not only of proliferation, but also of differentiation.12 Recently, it has become clear that the intensity of the Raf signal determines whether a cell will divide or arrest.23,37 Our results in hematopoietic cells are consistent with this concept, as activation of ΔRaf-1:ER is clearly necessary for cell division (Fig 3). Moreover, when we expressed a more highly active version of ΔRaf-1:ER (ΔRaf-1:ER Y340D, Y341D23), which led to a higher level of ERK activity, we observed cell-cycle arrest, even in the presence of IL-3 or IL-4 (data not shown).
The role of JNK in cell-cycle progression is less clear, but it is clearly activated not only by stress, but also by growth stimuli.10 In our system, activation of JNK was not sufficient for cell-cycle progression in the presence of IL-4 as expression of an activated V12Rac-1, which activates JNK in Ba/F3 cells,38 did not synergize with IL-4 to support the long-term growth of Ba/F3 cells (M.K. Levings, R.A. Salmon, Y. Quin, and J.W. Schrader, unpublished data).
Two signaling pathways activated by IL-4, the PI3′K pathway and the Jak-STAT pathway, may synergize with Raf-1 activity to promote growth. STAT6 is required for the downregulation of the cell-cycle inhibitor p27Kip1 by IL-4.39 The PI3′kinase pathway promotes cell survival,40 but this effect is unlikely to be the basis of the synergy with Raf-1 as cells expressing active Raf-1 alone exhibit increased survival. Recently, we have shown that IL-4 upregulates levels of c-myc mRNA through a PI3′kinase-dependent mechanism (Wieler and Schrader, submitted). This IL-4-dependent increase in c-myc mRNA is a good candidate for an IL-4–dependent pathway that could synergize with Raf-1 to promote growth.
We were surprised to find that whereas ERK activity was not detectable above control levels in cells growing in IL-3 (Fig 5), these cells exhibited levels of JNK activity equivalent to ΔRaf-1:ER cells that had been stimulated with IL-4 plus 4HT (Figs 6 through 8). These data show that, at least in Ba/F3 cells growing in mitogenic concentrations of IL-3, high levels of JNK activity do not inhibit cell-cycle progression or induce apoptosis, and, in fact, correlate with proliferation. However, the high level of JNK activity we observed in parental Ba/F3 cells is not a general phenomenon. Thus under similar conditions, other IL-3–dependent cell lines, IL-3–dependent primary mast cells, or IL-2–dependent primary T cells have undetectable levels of JNK activity (data not shown). One potential explanation for the discrepancy in levels of JNK activity may be that Ba/F3 cells do not express a critical phosphatase such as M3/6 that normally downregulates JNK activity.41
The activation of JNK after stimulation of Raf-1 activity and addition of IL-4 was unexpected as neither Raf nor IL-4 alone are able to activate upstream activators of JNK.10,30 38 We were unable to find any evidence to suggest that activation of JNK was caused by stress or the production of an autocrine factor (Figs 6B and 7). We could not detect a factor in media conditioned by ΔRaf-1:ER cells that had been activated with 4HT in either a biological assay or a JNK kinase assay (data not shown). The results of cell-mixing experiments shown in Fig 7 argue strongly against an indirect mechanism of activation of JNK through induction of an autocrine factor or cell-surface bound molecule. There remains, however, the formal possibility that the activation of JNK is caused by the production of an autocrine factor that acts on a receptor that is only expressed in cells expressing activated Raf-1.
Current evidence favors the view that ERK and JNK are activated by distinct, nonoverlapping mechanisms. Our data suggest that this may not always be the case, because in the cells studied here, activation of Raf-1 is clearly able to influence JNK activity (Figs 6 through 8). The level at which the combination of Raf-1 and IL-4 influences JNK and/or upstream activators of JNK, such as MKK4&7 and/or MEKK1, remains to be determined. Nor is it clear whether it is Raf-1 itself or a downstream kinase (ie, MEK or ERK) that can synergize with IL-4 to activate JNK. The observation that the synergistic effect of IL-4 and Raf-1 on JNK activity was not evident after exposure to IL-4 for 10 minutes (Fig 9), argues against an acute effect of IL-4 and suggests that there may be a requirement for the synthesis of a new protein such as a kinase or another regulator of kinase cascades. Finally, it is also conceivable that the observed increase in JNK activity does not result directly from the activity of Raf and IL-4, but is rather a consequence of cell-cycle progression.
IL-4 stimulated increases in the lipid products of PI3′kinase activity could be involved in activation of Rac-1 through the pleckstrin homology domains in Rho family exchange factors.42,43However, these phospholipid products alone are not sufficient to activate JNK or induce Rac-mediated transcription factor pathways.43 Although Frost et al44 did not find any evidence for synergy between Raf-1 and V12Rac-1 orV12Cdc42 in activation of JNK, it is possible that overexpression of these mutants provided a saturating signal that masked any potential cooperative effect with Raf-1. It would be interesting to see if coexpression of an activated PI3′kinase and activated Raf would lead to activation of JNK, and what effects addition of PI3′kinase inhibitors would have on JNK activition in our system.
In conclusion, we have demonstrated that the failure of IL-4 to support long-term cellular growth can be complemented by expression of activated Raf-1. These data support the notion that Raf-1 activity is necessary but not sufficient for cell-cycle progression. Our observation that IL-4 and Raf-1 synergize to induce activation of JNK raises the possibility that JNK activity is also necessary for cell-cycle progression and that it may be partially regulated downstream of Raf-1. The activation of JNK did not appear to involve early events in IL-4 signal transduction and we could not find evidence for the involvement of autocrine mechanisms. Further identification of the pathway(s) activated by IL-4 that interact with the Raf kinase cascade to activate JNK could provide new insights into the mechanisms that regulate JNK activity and proliferation.
ACKNOWLEDGMENT
We thank Martin McMahon for the ΔRaf-1:ER and ΔRaf-1:ER K70W expression vectors, helpful discussions, and critical reading of the manuscript. We thank Rob Kay for the Q61KN-Ras cDNA, Leonard Zon for the GST-JNK-1 cDNA, and Ruth Salmon and Ian Foltz for critical reading of the manuscript.
Supported by grants from the Medical Research Council of Canada and The Canadian Arthritis Society.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John W. Schrader, MB, PhD, The Biomedical Research Centre, 2222 Health Sciences Mall, University of British Columbia, Vancouver, British Columbia, Canada, V6T 1Z3; e-mail:john@brc.ubc.ca.

![Fig. 3. Activation of ▵Raf-1:ER in combination with IL-4 stimulates DNA synthesis and the long-term growth of Ba/F3 cells. (A) Ba/F3 or ▵Raf-1:ER cells were washed free of IL-3 and plated at 250 cells/well in a Terasaki microtitre plate in medium alone or IL-4, with increasing concentrations of 4HT. After 40 hours, the cells were pulsed with [3H]-thymidine for an additional 8 hours, harvested, and counted in a scintillation counter. (B) ▵Raf-1:ER or (C) ▵Raf-1:ER K70W cells were washed free of IL-3 and incubated in IL-3, IL-4, 100 nmol/L 4HT, IL-4 plus 100 nmol/L 4HT or without factor (MA) at a density of 1 × 105 cells/mL. Cells were counted in triplicate at the indicated times and diluted as appropriate to maintain a consistent density. The results are representative of several independent experiments, with two independent clones. Error bars represent the SEM of triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/11/10.1182_blood.v93.11.3694/4/m_blod41133003x.jpeg?Expires=1769208709&Signature=trlxy4k8uuuBBzJUXEyvHA6sYa9uNPB4PLR0YaKnjpc2r2ugAezdunIVpE6r4sJbWBx3A~VzPuMp43d1FeGTbChJIRHXUCbjsguUjzQU-PMu-g8Cu53FM5JTMXDH~xk80PxSeshcwJvHMUuVUMqYsv5SjN4UjSzFXeZtk6A70j7EpiIXQY~Q-1pEJ1HT97JJoTBx~pMjO2fMYMEPM979PhMWcaFKo3L1L4ib2JxYNBoNl1X7eXTgMKAwmrSztet8tAGAoqELrKEueHTGLnhjD9g7wmfAt8JV5czdjG6nQXs374YZ3ih9XFfEZnHwdxlPaNWZsB2-Hj5HvBpR-rQHmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal