The success of bone marrow transplantation (BMT) from HLA-disparate donors depends on the development of new strategies able, on one hand, to efficiently prevent graft-versus-host disease (GVHD) and, on the other hand, to protect leukemic patients from relapse and infections. Using an immunotoxin (IT) directed against the chain (p55) of the human interleukin-2 receptor (RFT5-SMPT-dgA), we previously showed that it is possible to kill mature T cells activated against a specific HLA complex by a one-way mixed lymphocyte culture (MLC). The present study was performed to investigate whether this protocol of allodepletion affects the capacity of residual T cells to display antileukemia and antiviral activity evaluated by limiting dilution assays (LDA), measuring the frequency of cytotoxic T-lymphocyte precursors (CTLp) directed against autologous leukemic blasts (LB) and cytomegalovirus (CMV)- and Epstein-Barr virus (EBV)-infected target cells. Antileukemia activity was evaluated in peripheral blood mononuclear cells (PBMC) of 3 patients treated for acute myeloid leukemia who had developed a high frequency of LB-reactive CTLp after either autologous or allogeneic BMT. Results demonstrate that (1) depletion with RFT5-SMPT-dgA efficiently inhibited MLC; (2) fresh PBMC of patients yielded a high frequency of LB-reactive CTLp comparable to that of the mock-treated PBMC; and (3) effector cells obtained after allodepletion fully retained the capacity to lyse pretransplant LB. By contrast, the frequency of CTLp directed against patient’s pretransplant BM remission cells was always undetectable. Data obtained in 4 healthy donors showed that specifically allodepleted T cells recognized and killed autologous CMV-infected fibroblasts and autologous EBV–B-lymphoblastoid cell lines. In conclusion, our data indicate that allodepletion using RFT5-SMPT-dgA efficiently removed alloreactive cells, while sparing in vitro antileukemic and antiviral cytotoxic responses.
HEMATOPOIETIC STEM cell transplantation (HSCT) is a commonly used therapy for malignant diseases and inborn errors. Unfortunately, less than one third of patients eligible for this procedure have an HLA-identical related donor. The feasibility of HSCT from related HLA-partially matched donors has been demonstrated in pediatric patients receiving HSCT for inborn errors, while worse results have been obtained in patients with malignancy.1Graft-versus-host disease (GVHD) remains a major problem with these transplants. Even though T-cell depletion of stem cells reduces the incidence and severity of this complication,2-4 it increases the risk of graft rejection and plays a major role in prolonging the period of immunodeficiency after transplantation. The incidence of rejection can be reduced either by increasing the number of stem cells infused through mobilization with granulocyte colony-stimulating factor (G-CSF)5 or by the administration of immunosuppressive agents to the recipients. In particular, anti-thymocyte globulin (ATG), Campath-1G,6 or monoclonal antibodies (MoAbs) directed against adhesion molecules such as anti–LFA-1 plus anti-CD27,8 proved to be of value in increasing the recipient’s immunosuppression. For the time being, the significant delay in immune reconstitution, due to removal of mature T cells from donor marrow, in vivo immunosuppression, and HLA disparity between donor and recipient, remains the major problem of HSCT from HLA-disparate donors, because it contributes to the dramatic incidence of life-threatening viral and fungal infections observed after this type of HSCT and, in leukemia patients, to disease recurrence.9,10 The development of new strategies able, on one hand, to efficiently prevent GVHD and, on the other hand, to protect the patients from relapse and infection by accelerating their immunological reconstitution could both extend the applicability of HSCT and increase the success of this procedure. In the last few years, several groups have attempted to develop different methods of T-cell manipulation to block and/or to kill mature T cells activated by HLA antigens. In particular, two different ex vivo approaches have been recently proposed for this aim. The first approach tries to induce host alloantigen-specific anergy in human donor T cells before allogeneic HSCT by using CTLA4-Ig either alone11 or in combination with cyclosporine A.12 The second approach tries to eliminate alloreactive T cells after specific activation through their killing13,14 or fluorescence-activated cell sorting,15,16 while sparing T cells with other functions. In a previous human preclinical study, we demonstrated that allospecific T-cell depletion by using an immunotoxin (IT), directed against the p55 chain of interleukin-2 (IL-2) receptor, was feasible, reproducible, and specific.14 Even though the proliferative response to alloantigens does not predict with absolute reliability the development of GVHD, the marked inhibition of alloreactivity through this IT provided a rational basis for considering the use of this approach in humans. The spared T cells were still able to proliferate against third-party cells, Candida, and cytomegalovirus (CMV) antigens.17 Moreover, in vivo studies in a murine animal model showed that this particular T-cell depletion was efficient, at least partially, in preventing both graft rejection and GVHD in a complete haplotype-mismatched combination.18 However, so far, few data are available regarding the possibility to maintain antileukemia reactivity after elimination of alloreactive T cells.19
The clinical and experimental data reported in the literature on the possibility of separating GVHD and graft-versus-leukemia reaction (GVLR) still remain controversial.20-22 We previously reported23 that cytotoxic T-cell clones reactive to autologous leukemic blasts (LB) but not to autologous bone marrow remission cells (BMRC) can be obtained from peripheral blood mononuclear cells (PBMC) of leukemic children by in vitro lymphocyte stimulation with autologous tumor cells and recombinant IL-2 (rIL-2).23 Moreover, we showed that cytotoxic T-lymphocyte precursors (CTLp), specifically reactive towards recipient LB, were undetectable or low in donor peripheral blood, although their frequency reached high values in the recipients after transplantation.24 This provided evidence for an in vivo expansion of donor T cells able to recognize leukemic recipient cells.
The aim of this study was to assess whether in vitro alloreactive T-cell depletion could affect the capacity of spared T cells to kill LB or virus-infected cells. To address these issues, we established a limiting dilution assay (LDA) to measure the frequency of CTLp directed against autologous LB, CMV-infected fibroblasts, and Epstein-Barr virus lymphoblastoid cell lines (EBV-LCL) before and after depletion of alloreactive T cells. Antileukemia activity was assessed in PBMC of 3 patients with acute myeloid leukemia (AML) who had developed a high frequency of LB-reactive CTLp after either autologous or allogeneic HSCT. PBMC of patients were activated in vitro in a one-way mixed lymphocyte culture (MLC) against PBMC of their father, depleted of activated T cells by an anti-CD25-ricin α chain IT (RFT5-SMPT-dgA),25,26 and then tested for antileukemia activity. This new IT has been previously used in a murine model27 and in a Food and Drug Administration-approved clinical phase I trial on patients with refractory Hodgkin’s lymphoma.28 Reactivity against virus-infected cells was evaluated in PBMC of healthy controls after allodepleted MLC. We showed that allodepletion using RFT5-SMPT-dgA efficiently removed alloreactive T cells but did not significantly affect in vitro antileukemia and antivirus responses.
MATERIALS AND METHODS
Isolation of PBMC and BM cells from patients and healthy subjects.
Three children with AML were studied to evaluate antileukemia activity after elimination of alloreactive T cells activated against paternal HLA-antigens. One patient (BP) had been treated with autologous BM transplantation (BMT), whereas patients ER and GA had received allogeneic BMT from HLA-identical sibling donors. Patients were analyzed 6 months after BMT, when the presence of sizeable values of LB-directed CTLp was documented. Persistence of antiviral activity was evaluated in PBMC of 4 healthy donors.
Cell preparation and cell line establishment.
Heparinized BM aspirate containing greater than 90% LB was obtained from patients at the time of diagnosis and BMRC were collected after demonstration of complete hematological remission. PBMC of patients were collected 6 months after either allogeneic or autologous BMT, while patients were in remission. BMRC and PBMC were isolated by Ficoll-hypaque density gradient of anticoagulated whole blood, cryopreserved in fetal calf serum (FCS; GIBCO Ltd, Paisley, UK) supplemented with 10% dimethyl sulfoxide (DMSO), and stored in liquid nitrogen.
IT.
RFT5-SMPT-dgA. RFT5 is a murine anti-CD25 MoAb (IgG1) selected from a group of 25 antibodies based on its ability to form a potent IT and to stain only activated T cells in a panel of 28 normal human tissues.25 This IT was prepared by using the hindered heterobifunctional crosslinker N-succinimidyloxycarbonyl-α-methyl-(2-pyridyldithio) toluene (SMPT)29 and chemically deglycosylated ricin α chain (dgA) according to published reports.30
Cell activation and in vitro treatment with RFT5-SMPT-dgA.
The experimental system to activate T lymphocytes was a one-way MLC, as previously described.14 Briefly, 25 × 106PBMC from patients or healthy donors (A) were incubated at 37°C for 2 days with 25 × 106 irradiated (3,000 rads) stimulating PBMC from haploidentical parents referred as B*. Th medium used was RPMI 1640 (GIBCO) supplemented with 2 mmol/L L-glutamine, 50 μg/mL gentamicine, and 10% human AB serum (RPMI-HS). All cultures were performed in 25-cm2 flasks (Corning, Corning, NY) in a final volume of 20 mL. The unstimulated control was performed with 25 × 106 irradiated autologous PBMC referred as A*.
After in vitro activation, the cells were harvested, washed twice, resuspended at 10 × 106 cells/mL, and treated overnight at 37°C with the IT in RPMI 1640 containing 20 mmol/L NH4Cl (pH 7.8) to increase the cytotoxicity of RFT5-SMPT-dgA. After two washes, cells were resuspended in RPMI-HS at 2.5 × 106 cells/mL and used immediately for proliferation and LDAs.
Study of MLC inhibition.
Two hundred microliters of treated and mock-treated cell mixtures (AB* and AA*) were plated in duplicate in 96-well, round-bottomed microtiter plates (Limbro; Flow Lab, Scotland) and incubated at 37°C in 5% CO2 until day 6.
One hundred microliters of the same cell mixtures was also cocultured in duplicate with 100 μL of irradiated (3,000 rads) PBMC of a third-party HLA nonidentical subject referred as C* and cultured for 6 days (ie, day 8 from the start of each experiment). The cultures were pulsed with 1 μCi of (3H) thymidine (Amersham, Amersham, UK) for 18 hours, harvested, and counted in a liquid scintillation counter (MINAXI-TRICARB 4000; Packard Instruments, Zürich, Switzerland).
The MLC results (mean cpm of duplicate cultures) were expressed as the percentage of the respective controls according to the formula: (AB* [IT-treated] − AA* [IT-treated])/(AB* [untreated] − AA* [untreated]) × 100. Similarly, the proliferation against third-party cells (C*) was calculated as the percentage of the mock-treated sample.
LDA for evaluation of LB-reactive CTLp.
To evaluate the persistence of antileukemia activity after allodepletion, fresh PBMC or cultured-treated and mock-treated patient cells were seeded as responder cells with autologous LB used as stimulator cells in 96-well round-bottom microplates. Briefly, decreasing numbers of responder cells (4 × 104, 104, 5 × 103, 2.5 × 103, and 1.25 × 103) were seeded together with 2 × 104 irradiated LB (7,000 rads). When IT- or mock-treated cells were used as responders, 2 × 104irradiated autologous PBL were added to the cultures as feeder cells. Control wells contained irradiated autologous LB without responder cells. The medium used was RPMI-HS supplemented with 100 U/mL of rIL-2 (Hoffman-La Roche, Basel, Switzerland). The cultures were incubated at 37°C in 5% CO2. On day 10, wells were accurately resuspended, and 100 μL of each well was transferred for split experiments. Cultures were then tested for cytolytic activity against both LB and autologous BMRC.
Production of EBV–B-lymphoblastoid cell line (B-LCL).
PBMC were incubated with EBV-containing supernatant from the B95.8 cell line (American Type Culture Collection, Rockville, MD) in the presence of 800 ng/mL of cyclosporin A in RPMI 1640 medium supplemented with 2 mmol/L L-glutamine, 50 μg/mL gentamicin, and 10% FCS (RPMI-FCS). Cells were continuously incubated at 37°C, 5% CO2 for 3 to 4 weeks. Each week, 2 mL of culture medium was removed and 2 mL of fresh medium was added until growth of B-LCL was established.
LDA for evaluation of CMV and EBV-specific CTLp.
To evaluate CTLp frequency to CMV-infected fibroblasts, fresh PBMC or cultured IT-treated and mock-treated cells obtained from healthy controls were seeded in a final volume of 200 μL in 96-well round-bottom microplates. Autologous fibroblast were infected with AD169 strain CMV following a previously described method.31A decreasing number of responder cells (104, 5 × 103, 2.5 × 103, 1.25 × 103, and 0.6 × 103) were stimulated with 2 × 103 autologous infected fibroblasts. When the IT- or mock-treated cells were used as responders, 4 × 104 irradiated (3,000 rads) were added as feeder cells. On days 4 and 9, 40 U/mL rIL-2 was added to the cultures. On day 12, the plates were accurately splitted as described above and tested against CMV-infected and mock-infected fibroblasts. For evaluation of EBV-specific CTLp, responder cells were stimulated with autologous irradiated B-LCL following a previously described method.32
Cytotoxicity assay.
At the end of the cultures, effector cells were assayed for cytolytic activity against 51Cr-labeled targets. Target cells included autologous LB, autologous BMRC, CMV-infected or mock-infected fibroblasts, and EBV B-LCL. Briefly, 2 to 3 × 106 LB, autologous BMRC, and EBV B-LCL or 5 × 105 fibroblasts were labeled with 100 to 200 μCi 51Cr for 2 hours, washed four times, and added to the wells. Plates were incubated for 5 hours at 37° and then centrifuged at 200g for 10 minutes. Finally, 100 μL of supernatant was collected from each well and counted for 1 minute in a gamma-counter. To provide necessary controls, spontaneous and total 51Cr release from target cells were also determined. Spontaneous release from all types of target cells was consistently less than 20%.
Calculation of CTLp frequency.
Assay wells were defined as positive when 51Cr release exceeded the average plus 3 sD of control wells. The frequency of responding cells was determined by maximum likelihood estimation using a statistical program and the variance by the use of 95% confidence limits.33
Generation of leukemia-reactive T-cell clones.
LB-reactive effector cells of patient ER were recovered from pooled positive wells obtained from 10-day LDA of both untreated and allodepleted cultures and seeded into Terasaki trays at 0.3 cells/well in the presence of rIL-2 (200 U/mL), phytohemagglutinin (PHA; GIBCO; M-form 1:100), 2 × 105/mL irradiated (7,000 rads) autologous LB, and 5 × 105/mL of allogeneic irradiated (3,000 rads) feeder cells. After 12 to 14 days of culture, all growing wells were harvested and expanded by cultivation in 96-well flat-bottom plates with 106/mL irradiated allogeneic feeder cells in HS-RPMI containing 200 U/mL rIL-2 and PHA. Conditions for maintenance of T-cell clones (TCC) have been described elsewhere.34 The clones thus obtained were screened for their capacity to lyse recipient LB in a 51Cr release assay. Thereafter, LB-reactive TCC were further characterized for their surface phenotype and specificity.
MoAbs for phenotyping and blocking experiments.
MoAbs used in this study included anti-Leu4(CD3)-fluorescein isothiocyanate (FITC) or -phycoerythrin (PE), anti-Leu 3a(CD4)-PE, anti-Leu-2a(CD8)-PE, anti-Leu28 (CD28)-PE, anti-BB-1:B7 (CD80)-FITC, anti-TCR αβ-FITC, anti-TCR γδ-FITC (Becton Dickinson, Mountain View, CA), and anti-CD86-FITC (Pharmigen, San Diego, CA). Phenotypic analysis was performed by means of direct or indirect immunofluorescence on a FACScan flow cytometer (Becton Dickinson). Blocking experiments of cytolytic activity with TR66 anti-CD3 and with anti-HLA class I MoAb (W6/32) were performed as follows. (1) Target cells were previously incubated with anti-HLA (25 μg/mL) for 30 minutes at 4°C. (2) Effector cells were previously incubated with anti-CD3 MoAb (1 μg/mL) for 30 minutes at 4°C. Subsequently, the same concentration of each MoAb was added to the cultures in a 4-hour standard cytotoxicity assay.
RESULTS
Specific inhibition of an MLC (AB*).
IT-induced specific inhibition of MLC was verified before assaying each limiting dilution experiment for evaluation of leukemia-specific or virus-specific CTLp frequency. The functional depletion of A anti-B reactive cells was tested in a 2-day AB* MLC. The proliferative capacity of treated T cells towards third-party cells (C*), unrelated to B*, was assessed to evaluate the spared T-cell reactivity towards unrelated HLA antigens. The residual proliferative capacity of responder cells versus B* ranged between 0% and 1.5% of the control. By contrast, the alloreactive response against C* was almost completely preserved (Table 1).
Residual Proliferation of IT–Treated PBL in Primary MLC
| Experiment No. . | AB*/B* . | AB*/C* . | ||
|---|---|---|---|---|
| Untreated (cpm ×10−3) . | Treated (cpm ×10−3) (%) . | Untreated (cpm ×10−3) . | Treated (cpm ×10−3) (%) . | |
| 1 (pt BP) | 17.4 | 0.3 (1.7) | 13.6 | 12.2 (70.5) |
| 2 (pt ER) | 47.4 | 0.9 (1.9) | 43.6 | 51.6 (108) |
| 3 (pt GA) | 31.5 | 0.6 (1.9) | 27.8 | 30.8 (96.7) |
| 4 (donor 1) | 16.4 | 0.3 (1.8) | 25.2 | 24.1 (150) |
| 5 (donor 2) | 51.7 | 0.5 (0.9) | ND | ND |
| 6 (donor 3) | 21.3 | 0.4 (1.9) | ND | ND |
| 7 (donor 4) | 13.1 | 0.2 (1.5) | 7.2 | 19.1 (146) |
| Experiment No. . | AB*/B* . | AB*/C* . | ||
|---|---|---|---|---|
| Untreated (cpm ×10−3) . | Treated (cpm ×10−3) (%) . | Untreated (cpm ×10−3) . | Treated (cpm ×10−3) (%) . | |
| 1 (pt BP) | 17.4 | 0.3 (1.7) | 13.6 | 12.2 (70.5) |
| 2 (pt ER) | 47.4 | 0.9 (1.9) | 43.6 | 51.6 (108) |
| 3 (pt GA) | 31.5 | 0.6 (1.9) | 27.8 | 30.8 (96.7) |
| 4 (donor 1) | 16.4 | 0.3 (1.8) | 25.2 | 24.1 (150) |
| 5 (donor 2) | 51.7 | 0.5 (0.9) | ND | ND |
| 6 (donor 3) | 21.3 | 0.4 (1.9) | ND | ND |
| 7 (donor 4) | 13.1 | 0.2 (1.5) | 7.2 | 19.1 (146) |
Data reported represent the mean of duplicate cultures for each independent experiment. Standard deviation was less than 10% of mean values.
Evaluation of LB-reactive CTLp frequency.
The frequency of CTLp directed against recipient LB in peripheral blood of the patients was investigated immediately after treatment with the IT. Baseline LB-reactive CTLp frequency was 1/14.296, 1/10.911, and 1/36.156 in the 3 patients tested, respectively. After allospecific T-cell depletion, an apparent increase in LB-reactive CTLp frequency was observed in all patients analyzed, with frequencies being 1/2.480, 1/5.141, and 1/24.374, respectively (Fig 1). Undetectable frequencies of CTLp were found against autologous BMRC both before and after treatment with IT (data not shown).
Surface phenotype and cytolytic activity of LB-reactive TCC.
Effector subsets of cytotoxic activity against recipient LB before and after T-cell–specific allodepletion were characterized by cloning in limiting dilution pooled positive wells from patient ER. The growing clones were expanded and then screened and selected for their capacity to lyse recipient LB but not BMRC in a cytotoxicity assay. Different subsets of LB-reactive TCC could be defined on the basis of the surface phenotype. In untreated cultures (when effector cells were fresh PBMC), we were able to isolate 5 LB-reactive TCC. Two TCC were CD3+/TCRαβ+/CD8+ cells, 1 TCC was CD3+/TCRγδ+/CD8+, 1 TCC was CD3+/TCRαβ+/CD4/CD8 double negative (DN), and the last was CD3+/TCRγδ+/CD4/CD8DN cells. Six TCC were obtained from cultures after depletion of alloreactive T cells: 4 of them were CD3+/TCRαβ+/CD8+ lymphocytes, 1 TCC was CD3+/TCRγδ+/CD8+, and 1 displayed CD3+/TCRγδ+/CD4/CD8DN phenotype.
LB-reactive CTLp frequency of PBMC (▪), IT-treated (▴), and mock-treated (•) cultures of the 3 patients (PB, ER, and GA). The fraction of negative wells was plotted on a logarithmic scale against the number of responder cells per well. Frequency and 95% confidence limits are also reported.
LB-reactive CTLp frequency of PBMC (▪), IT-treated (▴), and mock-treated (•) cultures of the 3 patients (PB, ER, and GA). The fraction of negative wells was plotted on a logarithmic scale against the number of responder cells per well. Frequency and 95% confidence limits are also reported.
Some LB-reactive TCC derived from either untreated and IT-treated cultures could be expanded sufficiently to be tested in blocking experiments with anti-CD3 and anti-HLA class I MoAbs (Table 2). The killing capacity of the αβ+/DN TCC, γδ+/DN TCC, and γδ+/CD8+ TCC was not affected by the addition of the two MoAbs. In contrast, the addition of these MoAbs strongly reduced cytolytic activity of αβ+/CD8+ TCC.
Effect of the Addition of Anti-CD3 and Anti-HLA Class I MoAbs on the Cytolytic Activity of LB-Reactive TCC Against Autologous Pretransplant LB
| TCC No. . | Phenotype . | % of Specific Lysis . | ||
|---|---|---|---|---|
| Untreated . | +Anti-CD3 . | +Anti-HLA Class I . | ||
| 6* | TCR αβ+/CD8+ | 35 | 2 | 6 |
| 8* | TCR αβ+/CD8+ | 68 | 9 | 8 |
| 13* | TCR αβ+/CD8−/CD4− | 26 | 32 | 28 |
| 15* | TCR γδ+/CD8+ | 26 | 30 | 21 |
| 2† | TCR αβ+/CD8+ | 58 | 26 | 8 |
| 7† | TCR αβ+/CD8+ | 36 | 6 | 4 |
| 15† | TCR γδ+/CD8+ | 15 | 12 | 17 |
| 18† | TCR γδ+/CD8−/CD4− | 35 | 30 | 25 |
| TCC No. . | Phenotype . | % of Specific Lysis . | ||
|---|---|---|---|---|
| Untreated . | +Anti-CD3 . | +Anti-HLA Class I . | ||
| 6* | TCR αβ+/CD8+ | 35 | 2 | 6 |
| 8* | TCR αβ+/CD8+ | 68 | 9 | 8 |
| 13* | TCR αβ+/CD8−/CD4− | 26 | 32 | 28 |
| 15* | TCR γδ+/CD8+ | 26 | 30 | 21 |
| 2† | TCR αβ+/CD8+ | 58 | 26 | 8 |
| 7† | TCR αβ+/CD8+ | 36 | 6 | 4 |
| 15† | TCR γδ+/CD8+ | 15 | 12 | 17 |
| 18† | TCR γδ+/CD8−/CD4− | 35 | 30 | 25 |
Results are expressed as the percentage of specific lysis at an E:T ratio of 5:1.
TCC isolated from untreated cultures.
TCC isolated from IT-treated cultures.
Evaluation of virus-specific CTLp frequency.
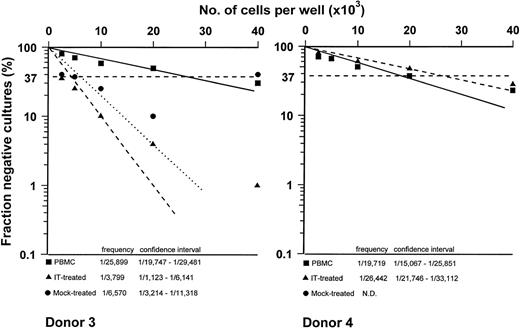
The capacity to kill autologous CMV-infected fibroblasts was evaluated in PBMC from 2 healthy controls. As shown in Fig 2, we observed that allodepletion did not affect the frequency of CMV-specific precursors, because in the first subject it was 1/19.834 in untreated cells versus 1/15.212 after allodepletion and in the second subject frequencies were 1/5.052 and 1/4.566, respectively. Reactivity against mock-infected fibroblasts was always undetectable. Similar results were obtained against EBV-LCL (Fig 3). In particular, CTLp frequencies were 1/25.899 and 1/17.719 in PBMC of the 2 donors tested, and they reached values of 1/3.799 and 1/26.442 after T-cell allodepletion, respectively.
CMV-specific CTLp frequency of PBMC (▪), IT-treated (▴), and mock-treated (•) cultures of the 2 donors. The fraction of negative wells was plotted on a logarithmic scale against the number of responder cells per well. Frequency and 95% confidence limits are also reported.
CMV-specific CTLp frequency of PBMC (▪), IT-treated (▴), and mock-treated (•) cultures of the 2 donors. The fraction of negative wells was plotted on a logarithmic scale against the number of responder cells per well. Frequency and 95% confidence limits are also reported.
EBV-specific CTLp frequency of PBMC (▪), IT-treated (▴), and mock-treated (•) cultures of the 2 donors. The fraction of negative wells was plotted on a logarithmic scale against the number of responder cells per well. Frequency and 95% confidence limits are also reported.
EBV-specific CTLp frequency of PBMC (▪), IT-treated (▴), and mock-treated (•) cultures of the 2 donors. The fraction of negative wells was plotted on a logarithmic scale against the number of responder cells per well. Frequency and 95% confidence limits are also reported.
DISCUSSION
The present study demonstrates that allospecific T-cell depletion obtained by using RFT5-SMPT-dgA does not affect the frequency of CTLp directed against autologous LB in transplanted patients who had developed a sizeable frequency of LB-directed CTLp after transplantation. Moreover, the spared T cells maintained a high frequency of CTLp directed against EBV- and CMV-infected cells. Treatment with RFT5-SMPT-dgA results in strong inhibition of primary MLC response from PBMC of subjects used to evaluate antileukemia and antiviral activities, whereas residual T cells maintain the capacity to proliferate in the presence of third-party stimulating cells.
In a previous study,18 we demonstrated that specific depletion of donor T cells reactive against the host led to a reduction in the incidence and severity of GVHD after haploidentical HSCT in mice. The persistence of antileukemia activity after removing host-specific reactive T cells is crucial for successful allogeneic HSCT in patients with malignancy. A number of clinical and experimental data indicate that mature adoptively transferred donor lymphocytes are involved in the generation of both GVHD and GVLR after HSCT. In particular, clinical evidence suggests the existence of a strong relationship between GVHD and GVLR.20-22 After allogeneic HSCT from HLA-matched sibling donors, it is believed that non–MHC-encoded minor histocompatibility antigens (mHAg) are involved in both GVHD and GVLR activities. Although widely distributed mHAg account for the GVLR associated to GVHD, tissue-restricted or leukemia-specific antigens can elicit a specific GVLR,35-42and it has been demonstrated that both CD4+ and CD8+ CTL recognizing mHAg in a classical MHC-restricted fashion can be generated in vitro.35,36 In particular, mHAg-specific CD8+ CTL can display strong lysis of mature leukemia cells as well as suppress, together with CD4+mHAg-specific CTL, the growth of clonogenic leukemia precursor cells.37 38
We have previously described how CTLp reactive towards recipient LB can be detected in PBMC of children receiving allogeneic HSCT.24 LB-reactive CTLp developed in the first months after transplant and persisted for at least up to 18 months in patients in hematological remission.24 The GVLR we observed in vitro seems not to be strictly correlated with GVHD, given that the emergence of a high frequency of LB-reactive CTLp was not related to the occurrence and severity of clinical GVHD. Results of the present work, obtained in patients who have developed in vivo antileukemia activity, demonstrate that, notwithstanding depletion of alloreactive T cells, a high frequency of precursor cells able to kill LB is still present among spared lymphocytes. Our data confirm results recently reported by Mavroudis et al,19 who demonstrated the maintenance of antileukemia reactivity after depletion of alloreactive T cells through an IT targeting the α-chain of IL-2 receptor. Moreover, evaluation of LB-reactive TCC documents that clones derived from allodepleted cultures exhibit surface phenotype and functional characteristics similar to clones derived from untreated cultures. In particular, we demonstrate that LB-reactive, CD3+/CD8+, or CD3+/CD4-CD8 DN TCC expressing either TCR αβ or γδ are involved in the generation of in vitro antileukemia activity obtained from untreated and T-cell allodepleted cultures. These results confirm the data on LB-directed TCC previously obtained in children who have received allogeneic HSCT. They demonstrate that several types of CTL clones can contribute to antileukemia activity in vitro, because we also isolated, together with CD3+/αβ+/CD8+ MHC-restricted TCC, TCC expressing TCR αβ or γδ, whose cytolytic activity is not affected by the addition of MoAb directed against HLA class I antigens and the CD3 complex. The isolation of a number of HLA-unrestricted TCC that use a TCR/CD3-independent recognition pathway suggests that some T-lymphocyte subsets may use alternative mechanisms to kill malignant cells. These nonconventional recognition and activation pathways could be due to the variable expression of surface molecules on LB used as stimulator and target cells. In particular, LB of patients used in this study express neither CD80 nor CD86, whereas LB-directed TCC displayed a CD28− or CD28dim phenotype (data not shown). It is indeed well known that CD80-CD86 costimulation pathway plays a critical role in HLA-restricted, TCR-mediated activation of T cells.43-45The inefficient delivery of crucial costimulatory signals may allow the development of alternative pathways of target cell recognition. We can, therefore, hypothesize that both HLA-restricted CTL directed towards mHAg and other CTL subsets, such as those expressing γδ/αβ TCR and using a TCR/CD3-independent activation pathway, are involved in mediating GVLR in vitro and in sustaining in vivo anticancer immunosurveillance.46 47
Persistence of a high frequency of LB-directed CTLp after removal of alloreactive T cells in patients receiving HSCT provides further support to the possibility that GVLR and GVHD can be at least partially separated and that some lymphocyte subsets are able to selectively recognize leukemia-specific antigens, heat-shock proteins, or overexpressed tissue-restricted molecules on tumor cells. Whether these lymphocytes can contribute in vivo to the eradication or immune control of clonogenic malignant cells, as well as the capacity of this approach to prevent GVHD occurrence in human beings, remains to be proven in a clinical study.
In a previous work, we have shown that the proliferative response of IT-treated cells toward candidin and CMV, as well as cytotoxic activity against EBV, was conserved.17 In the present study, we demonstrate that the number of cytotoxic precursor cells evaluated by LDA and directed against both CMV-infected fibroblasts and EBV-LCL are not affected by such T-allospecific depletion. It has been previously reported that (1) among the population of T cells specific for allogeneic MHC, there is a mix of virgin and experienced cells; (2) TCRs are broadly cross-reactive; and (3) many T cells specific for environmental antigens also cross-react on foreign MHC.48 49 On the basis of these data, it could be theoretically hypothesized that T-cell allodepletion be associated with reduction of the number of T cells specific for viral antigens. On the contrary, present data demonstrate that virus-specific precursor cells are not impaired by the strategy of T-cell depletion here described.
In conclusion, our data indicate that treatment with RFT5-SMPT-dgA is able to delete alloantigen specific T cells while sparing in vitro antiviral and antileukemia activity. Use of this IT is now under evaluation in a phase I clinical trial aimed at testing the feasibility of this approach for extending the applicability of allogeneic HSCT from HLA-disparate donors.
Supported in part by grants from Associazione Italiana Ricerca sul Cancro (AIRC) to F.L. and to R.M. and by Grants No. 261RFM95/01, 390RFM96/01, and 010RCR97/01 from IRCCS Policlinico San Matteo to F.L. and R.M.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Daniela Montagna, PhD, Laboratorio di Immunologia, Dipartimento di Scienze Pediatriche, IRCCS Policlinico San Matteo, P.le Golgi 2, 27100 Pavia, Italia.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal