Abstract
Mobilization of nuclear factor-κB (NF-κB) activates transcription of genes encoding endothelial adhesion molecules and chemokines that contribute to monocyte infiltration critical in atherogenesis. Inhibition of NF-κB has been achieved by pharmacological and genetic approaches; however, monocyte interactions with activated endothelium in shear flow following gene transfer of the NF-κB inhibitor IκB- have not been studied. We found that overexpression of IκB- in endothelial cells using a recombinant adenovirus prevented tumor necrosis factor- (TNF-)–induced degradation of IκB- and suppressed the upregulation of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin mRNA and surface protein expression and the upregulation of transcripts for the chemokines monocyte chemoattractant protein 1 (MCP-1) and growth-related activity- (GRO-) by TNF-. This was associated with a reduction in endothelial MCP-1 secretion and GRO- immobilization. Adhesion assays under physiological shear flow conditions showed that firm arrest, spreading, and transmigration of monocytes on TNF-–activated endothelium was markedly inhibited by IκB- overexpression. Inhibition with monoclonal antibodies and peptide antagonists inferred that this was due to reduced expression of Ig integrin ligand as well as of chemokines specifically involved in these events. In contrast, rolling of monocytes was increased by IκB- transfer and was partly mediated by P-selectin; however, it appeared to be unaffected by the inhibition of E-selectin induction. Thus, our data provide novel evidence that selective modulation of NF-κB by adenoviral transfer of IκB- impairs the expression of multiple endothelial gene products required for subsequent monocyte arrest and emigration in shear flow and thus for monocyte infiltration in atherosclerotic plaques.
MONOCYTE ADHESION and emigration involves sequential and overlapping interactions of signal molecules and is fundamental in the initial pathogenesis and progression of atherosclerosis.1-3 Whereas monocyte rolling on endothelium is mediated by selectins, activation of β1 and β2 integrins that bind to their endothelial ligands, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), respectively, is critical for firm adhesion and transmigration.2,3 The expression of VCAM-1 and ICAM-1 has been demonstrated in coronary atherosclerotic lesions, particularly in areas of neovascularization, and may contribute to leukocyte recruitment.4,5 Moreover, the CC chemokine monocyte chemoattractant protein 1 (MCP-1), which can trigger chemotaxis and extravasation of monocytes via its receptor CCR2, has been detected in atherosclerotic plaques.6-9 In addition, the induction and immobilization of the CXC chemokine growth-related activity-α (GRO-α) can induce monocyte adhesion to endothelium stimulated by minimally modified low-density lipoprotein (LDL), implying a role in monocyte recruitment during atherogenesis.10 Inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), upregulate the endothelial adhesion molecules ICAM-1, VCAM-1, and E-selectin and the production of chemokines, eg, MCP-1, at the level of gene transcription involving the binding of nuclear factor-κB (NF-κB) to motifs in the promoter regions.11-14 Activation and nuclear translocation of NF-κB occurs in endothelial cells and requires the phosphorylation of its inhibitor IκB-α, which is degraded by a proteasome-dependent pathway.15-18
Inhibition of NF-κB by increasing levels of IκB-α in endothelial cells has been suggested to be of potential use in suppressing the inflammatory response.19-22 Adenovirus-mediated gene transfer may be a potentially specific and effective method of gene delivery into local areas of inflammation, such as atherosclerotic plaques or after balloon angioplasty.23-25 We used an adenoviral vector encoding IκB-α to inhibit NF-κB activation and found that gene transfer of IκB-α in endothelial cells impaired the TNF-α–induced upregulation of adhesion molecule and chemokine expression. This was associated with a reduction in firm adhesion and transmigration but not in rolling of monocytes on activated endothelium in shear flow. These data extend the understanding of the mechanisms of action exerted by adenovirus-mediated inhibition of NF-κB as a potential therapy in the prevention of atherogenesis and its consequences.
MATERIALS AND METHODS
Cell culture and reagents.
Human umbilical vein endothelial cells (HUVEC) were used at passages 2 to 4 and grown in low serum PromoCell medium,26 and Mono Mac 6 cells (from Dr H.W.L. Ziegler-Heitbrock, Institut für Immunologie, Munich, Germany) were maintained as described.27,28 Monocytes were isolated from healthy human donors by NycoPrep density gradient centrifugation (Nycomed, Norway) and separated from platelets by multiple low gravity washes, as described.29 This protocol resulted in a purity of greater than 85% monocytes and a minimal contamination with platelets (<5%), as assessed by light scatter, staining for CD14 and P-selectin, and subsequent flow cytometric analysis that showed an overwhelming majority of P-selectin–negative monocytes (data not shown).
The ICAM-1 monoclonal antibody (MoAb) RR1/130 was from Dr R. Rothlein (Boehringer Ingelheim, CT). The β2 MoAb TS1/18 was from Dr L.B. Klickstein (Brigham & Women’s Hospital, Boston, MA),31 the α4 MoAb HP1/2 from Dr R. Lobb (Biogen, Cambridge, MA),32 and the blocking G1 and nonblocking S12 MoAbs to P-selectin from Dr R. McEver (University of Oklahoma).33,34 The peptide analogues 8-73 GRO-α and 9-76 MCP-135 36 were kind gifts from Dr I. Clark-Lewis (University of British Columbia, Vancouver, Canada). MoAbs to VCAM-1, E-selectin, MCP-1, or GRO-α and isotype controls were from Canon or R&D Systems (Wiesbaden, Germany) and reagents were from Sigma Chemical Co (Deisenhofen, Germany), unless otherwise stated.
Overexpression of adenovirally encoded IκB-α in endothelial cells.
Construction of the adenoviral vector encoding for IκB-α (rAd.IκB-α) and infection of endothelial cells were performed as previously described.20 Briefly, confluent HUVEC were washed with phosphate-buffered saline (PBS) and incubated with the adenovirus (multiplicity of infection [moi] of 100) in PBS for 30 minutes at 37°C, washed, and then cultured for 48 hours. Infection with a control adenoviral vector37 encoding green fluorescence protein (rAd.GFP) was performed at an equivalent moi of 100, and effective transduction was confirmed by analyzing GFP expression by flow cytometry, as described.37 Unless otherwise stated, cells were stimulated with TNF-α (100 U/mL) for 4 hours. Overexpression of IκB-α may induce apoptosis in TNF-α–activated endothelial cells by suppressing induction of inhibitor of apoptosis proteins38; however, cell viability was determined to be greater than 95% under all conditions. For Western blot, cells left untreated or activated with TNF-α for 1 hour were lysed in sample buffer containing protease inhibitors and lysates were separated by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose membranes and reacted with an MoAb to IκB-α (Santa Cruz Biotechnology, Heidelberg, Germany). Blots were developed with chemiluminescence (ECL; Amersham, Braunschweig, Germany).
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was isolated by phenol/chloroform/isoamylalcohol extraction and cDNA was reverse transcribed from 2 μg RNA. PCR was performed and PCR products were analyzed by agarose gel electrophoresis and quantitated by high-performance liquid chromatography (HPLC) as described.26,39 Primer sequences were TTGCAGACCCTGCAGGGAAT (GRO-α, sense), TGGATTTGTCACTGTTCAGC (GRO-α, antisense), GTCTCTGCAACGCTTCTGTGCC (MCP-1, sense), AGTCG TGTGTCTTGGGTTGTGG (MCP-1, antisense), AGTAATAGTCCTCCTCATCATG (E-selectin, sense), and ACCATCTCAAGTGAAGAAAGAG (E-selectin, antisense) and as published for ICAM-1, VCAM-1, and β-actin.39
Flow cytometry and quantification of MCP-1 protein.
Confluent HUVEC were trypsinized, washed, and reacted with saturating concentrations of MoAb for 30 minutes on ice, washed, and stained with fluorescein isothiocyanate (FITC)-or phycoerythrin (PE)-conjugated goat antimouse IgG (Boehringer Mannheim, Mannheim, Germany), washed, and analyzed in a FACScan (Becton Dickinson, Heidelberg, Germany).26 39 The concentration of MCP-1 protein present in the HUVEC supernatants was determined using a sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems) performed according to the manufacturer’s protocols.
Monocyte adhesion and transmigration on endothelium in shear flow.
Laminar flow assays were performed as previously described.3,29,40 HUVEC were grown to confluence in 35-mm petri dishes that were assembled as the lower wall in a parallel wall flow chamber and mounted on the stage of an Olympus IMT-2 inverted microscope (Olympus Optical, Hamburg, Germany) with 20× and 40× phase contrast objectives. Monocytes (0.5 × 106/mL) or Mono Mac 6 cells (106/mL) suspended in Hanks’ buffered salt solution containing 10 mmol/L HEPES, pH 7.4, 0.5% human serum albumin, and 1 mmol/L Mg2+, 1 mmol/L Ca2+ added shortly before the assay) were kept in a heating block at 37°C during assays and were perfused into the flow chamber at a rate of 1.5 dyn/cm2 for 5 minutes. The number of firmly adherent cells after 5 minutes was quantitated in multiple fields (at least 5 per experiment) by analysis of images recorded with a long integration JVC 3CCD video camera and a JVC SR L 900 E video recorder (JVC, Japan), and expressed as cells per square millimeter. The type of adhesion analyzed was restricted to primary, ie, direct interactions of monocytes with endothelium. Consistent with findings using a similar flow chamber,3secondary attachment to already adherent monocytes that is mediated by L-selectin and results in the formation of linear strings41-43 occurred only sporadically at 1.5 dyn/cm2, accounting for a very small component of total interactions. The extent of secondary tethers may vary due to differences in the dimension, profile, and other design characteristics of the flow chambers used. The number of cells remaining bound, undergoing shape change, or transmigrating after 5-minute intervals was determined in high power fields, as described,2 and expressed as the percentage of cells that had firmly adhered. As an inverse measure of firm arrest, the number of cells rolling at reduced velocity on endothelium was determined within the last 30 seconds of the 5-minute intervals and was expressed as the percentage of all cells interacting with HUVEC in the field. Pretreatment with MoAbs was performed at 10 μg/mL. Data are expressed as the mean ± SD.
Statistics.
Statistical significance was determined by analysis of variance, and differences with P < .05 were considered to be significant.
RESULTS
Degradation and adenoviral overexpression of IκB-α in activated HUVEC.
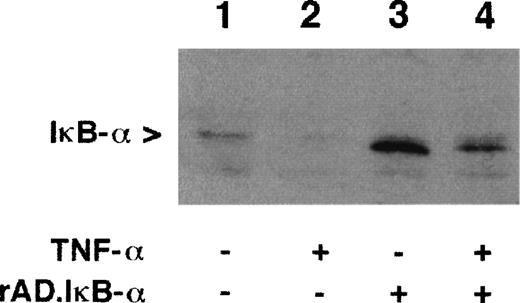
To study the effects of IκB-α overexpression, HUVEC were infected with a recombinant adenovirus encoding IκB-α.20 After 48 hours in culture, cells were challenged with TNF-α (100 U/mL) for 1 hour and the expression of IκB-α was determined by Western blot. In unstimulated cells, IκB-α was observed as a band of 37 kD (Fig 1, lane 1). Treatment of cells with TNF-α resulted in a marked decrease in detectable IκB-α compared with untreated cells, consistent with the degradation of IκB-α (Fig1, lane 2).17 18 Cells infected with rAd.IκB-α expressed substantially more IκB-α than untreated cells, indicating the high efficiency of infection and protein expression (Fig 1, lane 3). Stimulation with TNF-α after rAd.IκB-α infection decreased the expression of IκB-α, which, however, remained higher than in uninfected cells (Fig 1, lane 4). This was confirmed by densitometrical analysis (data not shown). These data suggest that overexpression of IκB-α prevents a substantial IκB-α degradation and NF-κB mobilization induced by TNF-α.
Expression of IκB- in HUVEC. Confluent HUVEC were left untreated (lanes 1 and 2), infected with rAd.IκB- (moi 100; lanes 3 and 4), and/or stimulated with TNF- for 1 hour (100 U/mL; lanes 2 and 4). Cell lysates were separated by 12.5% SDS-PAGE and Western blot was performed with MoAb to IκB-. Shown is a representative experiment.
Expression of IκB- in HUVEC. Confluent HUVEC were left untreated (lanes 1 and 2), infected with rAd.IκB- (moi 100; lanes 3 and 4), and/or stimulated with TNF- for 1 hour (100 U/mL; lanes 2 and 4). Cell lysates were separated by 12.5% SDS-PAGE and Western blot was performed with MoAb to IκB-. Shown is a representative experiment.
IκB-α inhibits mRNA transcription of endothelial signal molecules.
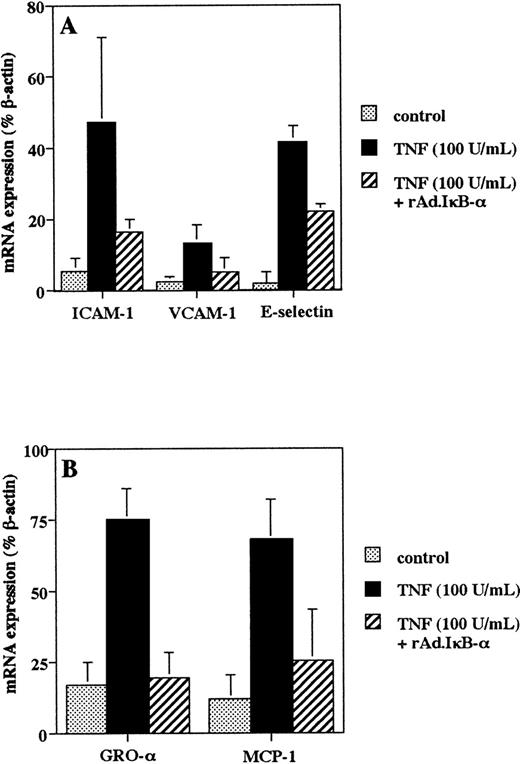
Activation of NF-κB is involved in upregulating the mRNA transcription of endothelial adhesion molecules and chemokines important in monocyte adhesion and transmigration.11-14 44-46 We used RT-PCR analysis with HPLC quantification to study the effect of IκB-α on the induction of mRNA transcripts. Treatment of HUVEC with TNF-α (100 U/mL) for 4 hours regulated ICAM-1, VCAM-1, and E-selectin mRNA expression (Fig 2A), whereas overexpression of IκB-α resulted in a reduction in mRNA levels after TNF-α stimulation (Fig 2A). Similarly, TNF-α increased the mRNA transcription of GRO-α and MCP-1, whereas infection with rAd.IκB-α inhibited these effects (Fig 2B).
Adenoviral IκB- transfer inhibits TNF-–induced endothelial adhesion molecule and chemokine mRNA expression. Cells were left untreated, infected with rAd.IκB-, and/or stimulated with TNF- (100 U/mL) for 4 hours. RT-PCR was performed using specific primers for ICAM-1, VCAM-1, and E-selectin (A); for MCP-1 and GRO- (B); and for β-actin. Quantification was perfomed by HPLC analysis, and mRNA expression was reported as the percentage of β-actin serving as an internal standard. Data are the mean ± SD of three separate experiments.
Adenoviral IκB- transfer inhibits TNF-–induced endothelial adhesion molecule and chemokine mRNA expression. Cells were left untreated, infected with rAd.IκB-, and/or stimulated with TNF- (100 U/mL) for 4 hours. RT-PCR was performed using specific primers for ICAM-1, VCAM-1, and E-selectin (A); for MCP-1 and GRO- (B); and for β-actin. Quantification was perfomed by HPLC analysis, and mRNA expression was reported as the percentage of β-actin serving as an internal standard. Data are the mean ± SD of three separate experiments.
Inhibition of TNF-α–induced adhesion molecule expression by IκB-α.
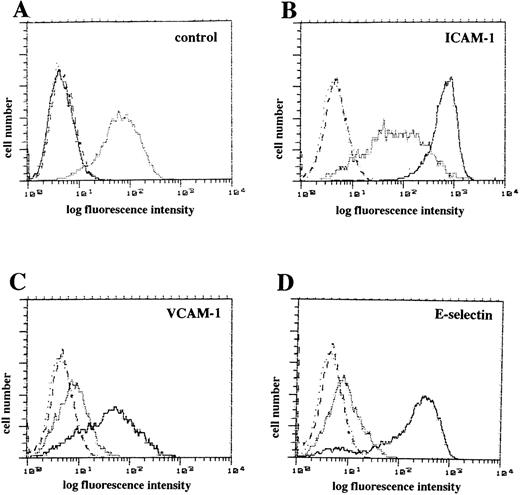
We determined whether rAd.IκB-α infection impaired the surface expression of endothelial adhesion molecules by flow cytometric analysis. Untreated HUVEC expressed moderate levels of ICAM-1 and low levels of VCAM-1 and E-selectin (Fig 3A). Stimulation with TNF-α (100 U/mL for 4 hours) markedly increased the expression of ICAM-1, VCAM-1, and E-selectin (Fig 3B through D). Infection with rAd.IκB-α almost completely inhibited the upregulation of adhesion molecules induced by TNF-α (Fig 3B through D). Thus, overexpression of IκB-α prevented the upregulation of endothelial adhesion molecule surface expression.
Flow cytometric analysis of endothelial adhesion molecule after IκB- overexpression. (A) Untreated HUVEC were stained with MoAbs to ICAM-1 (stippled line), VCAM-1 (dashed line), E-selectin (dotted line), and isotype control (solid line). (B through D) Cells stimulated with TNF- (100 U/mL) for 4 hours were stained with MoAbs (solid line) to ICAM-1 (B), VCAM-1 (C), E-selectin (D), or isotype control (B through D, dotted lines). Cells infected with rAd.IκB- and stimulated with TNF- (100 U/mL for 4 hours) were stained with MoAbs (stippled line) to ICAM-1 (B), VCAM-1 (C), E-selectin (D), or isotype control (B through D, dashed lines). Shown is a representative experiment.
Flow cytometric analysis of endothelial adhesion molecule after IκB- overexpression. (A) Untreated HUVEC were stained with MoAbs to ICAM-1 (stippled line), VCAM-1 (dashed line), E-selectin (dotted line), and isotype control (solid line). (B through D) Cells stimulated with TNF- (100 U/mL) for 4 hours were stained with MoAbs (solid line) to ICAM-1 (B), VCAM-1 (C), E-selectin (D), or isotype control (B through D, dotted lines). Cells infected with rAd.IκB- and stimulated with TNF- (100 U/mL for 4 hours) were stained with MoAbs (stippled line) to ICAM-1 (B), VCAM-1 (C), E-selectin (D), or isotype control (B through D, dashed lines). Shown is a representative experiment.
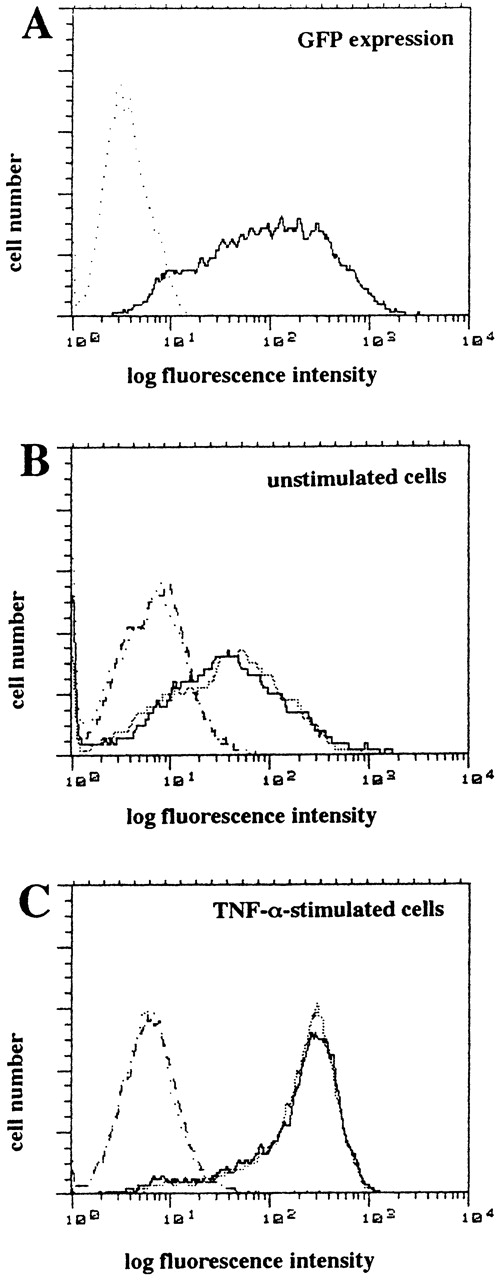
To control for effects of adenoviral infection itself, we infected HUVEC with the control vector rAd.GFP37 encoding GFP at an equivalent moi of 100 under identical conditions. Flow cytometric analysis showed a marked GFP expression in greater than 90% of HUVEC after 48 hours, confirming a high transduction efficiency (Fig 4A). Notably, infection of HUVEC with rAd.GFP under these conditions did not affect the surface expression of ICAM-1 in unstimulated HUVEC (Fig 4B) or in HUVEC stimulated with TNF-α (100 U/mL) for 4 hours (Fig 4C).
Flow cytometric analysis of endothelial adhesion molecule after infection with control vector rAd.GFP encoding GFP. (A) The expression of GFP in HUVEC was analyzed by flow cytometry after infection with rAd.GFP (solid line) or no treatment (dotted line) after 48 hours. (B and C) HUVEC infected with or without GFP control adenovirus were stimulated without (B) or with (C) TNF- (100 U/mL) for 4 hours and were stained with MoAb to ICAM-1 (solid or stippled line) or isotype control (dotted or dashed line). Shown are representative histograms.
Flow cytometric analysis of endothelial adhesion molecule after infection with control vector rAd.GFP encoding GFP. (A) The expression of GFP in HUVEC was analyzed by flow cytometry after infection with rAd.GFP (solid line) or no treatment (dotted line) after 48 hours. (B and C) HUVEC infected with or without GFP control adenovirus were stimulated without (B) or with (C) TNF- (100 U/mL) for 4 hours and were stained with MoAb to ICAM-1 (solid or stippled line) or isotype control (dotted or dashed line). Shown are representative histograms.
Effect of IκB-α on endothelial secretion of chemokines.
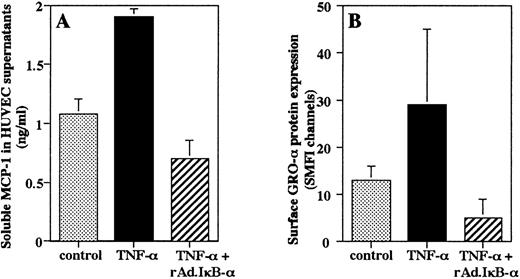
It has been shown that MCP-1 is secreted by endothelium as a soluble protein, whereas GRO-α can be immobilized on the endothelial surface.10 47 An ELISA of HUVEC supernatants showed that stimulation with TNF-α for 4 hours induced an increase in secretion of MCP-1 protein that was completely prevented by overexpression of IκB-α in HUVEC (Fig 5A). To determine the effects of rAd.IκB-α on immobilized GRO-α, we measured the amount of surface-associated protein by flow cytometric analysis. We found that TNF-α stimulation increased the amount of GRO-α associated with the endothelial surface that was inhibited by overexpression of IκB-α (Fig 5B).
Endothelial production of chemokines. HUVEC were left untreated, were infected with rAd.IκB- and/or stimulated with TNF- (100 U/mL) for 4 hours. (A) Quantification of soluble MCP-1 was performed by ELISA. Data are the mean ± SD of three separate experiments performed in duplicate. (B) Flow cytometric analysis was performed to quantitate the amount of surface-associated GRO-. Data are the mean ± SD of three separate experiments.
Endothelial production of chemokines. HUVEC were left untreated, were infected with rAd.IκB- and/or stimulated with TNF- (100 U/mL) for 4 hours. (A) Quantification of soluble MCP-1 was performed by ELISA. Data are the mean ± SD of three separate experiments performed in duplicate. (B) Flow cytometric analysis was performed to quantitate the amount of surface-associated GRO-. Data are the mean ± SD of three separate experiments.
Effect of IκB-α on monocyte interactions with endothelium in shear flow.
Firm adhesion of monocytes to endothelium under shear flow involves the α4 and β2 integrins, known to interact with VCAM-1 and ICAM-1, respectively.2,3 Moreover, chemokines such as IP10, Mig, and eotaxin have been implicated in the arrest of T effector cells and eosinophils in shear flow, respectively.40,48 Recently, we have shown that surface-bound GRO-α is involved in monocyte arrest, whereas soluble MCP-1 mediates transmigration of monocytes on activated endothelium in shear flow.49 Hence, we studied the effects of IκB-α overexpression on monocyte adhesion to activated endothelium under physiological flow conditions. After 48 hours in culture, HUVEC infected with rAd.IκB-α or left untreated were stimulated with TNF-α (100 U/mL) for 4 hours and inserted as the bottom wall of a parallel flow chamber. Isolated human blood monocytes or monocytic Mono Mac 6 cells, which express a similar array of adhesion molecules and chemokine receptors,50 were perfused through the chamber at a constant shear rate of 1.5 dyn/cm2. After a 5-minute period of accumulation, the number of Mono Mac 6 cells firmly attached to uninfected HUVEC treated with TNF-α was determined to be 198 ± 46 cells/mm2(Fig 6A). By comparison, overexpression of IκB-α in HUVEC before TNF-α stimulation resulted in a significant inhibition in the number of cells firmly arrested after 5 minutes (59 ± 22 cells/mm2), whereas infection with the control vector rAd.GFP had no effect and thus did not confound cell arrest (Fig6A). Because leukocytes can bind to adherent leukocytes via L-selectin,41-43 only direct interactions of monocytes with endothelium were included in this analysis. As described,2a percentage of firmly attached cells was observed to undergo shape change or spreading and migrate towards interendothelial junctions and transmigrate. On uninfected HUVEC treated with TNF-α, 25.0% ± 5.7% of the firmly attached cells were found to undergo a shape change, spreading, or transmigration (Fig 6B). Infection of HUVEC with rAd.IκB-α but not with control GFP adenovirus resulted in a clear reduction in the fraction of cells spreading or transmigrating (Fig6B).
Effects of adenoviral-mediated IκB- expression on firm adhesion, transmigration, and rolling of monocytic cells on endothelial cells under physiological flow. HUVEC grown in 35-mm Petri dishes were left untreated (▩), were infected with rAd.IκB- (▪), or were infected with the control vector rAd.GFP encoding GFP (░). All cells were stimulated with TNF- (100 U/mL) for 4 hours. Mono Mac 6 cells were perfused at a constant flow rate of 1.5 dyn/cm2. (A) The firm, shear-resistant attachment of monocytes to HUVEC was determined by counting the number of cells firmly attached per field after a 5-minute period. (B) Cells undergoing shape change and spreading or transmigration were counted after 5 minutes and expressed as the percentage of cells initially attached. Data are the mean ± SD of three independent experiments. *P< .05 versus uninfected control.
Effects of adenoviral-mediated IκB- expression on firm adhesion, transmigration, and rolling of monocytic cells on endothelial cells under physiological flow. HUVEC grown in 35-mm Petri dishes were left untreated (▩), were infected with rAd.IκB- (▪), or were infected with the control vector rAd.GFP encoding GFP (░). All cells were stimulated with TNF- (100 U/mL) for 4 hours. Mono Mac 6 cells were perfused at a constant flow rate of 1.5 dyn/cm2. (A) The firm, shear-resistant attachment of monocytes to HUVEC was determined by counting the number of cells firmly attached per field after a 5-minute period. (B) Cells undergoing shape change and spreading or transmigration were counted after 5 minutes and expressed as the percentage of cells initially attached. Data are the mean ± SD of three independent experiments. *P< .05 versus uninfected control.
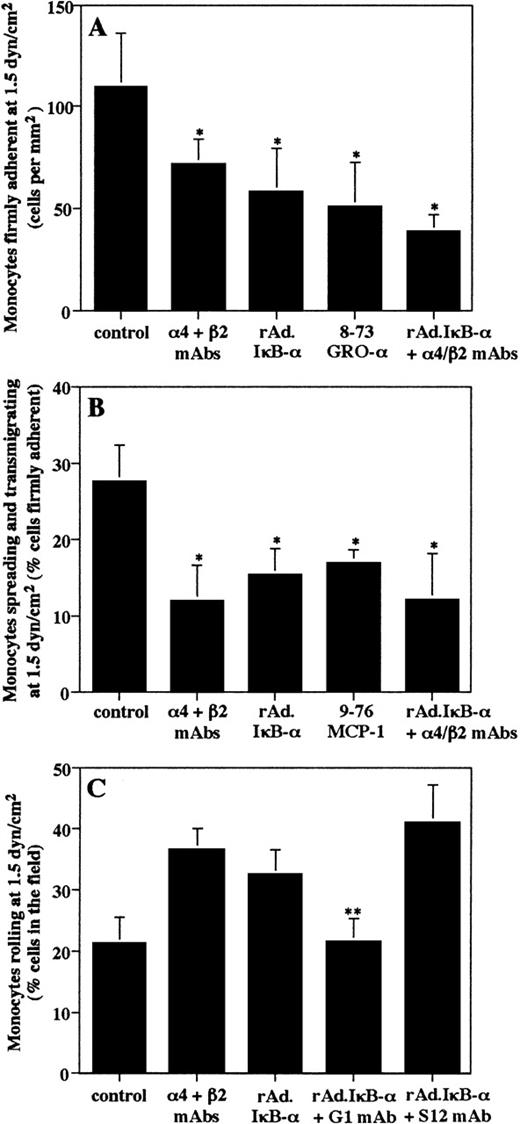
Analysis of experiments performed with isolated human blood monocytes showed that 110 ± 26 cells/mm2 had firmly attached after a 5-minute period on uninfected and TNF-α–treated HUVEC at a shear rate of 1.5 dyn/cm2 (Fig7A). Of these firmly attached cells, 27.5% ± 4.9% appeared to spread or to transmigrate between endothelial cells (Fig 7B). Consistent with previous results,2,3 a combination of α4 and β2 MoAbs markedly reduced firm arrest, as well as spreading and transmigration of monocytes (Fig 7A and B). Similarly, infection of HUVEC with rAd.IκB-α significantly inhibited the shear-resistant adhesion of monocytes to TNF-α–activated endothelium as compared with uninfected controls (Fig 7A). Subsequently, the percentage of attached cells that underwent spreading or transmigration was lower on HUVEC overexpressing IκB-α (Fig 7B). Consistent with recent findings,49 the inhibition with specific peptide antagonists showed that GRO-α is involved in monocyte arrest (Fig7A), whereas MCP-1 contributes to monocyte transmigration on activated endothelium in shear flow (Fig 7B). Control peptides had no effect (data not shown). Preincubation of monocytes with a combination of blocking α4 and β2 MoAb in addition to infection of HUVEC with rAd.IκB-α resulted in a slightly although not significantly greater inhibition of monocyte arrest and of spreading/transmigration as compared with infection with rAd.IκB-α or MoAb treatment alone (Fig7A and B). Taken together, these data infer that, beyond effects on endothelial Ig integrin ligands, the reduced chemokine expression in rAd.IκB-α–infected HUVEC is at least in part responsible for the inhibition of arrest and transmigration.
Effects of adenoviral-mediated IκB- expression on firm arrest, transmigration, and rolling of isolated blood monocytes on endothelial cells under physiological flow conditions. HUVEC were left untreated or were infected with rAd.IκB- and were stimulated with TNF- (100 U/mL) for 4 hours. Monocytes were perfused at a constant flow rate of 1.5 dyn/cm2 after pretreatment with or without MoAbs to 4 and β2, 8-73 GRO-, or 9-76 MCP-1 peptide analogues (1 μg/mL each) for 30 minutes on ice before being perfused. (A) The firm attachment of monocytes to HUVEC was determined by counting the number of cells attached per field after a 5-minute period. (B) Cells undergoing spreading or transmigration were counted after 5 minutes and expressed as the percentage of cells initially attached. (C) The number of rolling cells within the field was counted in the last 30 seconds of the 5-minute period and was expressed as the percentage of cells interacting with endothelium. HUVEC were pretreated with a blocking (G1) or nonblocking P-selectin (S12) MoAb for 30 minutes at 37°C. Data are the mean ± SD of at least three independent experiments. *P < .05 versus uninfected control. **P < .05 versus rAd.IκB-–infected HUVEC.
Effects of adenoviral-mediated IκB- expression on firm arrest, transmigration, and rolling of isolated blood monocytes on endothelial cells under physiological flow conditions. HUVEC were left untreated or were infected with rAd.IκB- and were stimulated with TNF- (100 U/mL) for 4 hours. Monocytes were perfused at a constant flow rate of 1.5 dyn/cm2 after pretreatment with or without MoAbs to 4 and β2, 8-73 GRO-, or 9-76 MCP-1 peptide analogues (1 μg/mL each) for 30 minutes on ice before being perfused. (A) The firm attachment of monocytes to HUVEC was determined by counting the number of cells attached per field after a 5-minute period. (B) Cells undergoing spreading or transmigration were counted after 5 minutes and expressed as the percentage of cells initially attached. (C) The number of rolling cells within the field was counted in the last 30 seconds of the 5-minute period and was expressed as the percentage of cells interacting with endothelium. HUVEC were pretreated with a blocking (G1) or nonblocking P-selectin (S12) MoAb for 30 minutes at 37°C. Data are the mean ± SD of at least three independent experiments. *P < .05 versus uninfected control. **P < .05 versus rAd.IκB-–infected HUVEC.
We next investigated the effect of IκB-α overexpression on selectin-mediated rolling interactions of monocytes on endothelium. Previous reports have demonstrated that rolling of monocytes on activated endothelium is largely mediated by interactions of L-selectin and P-selectin.2 3 On TNF-α–treated HUVEC, the rolling fraction of cells was found to be 21.2% ± 6.1% of cells in the field (Fig 7C). Because initial rolling is rapidly converted into integrin-mediated firm adhesion, cells were pretreated with MoAbs to α4 and β2 integrins to inhibit arrest and facilitate analysis of rolling. This resulted in an increase in the rolling fraction of cells, thus serving as an inverse measure of firm arrest. Overexpression of IκB-α in HUVEC followed by TNF-α stimulation similarly increased the rolling fraction of cells (34.5% ± 5.8% of cells in the field). Preincubation of rAd.IκB-α–infected HUVEC with a blocking MoAb to P-selectin caused a clear reduction in the rolling fraction of cells (Fig 7C) and consequently an inhibition in firm arrest (47 ± 14 cells/mm2). In contrast, the nonblocking P-selectin MoAb had no effect on rolling or firm arrest. Thus, these results show that P-selectin is involved in monocyte rolling, a prerequisite for firm adhesion.
DISCUSSION
We have found that overexpression of the cytoplasmic NF-κB inhibitor IκB-α suppressed the TNF-α–stimulated upregulation of ICAM-1, VCAM-1, and E-selectin and production of GRO-α and MCP-1 in endothelial cells. This was associated with a decrease in the adhesion and transmigration but not rolling of monocytes on activated endothelium in physiological flow and expands on previous findings that adenoviral gene transfer of IκB-α effectively inhibits NF-κB-dependent processes20 in a functionally relevant system. Whereas TNF-α stimulation of uninfected HUVEC markedly reduced the levels of IκB-α protein, most likely due to its proteolytic degradation,15-18 stimulation of rAd.IκB-α–infected cells with TNF-α decreased the levels of IκB-α that, however, remained higher than in control cells. IκB-α expressed by adenoviral gene transfer may be phosphorylated and thus more susceptible to TNF-α–induced degradation; however, as observed with LPS stimulation, the overwhelming proportion of IκB-α could not be processed.20 In comparison with the adenoviral techniques used here, retrovirus-mediated transfer of wild-type or a truncated form of IκB-α did not result in a marked overexpression in HUVEC.22 This may be due in part to the presence of the nuclear localizing sequence in the adenoviral vector, but also reflects the high efficacy of infection by the adenovirus.
To our best knowledge, this is the first study demonstrating the effects of IκB-α overexpression on leukocyte adhesion and transmigration in shear flow. As such, it clearly extends previous reports describing pharmacological and genetic approaches that inhibit NF-κB mobilization or increase the levels of IκB-α.19-22,51,52 As previously found,18,20,21 we show that inhibition of NF-κB mobilization by overexpression of IκB-α inhibited the induction of endothelial adhesion molecule mRNA and protein expression by TNF-α, in particular that of the integrin ligands ICAM-1 and VCAM-1. This was not due to apoptosis, because cell viability was unaltered after stimulation with TNF-α for 4 hours, whereas the onset of apoptosis occurs only after 6 hours.38 The effects were associated with a significant reduction in the ability of monocytic cells to firmly arrest and an increase in the rolling fraction of cells on rAd.IκB-α–infected HUVEC under physiological flow conditions. It has been shown that a combination of blocking α4 and β2 integrins inhibits the adhesion of monocytes2,3 and as an inverse measure of firm arrest, resulted in a shift to more rolling interactions on activated HUVEC in shear flow. Thus, the effects of IκB-α overexpression are likely due to reduced expression of VCAM-1 and ICAM-1, ligands for the α4 integrin VLA-4 and the β2 integrin LFA-1, respectively. In addition, subsequent spreading and transmigration of monocytes was reduced on rAd.IκB-α–infected HUVEC. Because transendothelial migration of leukocytes requires the coordinated regulation of integrin avidity, particularly that of VLA-4 and LFA-1,29 53 impaired expression of their ligands may also contribute to decreased transmigration across rAd.IκB-α–infected HUVEC.
Inhibition of NF-κB also suppressed the production of the chemokines MCP-1 and GRO-α in TNF-α–stimulated HUVEC, which are critically involved in monocyte adhesion and chemotaxis.14,44-46 Interestingly, overexpression of IκB-α appeared to be slightly more effective in inhibiting firm monocyte arrest on endothelium. Recent reports have shown that CXC chemokines IP10 and Mig mediate rapid and shear-resistant arrest of T effector cells, whereas blocking the eotaxin receptor CCR3 in combination with an α4 MoAb inhibited eosinophil arrest on activated endothelium.40,48 GRO-α can be immobilized to mmLDL-treated HUVEC and facilitate monocyte adhesion to endothelium,10 whereas monocyte transmigration appears to require a soluble gradient of MCP-1.47 Indeed, we have recently established a hierarchical involvement of chemokines in monocyte traffic on activated endothelium in shear flow, ie, monocyte arrest requires surface-bound GRO-α, whereas subsequent transmigration is mediated by MCP-1 presented in a soluble gradient created by removal of secreted protein in shear flow.49 We have confirmed here the contributions of these chemokines using peptide antagonists. Moreover, the combination of MoAb to integrins with peptide antagonists or with rAd.IκB-α–infection of HUVEC appeared to slightly accentuate the inhibition of monocyte arrest, as well as spreading and transmigration. Thus, the reduction in GRO-α due to NF-κB inhibition may contribute to decreased monocyte adhesion in shear flow. In addition, overexpression of IκB-α in HUVEC may result in a marked reduction in MCP-1 secretion that would impair the establishment of an MCP-1 gradient and thus monocyte transmigration. Hence, our data for the first time provide evidence that, in addition to effects on endothelial Ig integrin ligands, the reduced expression of specifically required chemokines in rAd.IκB-α–infected HUVEC contributes to the inhibition of monocyte arrest and transmigration in shear flow.
Interestingly, overexpression of IκB-α decreased firm adhesion but increased the rolling fraction of cells, indicating a shift to more rolling interactions. Blocking MoAb inhibition assays have shown that monocyte rolling on activated endothelium is largely mediated by P- and L-selectin, whereas no role for E-selectin was seen,2,3suggesting that interactions of P- or L-selectin are sufficient. Although effects of NF-κB mobilization on E-selectin expression are well described,11,18,39 the regulation of P-selectin expression by the NF-κB/IκB-α autoregulatory loop is not yet clear. Evidence with respect to the upregulation of P-selectin expression by TNF-α is not entirely conclusive.2,54,55Moreover, proteasome inhibitors and antioxidants have been shown to decrease the expression of endothelial P-selectin independently of NF-κB inhibition.55 We found that rolling interactions of monocytes were not affected by NF-κB inhibition in endothelial cells and that P-selectin, which was not inhibited by overexpression of IκB-α, plays a partial role in mediating monocyte rolling. These results are consistent with previous reports showing that rolling is not affected by E-selectin.2 3
Inhibition of NF-κB activation in endothelial cells has been suggested to be useful in dampening inflammatory responses.19-22 Notably, the inhibition of endothelial VCAM-1 and MCP-1 expression that can be specifically detected in atherosclerotic lesions4,5,9 appeared to be more prominent than that of ICAM-1, indicating a selective rather than general immunomodulatory effect. This was consistent with findings with PDTC or aspirin39,51 and may be due to differential contributions of a variant NF-κB site or dimer compositions to ICAM-1 transcription.13 Adenoviral gene transfer of IκB-α may offer advantages in its localized applicability,23-25 but deleterious effects of inhibiting NF-κB translocation in large cell populations cannot be excluded. Previous reports have confirmed the efficacy of adenoviral gene transfer in normal, atherosclerotic, or balloon-injured blood vessels.23-25 Although infection with control adenovirus alone did not increase ICAM-1 expression or cell arrest in our assays, adenovirus-mediated gene transfer itself may result in an inflammatory response and antigenicity,56which warrants further studies addressing the feasibility of adenoviral gene transfer in vivo. In conclusion, overexpression of IκB-α in endothelium may reduce extravasation of monocytes and other leukocyte subsets and be of potential use in limiting the inflammatory response in areas of atherosclerosis or after balloon injury.
ACKNOWLEDGMENT
The authors are grateful to Drs I. Clark-Lewis, R. Lobb, R. McEver, and R. Rothlein for providing valuable reagents and to Prof P.C. Weber for his continuing support.
Supported by Deutsche Forschungsgemeinschaft (We-1913/2) and August Lenz-Stiftung.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kim S.C. Weber, MD, Institut für Prophylaxe und Epidemiologie der Kreislaufkrankheiten, Ludwig-Maximilians Universität, Pettenkoferstrasse 9, D-80336 Munich, Germany; e-mail: kim.weber@klp.med.uni-muenchen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal