Transfusions (Tx) of Ultraviolet B (UVB)-irradiated peripheral blood mononuclear leukocytes (MNL) have been shown to induce humoral immune tolerance to major histocompatability complex (MHC) antigens (Blood 88:4375, 1996). To determine whether cellular immune tolerance to MHC antigens can be induced by the same approach, transplantation of bone marrow and spleen cells from tolerant donors across the H-2 barrier was conducted to study its effect on prevention of graft-versus-host disease (GVHD). After immune tolerance induction by four weekly Tx of UVB-irradiated BALB/c (H-2d) peripheral blood MNL into CBA/HT6 (H-2k) mice, bone marrow cells (BMC) and spleen MNL from tolerant or naive CBA mice were transplanted into lethally irradiated BALB/c mice. The transplanted mice were followed by measuring body weight, peripheral leukocyte counts, GVHD, survival, and cytokine response. All BALB/c recipient mice were fully engrafted with H-2k CBA donor cells after transplantation. The severity of GVHD was significantly attenuated in BALB/c mice transplanted with BMC and spleen MNL from tolerant CBA donor mice. The recovery of peripheral leukocyte and lymphocyte counts were faster and more complete in mice transplanted with cells from the tolerant donors. The serum cytokine profile after transplantation with tolerant donor cells showed increased interleukin-4 and reduced gamma interferon that are consistent with a polarized Th2 response. The results pooled from three separate experiments showed that BALB/c mice transplanted with 5 × 106 BMC and 4 × 105spleen MNL from tolerant CBA donors had better overall survival than the control group (72% v 17%, P = .018). The findings show that transplantation with bone marrow and spleen cells from tolerant H-2 disparate donor mice is associated with significant attenuation of GVHD and better outcomes. The results also support that transfusions of UVB-irradiated leukocytes may induce cellular immune tolerance.
IN SPITE OF THE WIDESPREAD use of allogeneic bone marrow transplant (BMT) in the treatment of various malignant and nonmalignant conditions, graft-versus-host disease (GVHD) continues to be a serious complication after allogeneic BMT. GVHD, whether acute or chronic, has been shown to occur in 30% to 60% of patients receiving histocompatible sibling-matched allogeneic BMT.1 Mortality attributed to GVHD has been reported in up to 50% of cases.2-4 The severity of GVHD is related to the degree of difference between hosts and donors across the major histocompatibility (MHC) barrier5,6 and the presence of donor T cells in marrow grafts.7-10 For this reason, T-cell depletion from marrow grafts has been successfully applied to prevent GVHD. Unfortunately, this approach has been associated with increased incidences of graft failure, more severe immunosuppression, and higher relapse rates of original malignancies.7,8 11-14 Therefore, developing a method for inducing specific humoral and cellular immune tolerance across the MHC barrier offers an attractive alternative approach to prevent or attenuate GVHD.
Irradiation of leukocytes with medium (UVB) or short (UVC) wavelength ultraviolet light has been shown to produce various immunomodulatory effects.15-21 These effects include inhibition of stimulator and responder function of leukocytes in mixed leukocyte culture (MLC),15,16 suppression of cytokine production,17 downregulation of cell membrane proteins such as class-II MHC antigens18 and cell adhesion molecules,19 reduced lymphocyte proliferative responses to mitogenic lectins,20 and inhibition of expression of costimulatory signals by antigen presenting cells.21 On the basis of these findings, UVC and UVB have been applied to inactivate leukocytes for reducing the immunogenicity of platelet concentrates.22-25 Results from these studies indicate that transfusions of platelet concentrates irradiated with UVB or UVC could induce humoral immune tolerance to allogeneic MHC antigens. However, the induced tolerance was either incomplete22 or inconsistent.24
To overcome the problems of inconsistency and partial tolerance, we investigated the effect of plasma and platelets on tolerance induction by UVB-irradiated leukocytes. Our study showed that the presence of plasma and platelets interfered with tolerance induction and that the use of highly purified peripheral mononuclear leukocytes (MNL) is critical for consistent induction of complete humoral immune tolerance to allogeneic MHC antigens.26 However, it is not known whether cellular immune tolerance can be induced by the same approach. To answer this question and to explore the potential application of this approach to allogeneic BMT, we conducted experiments to determine whether transplantation with bone marrow and spleen cells from H-2 disparate tolerant donor mice could prevent or attenuate the GVHD induced by allogeneic BMT in a murine model.
MATERIALS AND METHODS
Animals.
Eight-week old CBA/CaH-T6/J (CBA) mice with H-2k MHC haplotype and BALB/cByJ (BALB/c) mice with H-2d MHC haplotype were obtained from Jackson Laboratory (Bar Harbor, ME). BALB/c mice were also obtained from Harlan Sprague Dawley, Inc (Indianapolis, IN). All mice were housed in a temperature-controlled room (25°C) with a 12-hour interval light/dark cycle and fed ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee.
Preparation of UVB-irradiated peripheral mononuclear leukocytes.
Peripheral mononuclear leukocytes were prepared from freshly collected BALB/c venous blood by differential and Ficoll-hypaque density gradient centrifugation as described.22 MNL were suspended in phosphate buffered saline (PBS) and their concentrations were determined by hemacytometers after staining cells with propidium iodide solution.27 For irradiation of leukocytes by UVB, a Bioslink-UV irradiator (BIOS Corp, New Haven, CT) with a built-in dosimeter was used. The dose of UVB irradiation was 1,200 mJ/cm2. The irradiation was performed in an open sterile polypropylene container. The depth of cell suspension during UVB-irradiation was 1 mm. The cell suspensions were mixed manually by moving the tray back and forth during irradiation.
Induction of immune tolerance.
Immune tolerance was induced in CBA mice by four weekly intravenous injections of 2 × 105 UVB-irradiated BALB/c MNL in 100 μL PBS through a tail vein under light anesthesia with inhalation of methoxyflurane (Pitman-Moore Inc, Mundelein, IL). Preimmune serum samples were prepared from venous blood that was collected by retro-orbital bleeding 2 days before the first transfusion. One week after the last weekly transfusion of UVB-irradiated leukocytes, serum samples were collected to assess the development of antibodies to donor MHC antigens by flow cytometry as described.26 As reported previously,26 CBA mice became tolerant, if they did not develop any anti-H2d antibodies after four weekly transfusions of UVB-irradiated BALB/c MNL. To insure the development of tolerance, two transfused CBA mice were randomly selected and challenged with two weekly transfusions of 1 × 105untreated BALB/c MNL. We found that none of the challenged CBA mice became immunized to H-2d antigens of BALB/c mice as expected. In contrast, all control naive CBA mice developed anti–H-2d antibody after two transfusion challenges.
BMT.
Naive and tolerant CBA mice were used as donors of bone marrow and spleen cells. BALB/c mice were used as bone marrow recipients. BMC from CBA mice were prepared by flushing cells out of femurs and tibias using 25-gauge needles and syringes filled with RPMI 1640 medium as described.28 Spleen MNL were prepared from bone marrow donor mice by Ficoll gradient centrifugation.22 Fifty μL of spleen MNL containing specified numbers of cells were mixed with 5 × 106 bone marrow cells in a final volume of 200 μL. These cells were transplanted through a tail vein into each BALB/c mouse that had been lethally irradiated with 750 cGy gamma ray 5 to 6 hours before transplantation. The transplanted mice were fed with sterilized laboratory chow and acidified water. They were followed for engraftment of donor cells by immunofluorescence flow cytometry. Body weight, peripheral total and differential white cell count, GVHD, and survival were measured.
Immunofluorescence flow cytometry for detection of engraftment and characterization of lymphocyte subsets.
H-2 phenotypes of peripheral blood leukocytes were determined by immunofluorescence flow cytometry using fluorescein isothiocyanate (FITC)-labeled anti-H2d antibody and phycoerythrin (PE)-labeled anti-H2k antibody. Different subsets of lymphocytes including B cells, CD4+ helper T cells, and CD8+ cytotoxic T cells were measured using PE-labeled anti-B220, FITC-labeled anti-CD4, and PE-labeled anti-CD8 antibodies. All antibodies were purchased from PharMingen Corp (San Diego, CA). Peripheral leukocytes for flow-cytometric analysis were prepared by washing 20μL of whole blood with 1 mL PBS and lysing red cells with 1.5 mL of 0.85% ammonium chloride solution. After lysis of red cells, the remaining cells were washed twice with 0.5 mL PBS and resuspended in 100 μL PBS-0.02% azide-0.5% bovine serum albumin (BSA). Fifty μL of washed leukocytes were stained with appropriate concentrations of fluorescent antibodies for 30 minutes. After two washes, the cells were analyzed using a FACscan flow cytometer (Becton Dickinson, San Jose, CA).
Enzyme-linked immunoassays (EIA) for interleukin-4 (IL-4) and γ-interferon (γ-IFN).
For the assays, plastic wells of a microtiter plate were coated with 50 μL of 5μg/mL antimouse IL-4 or γ-IFN antibody in PBS-azide overnight at 4°C. The plates were washed three times using PBS-azide-0.2% Tween-20 and blocked with 150 μL PBS containing 10% newborn calf serum (NCS) for 30 minutes at room temperature. Thereafter, 50 μL of cytokine standards and diluted serum samples were added and incubated overnight at 4°C. Serum samples were diluted with equal volume of PBS-10% NCS. After overnight incubation, plates were washed and incubated sequentially with 50 μL of 5 μg/mL biotinylated anticytokine antibody diluted in PBS-Tween-1% BSA, strepavidin-peroxidase (Sigma Co, St Louis, MO), and o-phenylenediamine dihydrochloride peroxidase substrate (Sigma Co). Paired anti-IL-4 and anti-γIFN antibodies, recombinant murine IL-4 and γ-IFN used in the assays were obtained from PharMingen (San Diego, CA) and Endogen (Woburn, MA), respectively. The lowest concentrations of IL-4 and γ-IFN that can be measured by these two assays were 0.2 pg/mL, and 50 pg/mL, respectively.
GVHD scoring.
A scoring system was devised to grade GVHD in the transplanted mice. Hair and skin changes involving the head/neck, abdomen and tail, and changes in anal and perianal mucosa were scored according to severity from 0 to 3 as defined in Table 1. Mice with a total score of less than 3 were regarded as having mild GVHD, ≥3 to ≤5 as having moderate GVHD and total score of greater than 5 as severe GVHD.
Grading Criteria for GVHD in Transplanted Mice
| Criteria . | Grade 0 . | Grade 1 . | Grade 2 . | Grade 3 . |
|---|---|---|---|---|
| Hair loss, neck and head | Normal | Scattered loss (thinning) | Multi-patchy hair loss | Confluent hair loss (alopecia) |
| Hair loss, chest & abdomen Area involved | Normal | Scattered loss <20% | Multiple stripes ≥20%, <50% | Diffuse involvement ≥50% |
| Skin integrity tail | Normal | Hair loss, patchy-hyperkeratosis | Hair loss, diffuse-hyperkeratosis, areas of desquamation | Grade 2 changes plus necrosis |
| Perianal and anal mucosa | Normal | Perianal inflammation | Inflammation and prolapse of anal mucosa |
| Criteria . | Grade 0 . | Grade 1 . | Grade 2 . | Grade 3 . |
|---|---|---|---|---|
| Hair loss, neck and head | Normal | Scattered loss (thinning) | Multi-patchy hair loss | Confluent hair loss (alopecia) |
| Hair loss, chest & abdomen Area involved | Normal | Scattered loss <20% | Multiple stripes ≥20%, <50% | Diffuse involvement ≥50% |
| Skin integrity tail | Normal | Hair loss, patchy-hyperkeratosis | Hair loss, diffuse-hyperkeratosis, areas of desquamation | Grade 2 changes plus necrosis |
| Perianal and anal mucosa | Normal | Perianal inflammation | Inflammation and prolapse of anal mucosa |
RESULTS
Induction of GVHD.
Unlike humans, only a small number of T cells are present in murine bone marrow. According to our flow cytometric study, CD3+ T cells account for less than 1% of total bone marrow cells harvested from CBA mice. Therefore, transplantation of allogeneic bone marrow cells alone does not always result in clinically apparent GVHD in BALB/c recipient mice. Addition of appropriate numbers of spleen MNL to BMC before transplantation is necessary to induce clinically apparent GVHD. To determine the optimal numbers of spleen MNL that are required for inducing clinically significant GVHD, we first studied how different numbers of spleen MNL mixed with 5 × 106BMC affected the severity of GVHD. The results of our study indicate that moderate to severe GVHD can be induced by including 2.5 to 4 × 105 donor spleen MNL with 5 × 106BMC. Transplantation of 1 × 106 or more spleen MNL was associated with hyperacute GVHD in which most recipient BALB/c mice died within 8 days after transplantation. Inclusion of less than 1 × 105 spleen MNL was associated with nil to mild GVHD. GVHD was judged by the presence of hair loss, skin changes, body weight loss, and stunted weight gain and scored according to the criteria described in Table 1.
GVHD after transplantation with bone marrow and spleen cells from tolerant donors.
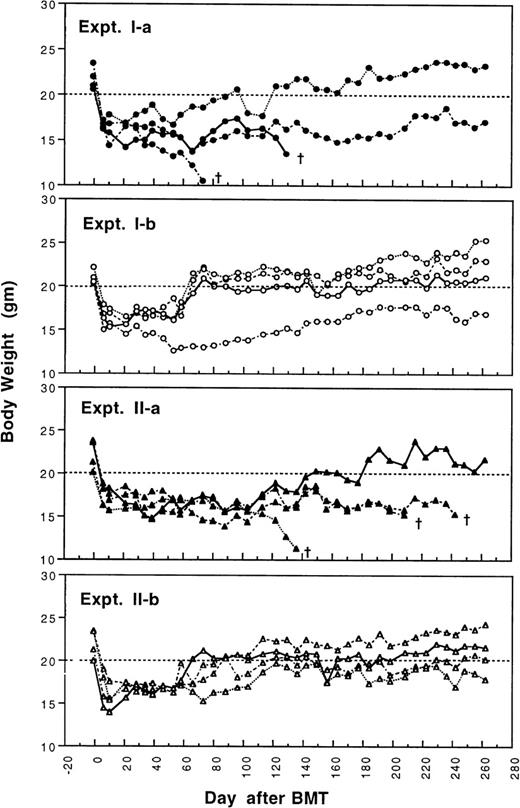
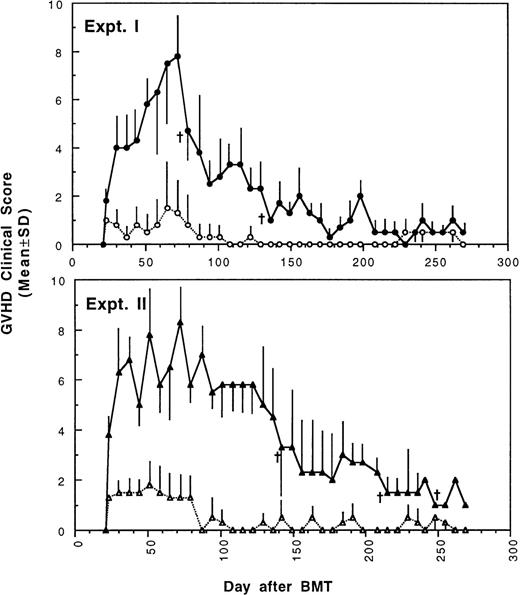
To determine whether GVHD can be prevented or attenuated by transplantation of cells from CBA mice tolerant to MHC antigens of BALB/c mice, we studied how the addition of 4 × 105or 2.5 × 105 spleen MNL from naive or tolerant CBA mice to their respective bone marrow cells affected the transplantation associated GVHD in recipient BALB/c mice. All transplanted BALB/c mice were followed weekly for changes in body weight and development of GVHD. As shown in Fig 1 and2, recipient BALB/c mice transplanted with two different doses of naive control spleen cells and 5 × 106 BMC had poor body weight gain and moderate to severe GVHD. Most BALB/c mice transplanted with two different doses of tolerant spleen MNL and 5 × 106 BMC had better body weight recovery (Fig 1) and significantly reduced GVHD (Fig2) when they were compared with recipients of naive control cells.
Changes of body weight after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow cells and either 2.5 × 105 (Experiment I) or 4 × 105 (Experiment II) spleen MNL from naive (Experiment Ia and IIa) or tolerant (Experiment Ib and IIb) CBA mice. There were four mice in each group. (†): Death of transplanted BALB/c mouse.
Changes of body weight after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow cells and either 2.5 × 105 (Experiment I) or 4 × 105 (Experiment II) spleen MNL from naive (Experiment Ia and IIa) or tolerant (Experiment Ib and IIb) CBA mice. There were four mice in each group. (†): Death of transplanted BALB/c mouse.
Development of GVHD after transplantation of lethally irradiated BALB/c mice with bone marrow and spleen MNL from naive (•,▴) or tolerant (○,▵) CBA mice as described in Fig 1. Experiment I: 5 × 106 bone marrow cells + 2.5 × 105 spleen MNL. Experiment II: 5 × 106 bone marrow cells + 4 × 105 spleen MNL. Each value is mean ± SD.
Development of GVHD after transplantation of lethally irradiated BALB/c mice with bone marrow and spleen MNL from naive (•,▴) or tolerant (○,▵) CBA mice as described in Fig 1. Experiment I: 5 × 106 bone marrow cells + 2.5 × 105 spleen MNL. Experiment II: 5 × 106 bone marrow cells + 4 × 105 spleen MNL. Each value is mean ± SD.
Engraftment and recovery of peripheral leukocytes.
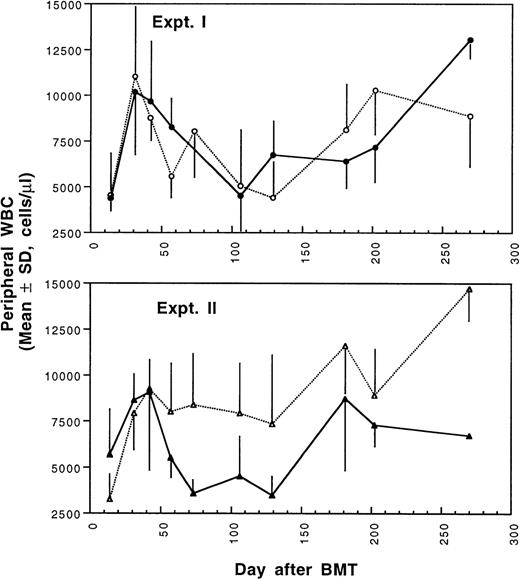
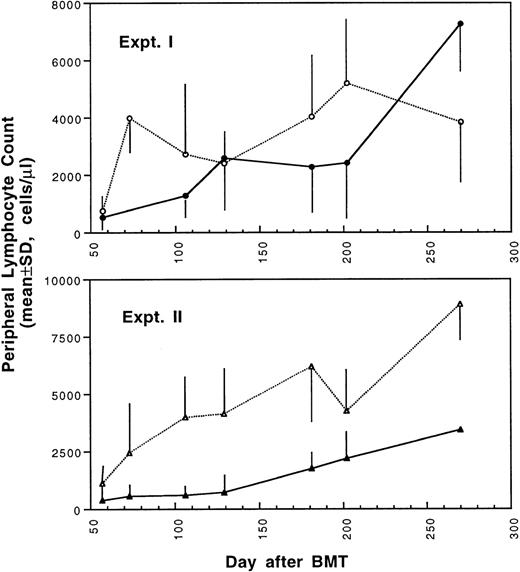
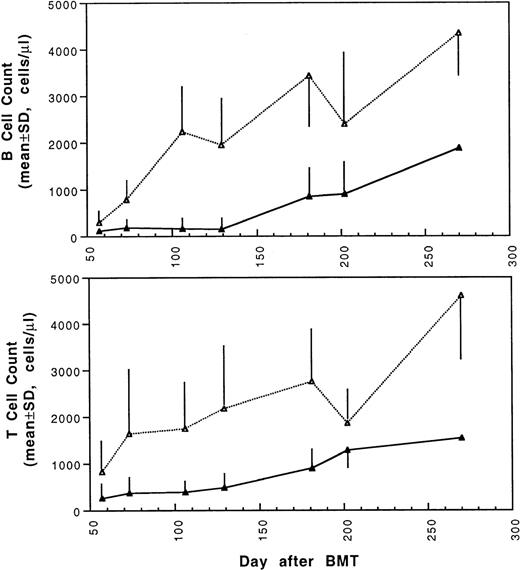
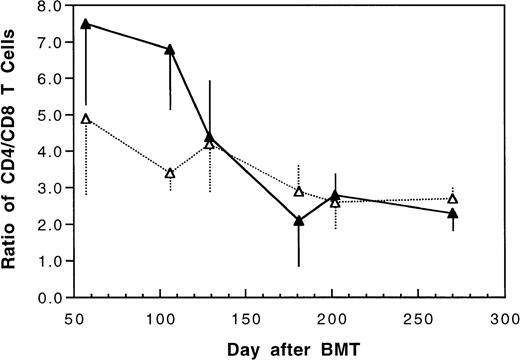
Two weeks after BMT, all H-2d recipient mice became completely engrafted with donor H-2k+ leukocytes in peripheral blood as assessed by immunofluorescence flow cytometry. To determine whether there was any difference in the rate of engraftment, peripheral total and differential leukocyte counts were followed. Peripheral lymphocyte counts including T and B cells subsets were measured according to peripheral blood total and differential leukocyte counts and immunophenotyping results for T and B cells by flow cytometry. As shown in Fig 3, there was no significant difference in the initial recovery of peripheral leukocyte counts between BALB/c recipient mice transplanted with either naive or tolerant donor bone marrow and spleen cells. Nevertheless, lymphocyte recovery was faster especially in recipients of 4 × 105 tolerant spleen MNL (Fig 4, Experiment II). BALB/c mice transplanted with 5 × 106BMC and 4 × 105 spleen MNL from tolerant donors also showed a faster and more complete recovery of T and B cells in the peripheral blood (Fig 5). In addition, it was noted that the recovery of CD8+ T cells was also faster after transplantation with bone marrow and spleen cells from tolerant donor mice (Fig 6). As shown in Fig 6, the recovery of CD8+ T cells in general lagged behind CD4+ T cells in our murine bone marrow transplantation model.
Changes of WBC counts in peripheral blood after transplantation of lethally irradiated BALB/c mice with bone marrow and spleen cells from naive (•,▴) or tolerant (○,▵) CBA mice as described in Fig 1. Experiment I: 5 × 106 bone marrow cells + 2.5 × 105 spleen MNL. Experiment II: 5 × 106 bone marrow cells + 4 × 105 spleen MNL. Each value is mean ± SD.
Changes of WBC counts in peripheral blood after transplantation of lethally irradiated BALB/c mice with bone marrow and spleen cells from naive (•,▴) or tolerant (○,▵) CBA mice as described in Fig 1. Experiment I: 5 × 106 bone marrow cells + 2.5 × 105 spleen MNL. Experiment II: 5 × 106 bone marrow cells + 4 × 105 spleen MNL. Each value is mean ± SD.
Changes of lymphocyte counts in peripheral blood after transplantation of lethally irradiated BALB/c mice with bone marrow and spleen cells from naive (•,▴) or tolerant (○,▵) CBA mice as described in Fig 1. Experiment I: 5 × 106 bone marrow cells + 2.5 × 105 spleen MNL. Experiment II: 5 × 106 bone marrow cells + 4 × 105 spleen MNL. Each value is mean ± SD.
Changes of lymphocyte counts in peripheral blood after transplantation of lethally irradiated BALB/c mice with bone marrow and spleen cells from naive (•,▴) or tolerant (○,▵) CBA mice as described in Fig 1. Experiment I: 5 × 106 bone marrow cells + 2.5 × 105 spleen MNL. Experiment II: 5 × 106 bone marrow cells + 4 × 105 spleen MNL. Each value is mean ± SD.
Changes of B and T cell counts in peripheral blood after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow and 4 × 105 spleen cells from naive (▴) or tolerant (▵) CBA mice. Each value is mean ± SD.
Changes of B and T cell counts in peripheral blood after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow and 4 × 105 spleen cells from naive (▴) or tolerant (▵) CBA mice. Each value is mean ± SD.
Changes of CD4/CD8 T cell ratio in peripheral blood after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow and 4 × 105 spleen cells from naive (▴) or tolerant (▵) CBA mice. Each value is mean ± SD.
Changes of CD4/CD8 T cell ratio in peripheral blood after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow and 4 × 105 spleen cells from naive (▴) or tolerant (▵) CBA mice. Each value is mean ± SD.
Survival after BMT.
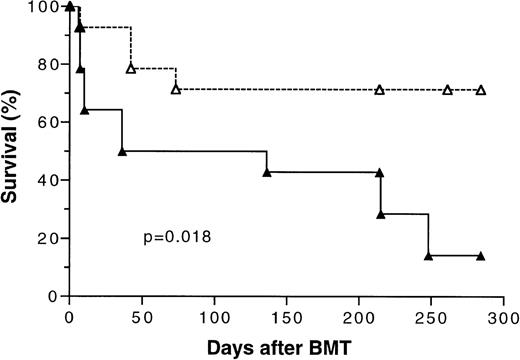
Because the initial results of our study (Fig 1) showed that transplantation of tolerant BMC and spleen MNL had a significant positive effect on overall survival, two additional bone marrow transplantation experiments using 5 × 106 BMC and 4 × 105 spleen MNL were conducted to confirm this finding. There were five mice in each experimental group. The survival data from these three separate experiments were pooled and analyzed. As shown in Fig 7, the overall long-term survival of mice transplanted with cells from tolerant donors was 72% versus 17% (P =.018) for those transplanted with naive cells.
Kaplan-Meier survival curve of lethally irradiated BALB/c mice transplanted with 5 × 106 bone marrow cells and 4 × 105 spleen MNL from naive (▴) or tolerant (▵) CBA mice. The data pooled from three separate experiments were analyzed. There were five mice in each treatment group in two experiments and four mice in one experiment. P value was determined by logrank test. A total of 14 mice were included in each treatment group.
Kaplan-Meier survival curve of lethally irradiated BALB/c mice transplanted with 5 × 106 bone marrow cells and 4 × 105 spleen MNL from naive (▴) or tolerant (▵) CBA mice. The data pooled from three separate experiments were analyzed. There were five mice in each treatment group in two experiments and four mice in one experiment. P value was determined by logrank test. A total of 14 mice were included in each treatment group.
T-cell cytokine response after BMT.
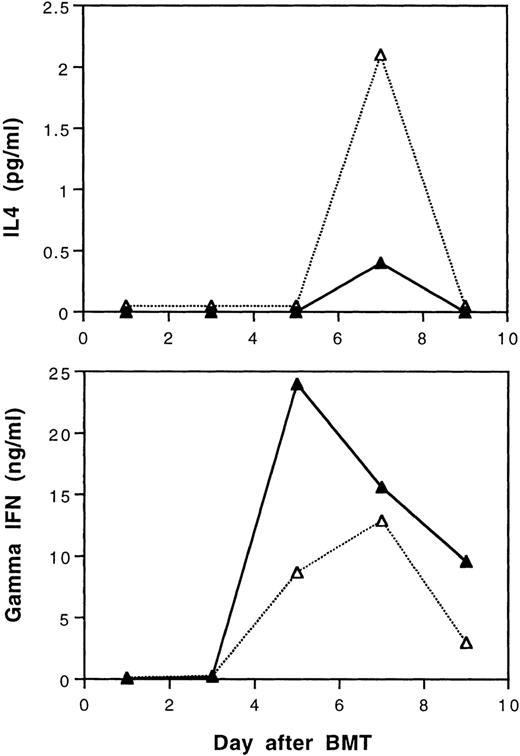
According to our recent study,29 tolerance induction by transfusions with UVB-irradiated BALB/c leukocytes polarizes T cells of CBA mice to produce of type-2 cytokines during in vitro stimulation by BALB/c spleen cells. It was of interest to study the serum levels of γ-IFN and IL-4 in BALB/c mice after transplantation with cells from tolerant CBA donors. In this study, three mice from each treatment group were randomly selected and sacrificed every other day until day 9 after BMT. Equal volumes of serum samples from three mice were pooled to obtain sufficient amounts of samples for assays of IL-4 and γ-IFN. The results show that recipient mice transplanted with tolerant BMC and spleen MNL had lower peak serum levels of γ-IFN and higher levels of IL-4 than those transplanted with cells from naive CBA donor mice after BMT, respectively (Fig 8).
Serum IL-4 and gamma interferon levels after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow and 4 × 105 spleen cells from naive (▴) or tolerant (▵) CBA mice. Three recipient mice from each treatment group were sacrificed every other day and equal volumes of serum samples were pooled for the assays. The sera were assayed in duplicate. Variations between duplicates were less than 17%. Similar results were obtained when the experiment was repeated.
Serum IL-4 and gamma interferon levels after transplantation of lethally irradiated BALB/c mice with 5 × 106 bone marrow and 4 × 105 spleen cells from naive (▴) or tolerant (▵) CBA mice. Three recipient mice from each treatment group were sacrificed every other day and equal volumes of serum samples were pooled for the assays. The sera were assayed in duplicate. Variations between duplicates were less than 17%. Similar results were obtained when the experiment was repeated.
DISCUSSION
T cells in allogeneic bone marrow are responsible for BMT-associated GVHD.7-10 Therefore, rendering donor T cells tolerant toward recipient MHC antigens offers an attractive approach to prevent or reduce GVHD. Our recent study showed that humoral immune tolerance to allogeneic MHC antigens can be induced without nonspecific immunosuppression by transfusions of UVB-irradiated leukocytes from H-2 disparate mice.26 It was therefore of interest to learn whether GVHD can be prevented by transplantation of bone marrow and spleen mononuclear cells from H-2 disparate donor mice that have been tolerized towards MHC antigens of BMT recipient mice. To answer this question, we conducted such a study. The results reported herein show that transplantation with bone marrow and spleen cells from tolerant CBA (H2k) donor mice to BALB/c (H2d) recipient mice is associated with significant attenuation of GVHD, better long-term survival, faster reconstitution of peripheral lymphocytes, and Th2 cytokine response immediately after transplantation. Because cell-mediated immunity plays an important role in the pathogenesis of GVHD, these findings suggest that transfusions of UVB-irradiated leukocytes may induce cellular immune tolerance. This conclusion is further substantiated by our recent study of cytotoxic T-cell activity against H-2d+ target cells in the CBA mice that had been tolerized by transfusions of UVB-irradiated BALB/c mononuclear leukocytes and subsequently challenged with two fully immunogenic doses of untreated BALB/c leukocytes (unpublished observation).
In our study, two different doses of spleen MNL (2.5 × 105 and 4 × 105) were used to induce GVHD. These two doses were selected for their ability to elicit significant degrees of acute GVHD without causing premature death so that the transplanted mice could be followed for an extended period of time. Among BALB/c recipient mice transplanted with cells from naive donor mice, we noted that recipient BALB/c mice transplanted with a lower number (2.5 × 105) spleen MNL had less severe and lasting GVHD (Fig 2). This finding confirms the quantitative importance of donor lymphocytes in eliciting GVHD as previously reported.30-33 The results of our study also showed that 1 month after BMT, peripheral leukocyte counts recovered to normal levels in most recipient mice regardless of whether donor cells were from naive or tolerant CBA mice. However, during the next 4 months peripheral leukocyte counts began to decline until GVHD largely resolved 150 days after BMT. In addition to GVHD, this decline of peripheral leukocyte counts was likely a result of delayed repopulation by more primitive long-term repopulating hematopoietic stem cells as observed in bone marrow transplant recipients.34 35 We also noted that recovery of peripheral T- and B-lymphocyte counts was faster in mice transplanted with bone marrow and spleen cells from the tolerized CBA donors (Fig 5). The cell counts and immunophenotyping results are consistent with the subjective clinical assessment of GVHD. All these findings support the beneficial effect of using cells from the tolerized donor mice for bone marrow transplantation across major MHC barrier. However, it is not known whether faster quantitative lymphoid reconstitution would translate to better immune function. Further studies in this regard are needed.
According to cytokine production profile, two subsets of helper (Th) and cytotoxic (Tc) T cells have been identified.36,37Secretion of γ-IFN and tumor necrosis factor-β (TNF-β) defines type-1 Th cells (Th1), and secretion of IL-4, IL-5, and IL-10 defines type-2 Th cells (Th2). Th1 cells are functionally involved in cell-mediated immunity, delayed-type hypersensitivity, production of IgG2a and IgG2b antibodies, and host defense to infection of intracellular pathogens.38-42 Th2 cells are involved in production of IgG1 and IgE antibodies, allergic conditions, and host defense to infection of extracellular pathogens.38-42 Th1 and Th2 responses during host-immune response have been shown to be mutually inhibitory.42 Thus, immune responses can be characterized by polarization toward Th1 or Th2 response.
Induction of tolerance by priming neonatal mice with allogeneic leukocytes has been associated with increased production Th2 cytokines and reduced secretion of Th1 cytokines.43 The finding indicates that tolerance induction leads the immune system towards an enhanced Th2 response.44-46 Our recent study of cytokine production in mixed leukocyte culture reactions using tolerized CBA spleen T lymphocytes as responder and BALB/c spleen leukocytes as stimulators showed an increase in IL4 and IL5 production (data not shown). In allogeneic BMT, donor lymphocytes with enhanced Th2 cytokine response have been associated with a much-reduced acute GVHD.47 For this reason, we measured serum IL-4 and γ-IFN levels after transplantation with bone marrow and spleen cells from tolerant or naive donor CBA mice. Our results showed an enhanced transient Th2 cytokine response in recipient mice transplanted with cells from tolerant donors. The results are consistent with our recent in vitro MLC finding and support the earlier reports that polarization of donor T cells to Th2 response is associated with reduced severity of acute GVHD.47-49 Nevertheless, we are unable to ascertain whether the observed differences in cytokine production were due to donor cells, recipient cells, or both. Although the polarized Th2 response in donors may contribute to protection of acute GVHD as suggested by various investigators,47-49 it is possible that donor Th2 cytokine response only reflects changes in the effector arm of the donor immune system and may not play a direct role in the prevention of GVHD associated with allogeneic BMT. The exact mechanism by which Th2 polarization of the bone marrow donor immune system could attenuate acute GVHD remains to be further elucidated.
In the present study, we found that acute GVHD can not be completely prevented by transplantation of bone marrow and spleen cells from tolerant CBA donors (Fig 2). This finding is not unexpected. Our recent MLC study using T cells from tolerant CBA mice as responders and gamma-irradiated or mitomycin-treated spleen leukocytes from BALB/c mice as stimulators indicated that T cells from tolerant CBA mice remain capable of producing type-1 T-cell cytokines such as γ-IFN but at a reduced level.29 Because type-1 T-cell cytokines have been implicated in the pathogenesis of acute GVHD,50 the reduced production of type-1 T-cell cytokines by T cells from tolerant donor mice might be responsible for the attenuated GVHD observed in our experiments.
In view of the feasibility and the safety of transfusing patients with UVB-irradiated platelet concentrates,51 the results of our study support the potential clinical application of UVB-irradiated leukocytes for tolerance induction in bone marrow donors to prevent GVHD. However, the safety and the ethical concerns of using leukocytes from patients with neoplastic diseases to induce tolerance in healthy bone marrow donors preclude such an approach. Nevertheless, tolerance induction in related bone marrow donors may be considered for patients who suffer from hereditary non-neoplastic hematological disorders, such as sickle cell anemia and thalassemia and who are not at risk of transmitting infectious diseases to their bone marrow donors. Another potential approach is to induce immune tolerance in patients using UVB-irradiated leukocytes from healthy bone marrow donors. The tolerized patients would then undergo bone marrow transplant using a nonmyeloablative conditioning regimen. Such an approach may allow the establishment of macro-mixed chimerism that is sufficient to ameliorate non-neoplastic hematological disorders without increasing the risk of graft rejection. At the same time, high-dose chemoradiotherapy related toxicity is avoided. Obviously, further animal studies are needed before the proposed approach can be applied to the clinical setting.
ACKNOWLEDGMENT
The authors are grateful to Sandra Donahue for her excellent technical assistance.
Supported by Grant R01 HL-58809 from the National Institutes of Health.
Address all correspondence to K.J. Kao, MD, Box 100275, Department of Pathology, Immunology & Laboratory Medicine, University of Florida, Gainesville, FL 32610; e-mail: kjkao@ufl.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal