During the process of normal hematopoiesis, proliferation is tightly linked to maturation. The molecular mechanisms that lead to production of mature effector cells with a variety of phenotypes and functions from a single multipotent progenitor are only beginning to be elucidated. It is important to determine how these maturation events are regulated at the molecular level, because this will provide significant insights into the process of normal hematopoiesis as well as leukemogenesis. Transcription factors containing the highly conserved homeobox motif show considerable promise as potential regulators of hematopoietic maturation events. In this study, we focused on identification and characterization of homeobox genes of the HOX family that are important in regulating normal human myeloid differentiation induced by the hematopoietic growth factor, granulocyte-macrophage colony-stimulating factor (GM-CSF). We have identified three homeobox genes, HOX A5, HOX B6, and HOX B7, which are expressed during early myelopoiesis. Treating bone marrow cells with antisense oligodeoxynucleotides to HOX A5 resulted in inhibition of granulocytic/monocytic hematopoiesis and increased the generation of erythroid progenitors. Also, overexpression of HOX A5 inhibited erythroid differentiation of the K562 cell line. Based on these observations, we propose that HOX A5 functions as an important regulator of hematopoietic lineage determination and maturation.
HOMEOBOX GENES encode proteins containing a conserved DNA binding domain of 61 amino acids that forms a helix-turn-helix motif, referred to as the homeodomain. Originally described in Drosophila, the homeobox genes are important regulators of embryonic development, with their spatio-temporal patterns of expression controlling the positioning of body axes, pattern formation, and the expression of many structural genes. In humans, the majority of homeobox genes are arranged in four clusters on chromosomes 2, 7, 12, and 17. These so-called class-1 human homeobox genes are highly homologous to the Antennapedia class ofDrosophila homeobox genes and are referred to as “HOX” genes (reviewed in Akam1 and De Robertis et al2).
Despite an increasing body of knowledge regarding hematopoietic growth factors and their receptors, details of the nuclear effectors associated with proliferative and differentiative signals are not yet clear. Members of the homeobox gene family show considerable promise as candidates for genes involved in the regulation of hematopoietic maturation events (reviewed in Lawrence and Largman3). Recent investigations have revealed differential, lineage-restricted patterns of HOX gene expression in leukemic cell lines.3,4A regulatory role for HOX genes in blood cell maturation can be inferred from experiments in which expression of a specific HOX gene has been blocked or amplified in leukemic cell lines. In such cases, the cell’s normal maturation pathway is subverted.5-9Moreover, abnormal homeobox gene expression has been described in a number of leukemias.3 10
The study of homeobox genes in leukemic cells provides convincing evidence that aberrant expression can play an important role during leukemogenesis. However, it is difficult to ascertain the normal function of these genes in hematopoiesis, because unregulated homeobox gene expression has been identified as playing a role in the transformed phenotype.3 10 Therefore, we have designed a reverse transcriptase-polymerase chain reaction (RT-PCR)–based strategy to identify HOX genes that are expressed during granulocyte-macrophage colony-stimulating factor (GM-CSF)–induced differentiation of normal human bone marrow cells. GM-CSF stimulates the proliferation and maturation of multipotential progenitors into cells of predominantely granulocytic and monocytic/macrophage lineages. We characterized the function of one of these genes, HOX A5, using an antisense oligodeoxynucleotide (ODN) strategy to suppress its expression in both colony assays and an ex vivo expansion regimen and by enforcing its expression in the K562 cell line. Our results suggest an important role for HOX A5 in the regulation of myeloid cell differentiation.
MATERIALS AND METHODS
RT-PCR screen for HOX gene expression in GM-CSF–stimulated primary bone marrow cells.
Human bone marrow was obtained from a normal donor following informed consent. Mononuclear cells were purified by gradient centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) for 1 hour at 400g. The resulting buffy coat was removed and washed twice with phosphate-buffered saline (PBS). Cells were then suspended in 0.9% methylcellulose containing Iscove’s modified Dulbecco’s media (IMDM) supplemented with 30% fetal calf serum (FCS) and 1 nmol/L human GM-CSF (generously provided by Dr Larry Souza, Amgen, Thousand Oaks, CA) and incubated at 37°C, 5% CO2, 100% humidity. After 4 days, GM-CSF–induced clusters containing 4 to 8 cells were picked, pooled, and washed twice in PBS. Approximately 5 × 103 cells were collected. mRNA was isolated from the pooled clusters using the QuickPrep Micro mRNA Purification Kit (Pharmacia) and resuspended in 16 μL of H2O. This mRNA (4 μL) was reverse transcribed into cDNA using AMV reverse transcriptase (Boehringer Mannheim, Indianapolis, IN) in a 20-μL reaction volume, per the manufacturer’s instructions.
This cDNA was used in a PCR reaction using degenerate primers complementary to conserved sequences belonging to the mammalianAntennapedia class of HOX genes: 5′ primer, 5′-AT(A/C/T)TA(C/T)CC(A/C/G/T)TGGATG-3′; and 3′ primer, 5′-TTTCAT(A/G)C(T/G)(A/G)C(G/T)(A/G)TT(C/T)TG(G/A)AACCA-3′. Oligonucleotide primers were synthesized on a PCR Mate oligonucleotide synthesizer (Applied Biosystems, Foster City, CA) and purified by affinity chromatography using a Nensorb column per the manufacturer’s instructions (DuPont, Boston, MA). To avoid the presence of false-positive PCR products resulting from genomic DNA contamination in the mRNA, this primer set spans an intron, and parallel cDNA reactions lacking reverse transcriptase were also performed as a control.
The PCR reaction mix contained 5 μL cDNA, 0.5 mmol/L of each 3′ and 5′ primer, 0.25 mmol/L of each dNTP (Boehringer Mannheim), 16.6 mmol/L (NH4)2SO4, 67 mmol/L Tris-HCl (pH 8.8; Sigma, St Louis, MO), 6.7 mmol/L MgCl2 (Sigma), 6.7 mmol/L EDTA (Sigma), 0.17 mg/mL bovine serum albumin (BSA; Sigma), 10% dimethyl sulfoxide (DMSO; Sigma), and 1.25 U of Taq polymerase (Boehringer Mannheim). PCR cycling was performed in a Perkin-Elmer Cetus (Norwalk, CT) thermocycler as follows: 94°C for 1 minute, 45°C for 1 minute, and 72°C for 2 minutes for 35 cycles, followed by a 72°C extension for 10 minutes. Five microliters of this reaction was then reamplified in a fresh reaction mix under the same conditions for 25 cycles. This reamplified PCR product was then directly ligated into the TA vector (Invitrogen, Carlsbad, CA). Clones were sequenced to identify the numbers and types of homeobox genes present. Complete sequences were identified with the GENBANK database (Bethesda, MD).
Analysis of HOX A5 expression in CD34+ cells.
Bone marrow was obtained from a normal, healthy volunteer after informed consent was received. CD34+ cells were purified using the Miltenyi Mini Macs CD34 antibody and column kit (Miltenyi Corp, Sunnyvale, CA). A portion of these CD34+cells was plated into colony assays containing IMDM, 0.3% agar, 20% FCS, 50 ng/mL each of interleukin-3 (IL-3), IL-6, stem cell factor (SCF), and granulocyte colony-stimulating factor (G-CSF) and 2 U erythropoietin (Epo). CD34+ cells (5 × 105) were plated into 2 mL of IMDM + 20% fetal bovine serum (FBS) with or without 1 nmol/L GM-CSF. After 72 hours, cells were harvested, and a portion of each population was plated into colony assays containing G-CSF, SCF, IL-3, IL-6, GM-CSF, and Epo. From 5 × 105 cells of each population, mRNA was extracted using the QuickPrep Micro mRNA Purification Kit (Pharmacia). mRNA samples were analyzed by HOX A5 semiquantitative RT-PCR.
HOX A5-specific semiquantitative RT-PCR.
HOX A5-specific primers (5′ primer, 5′-CGCCGGCAGCACCCACATCAG-3′; 3′ primer, 5′-TTCCGGGCCGCCTATGTTGT-3′) and glyceraldehyde phosphate dehydrogenase (GAPDH)-specific primers (5′ primer, 5′-TGATGACATCAAGAAGGTGGTGAAG-3′; 3′ primer, 5′-TCCTTGGAGGCCATGTGGGCCAT-3′)11 were synthesized and purified over a Nensorb column, as described above. mRNA was treated with RNase-free DNase in a buffer containing 10 mmol/L Tris-HCl, 1 mmol/L dithiothreitol (DTT), 10 mmol/L MgCl2, 0.025 U/μL RNase inhibitor (Boehringer Mannheim), and 5 ng/μL RNase-free DNase (Boehringer Mannheim) for 15 minutes at 37°C. DNase was inactivated and removed from the samples by heating to 70°C for 10 minutes, followed by the addition of EDTA to 10 mmol/L, phenol-chloroform extraction, and ethanol precipitation. Reverse transcription of equal amounts of mRNA was performed using SuperScript II reverse transcriptase and an oligo(dT) primer according to the manufacturer’s instructions (GIBCO-BRL, Gaithersburg, MD). Controls for DNA contamination consisted of the entire reverse transcription mix minus the reverse transcriptase and were performed in parallel. cDNA (5 μL) was added to a 50-μL PCR reaction mix containing 0.8 μmol/L HOX A5 or GAPDH primer pairs, 3.75 U Taq polymerase (GIBCO), 25 μmol/L of each deoxynucleotide, and 1.5 mmol/L MgCl2 in the manufacturer’s PCR buffer. HOX A5 PCR was performed in a thermocycler (Perkin-Elmer Cetus) under the following conditions: 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1.5 minutes for 45 cycles, followed by a 72°C extension for 10 minutes. GAPDH PCR was performed in parallel as follows: 94°C for 2 minutes, 55°C for 1.5 minutes, and 72°C for 1.5 minutes for 35 cycles, followed by a 72°C extension for 10 minutes. PCR products were separated by electrophoresis on a 3% NuSieve Agarose-TBE gel (FMC BioProducts, Rockland, ME). Gels were stained with ethidium bromide, and UV-visible bands were digitally recorded using a Speedlight Platinum gel documentation system (Lightools Research, Encinitas, CA) and quantitated using ImageQuant v1.1 software (Molecular Dynamics, Sunnyvale, CA). The results of titration experiments show parallel linear increases in signal with input concentrations of mRNA in the range of 0.1 to 5 μg. HOX A5 sequences were identified by a Southern analysis probe using a random-primed radiolabeled fragment of the first 485 nucleotides of HOX A5 cDNA, excluding the homeodomain. Southern blots were developed on a Molecular Dynamics PhosphorImager 445SI.
Cloning of HOX A5.
PCR primers specific for the HOX A5 sequence (5′ primer, 5′-AATGAGCTCTTATTTTGTAAACTC-3′, and 3′ primer, 5′-TCAGATACTCAGGGACGGAAGGC-3′) were synthesized and purified as described above. mRNA was extracted from the myelomonocytic cell line, U937, by the Pharmacia Micro QuickPrep kit and cDNA made using AMV reverse transcriptase (Boehringer Mannheim) per the manufacturer’s instructions. U937 cDNA (5 μL) was amplified in a PCR mix containing 2.5 U Taq polymerase (Boehringer Mannheim), 1 μmol/L of each HOX A5-specific primer, 200 μmol/L of each nucleotide, 1 mmol/L MgCl2, and 0.5% DMSO in the manufacturer’s supplied buffer. Cycling parameters are as follows: 94°C for 1.5 minutes, 50°C for 1.5 minutes, and 72°C for 1.5 minutes for 35 cycles, followed by a 72°C extension for 10 minutes. PCR products were subcloned into the TA vector as described above, and dideoxynucleotide sequencing was performed to confirm identity with published sequence.12 A putative isoform of HOX A5 was also isolated using this strategy. This cDNA contains a 4-nucleotide deletion at position 770, resulting in a protein truncated at the 3′ end of the homeodomain. No difference is observed when the full-length and truncated HOX A5 clones are compared in side-by-side biological assays.
Construction of K562-LA5SN–expressing cells.
HOX A5 cDNA was subcloned into the retroviral vector, LXSN (generously provided by Dusty Miller, Fred Hutchinson Cancer Research Center, Seattle, WA), to create the construct LA5SN. LA5SN was transfected using lipofectin into the ecotropic murine fibroblast packaging line, GPE. Culture supernatants from LA5SN-containing GPE cells were collected and used to infect the amphotropic murine fibroblast packaging line, PA317. K562 cells were transduced with LA5SN by cocultivation with irradiated PA317 packaging cells expressing LA5SN.
Antisense oligonucleotide preparation.
HOX A5 antisense (5′-TACAAAATAAGAGCTCAT-3′), HOX A5 sense (5′-ATGAGCTCTTATTTTGTA-3′), and HOX A5 nonsense (scrambled HOX A5 antisense: 5′-CACAACATAAGTAATTAG-3′) ODN were synthesized as described above, purified by butanol extraction and ethanol precipitation, and resuspended in serum-free hematopoietic stem cell medium (GIBCO-BRL). The antisense ODN was designed to recognize the initial 18 nucleotides of the HOX A5 mRNA, beginning with the translational start codon. The specificity of the antisense and sense oligodeoxynucleotides for HOX A5 sequences and the nonspecificity of the nonsense oligodeoxynucleotide for any known sequence was determined by screening GENBANK.
Assay of antisense ODN activity.
K562-LA5SN cells (107) were plated in 3 mL of X-Vivo-10 serum-free medium (BioWhittaker, Walkersville, MD) containing 15 μmol/L HOX A5 antisense, sense, or nonsense ODN for 24 hours. Control cultures containing no oligodeoxynucleotide were also prepared. Total RNA was extracted from treated cells using a urea lysis/sodium dodecyl sulfate (SDS) method. RNA was processed as described above for the semiquantitative PCR protocol and subjected to the same RT-PCR procedure, with the following modifications: 2 μL of cDNA and 2.5U of Taq polymerase were used per 50-μL PCR reaction, HOX A5 PCR was shortened to 35 cycles, and GAPDH PCR was shortened to 25 cycles. Quantitation of PCR products was performed using the Speedlight Platinum gel documentation system and ImageQuant v1.1 software, as described above.
Antisense HOX A5 ODN colony-forming unit–granulocyte-macrophage (CFU-GM) colony assay.
Human bone marrow was obtained from a normal donor after informed consent was received. Mononuclear cells were purified by gradient centrifugation on Ficoll-Hypaque (Pharmacia) for 40 minutes at 400g. The resulting buffy coat was removed and washed twice with PBS. Cells were preincubated overnight in indicated amounts of ODN in serum-free medium (HSCM; GIBCO-BRL). Colony assays were performed by plating 105 viable cells in HSCM containing 0.3% agar (DIFCO, Detroit, MI), 1 nmol/L GM-CSF, and antisense, sense, or nonsense ODN added at indicated concentrations in a total volume of 0.5 mL in triplicate into the wells of a 24-well plate. Colonies were incubated at 37°C, 5% CO2, 100% humidity for 14 days. CFU-GM were scored in a blinded fashion at day 14 when more than 50 cells were present.
Antisense HOX A5 ODN ex vivo expansion assay.
Ex vivo expansion assays were performed using a modification of our previously published procedure.13 Human bone marrow mononuclear cells from a normal donor were purified as described above. CD34+ cells were separated from the mononuclear cells using a Ceprate LC column per the manufacturer’s instructions (Cellpro, Bothell, WA). A portion of these CD34+ cells was plated into colony assays containing IMDM; 0.3% agar; 20% FCS; 50 ng/mL each of IL-3, IL-6, SCF, and G-CSF; and 2 U of Epo. The remaining CD34+ cells (1.5 × 104) were incubated in 0.5 mL HSCM containing 50 ng each of IL-3, IL-6, SCF, and G-CSF. Antisense, sense, or nonsense ODN to HOX A5 were added to a concentration of 15 μmol/L. Cells were incubated at 37°C, 5% CO2, 100% humidity for 10 days, and cultures were resupplemented with fresh medium, growth factors, and ODN after 5 days. On day 10, the cells were washed twice in PBS and plated in triplicate into 0.5-mL colony assays, with each well containing 5 × 103 cells in IMDM; 0.3% agar; 20% FCS; 50 ng each of IL-3, IL-6, SCF, and G-CSF; and 2 U Epo. Colony assays were incubated at 37°C, 5% CO2, 100% humidity for 14 days. CFU-GM and burst-forming units-erythroid (BFU-E) were scored in a blinded fashion on day 14, based on colony morphology and color.
Construction of FLAG-tagged HOX A5-Thy 1.2 expression vector.
A FLAG epitope adapter was created by synthesizing coding (5′-AAAGGATCCATGGACTACAAAGACGATGACGATAAAGAATTCATA-3′; FLAG-encoding sequence underlined) and complementary (5′-TATGAATTCTTTATCGTCATCGTCTTTGTAGTCCATGGATCCTTT-3′) single-stranded DNA on a PCR Mate oligonucleotide synthesizer (Applied Biosystems). These oligonucleotides were purified by affinity chromatography using a Nensorb column per the manufacturer’s instructions (DuPont) and annealed by heating to 95°C for 5 minutes, followed by slow cooling to room temperature. This adapter contains a 5′ BamHI and 3′ EcoRI sites for cloning purposes and an ATG translational start site. The FLAG epitope adapter was ligated to the 5′ end of the HOX A5 cDNA, and the resultant FLAG-HOX A5 construct was subcloned into the BSVprXThy vector,14 a derivative of the pCMV-thy-1 vector,15 both kindly provided by the laboratory of Dr Irvin Chen (UCLA, Los Angeles, CA). The BSVprXThy vector contains two cytomegalovirus (CMV) promoters, one driving expression of the cell surface antigen, murine Thy1.2, and the other driving expression of the HA nonapeptide and the HIV-1 vpr gene. The HA nonapeptide and the vpr gene were removed by HindIII/Xba I digestion and replaced by the FLAG-HOX A5 construct. Here, we refer to this FLAG-tagged HOX A5/Thy 1.2 coexpression vector as pFAT (for FLAG-A 5-Thy-1). The pCMV-thy-1 vector expressing only murine Thy 1.2 was used as a control.
Transient transfection and sorting of K562 cells with FLAG-tagged HOX A5.
K562 cells (107) were electroporated with 14 nmol/L pFAT or pCMV-thy-1 expression plasmids. Electroporations were performed in 1.2× RPMI-1640 containing 20% FBS at 250 V using a Bio-Rad GenePulser (Bio-Rad, Hercules, CA). Electroporated cells were placed in IMDM + 10% FBS. After 24 hours, electroporated cells were washed in PBS and replated in fresh medium. After electroporation (24 hours), cells were harvested for sorting. Sorting of Thy 1.2-positive cells was performed using the Miltenyi MiniMacs column system, employing magnetic beads conjugated to an antimouse Thy 1.2 antibody supplied with the kit. Purification was performed according to the manufacturer’s directions, with the exception that the purified Thy 1.2-positive eluate was run over a second MiniMacs column to further enrich the population for high-expression cells. Analysis of flow-through and eluate fractions from the Miltenyi columns for Thy 1.2-positive cell purity and recovery was performed by fluorescence-activated cell sorting (FACS; Becton Dickinson, Franklin Lakes, NJ) using antimouse Thy 1.2 (Caltag, Burlingame, CA) antibody. Expression of FLAG-tagged HOX A5 in column fractions was analyzed by Western blot using an anti-FLAG epitope antibody (Eastman Kodak, Rochester, NY). For the Western blots, 105 cells were resuspended in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled for 5 minutes, and centrifuged at high speed for a few seconds to pellet debris, and supernatants were loaded on a 10% SDS-PAGE.
Differentiation and analysis of K562 cells expressing FLAG-tagged HOX A5.
pFAT- or pCMV-thy-1–transfected K562 cells were maintained in IMDM + 10% calf serum at less than 5 × 104 cells/mL. Erythroid differentiation was induced by addition of 1 mmol/L sodium butyrate. To measure heme content, aliquots of butyrate-treated cells were removed from culture and stained with benzidine, as previously described.16 Glycophorin A cell surface expression was analyzed by FACS (Becton Dickinson) using specific monoclonal antibodies according to the manufacturer’s instructions (Dako, Carpenteria, CA). Monocyte differentiation was induced by the addition of 8 nmol/L 12-0-tetradecanoylphorbol 13-acetate (TPA; Sigma). TPA-treated cells were cytospun onto slides and stained for α-Napthyl acetate esterases according to the manufacturer’s directions (Sigma).
RESULTS
Expression of HOX A5 in GM-CSF–stimulated bone marrow.
To characterize the pattern of HOX gene expression in normal human bone marrow cells, progenitiors were stimulated with GM-CSF and harvested at the earliest timepoint at which the biological effect of this cytokine was discernable as a growth stimulus. To accomplish this, normal human bone marrow cells were cultured for 4 days in semisolid medium containing IMDM, 30% FCS, 0.9% methylcellulose, and 1 nmol/L GM-CSF. Under these conditions, colonies of greater than 50 cells consisting of mature neutrophils and/or monocytes form in 8 to 14 days. Clusters containing 4 to 8 immature cells were picked on day 4 using a micropipettor, pooled, and washed in PBS. Approximately 5 × 103 cells were harvested using this method. The timing of this harvest is important, because it represents the earliest time at which GM-CSF–stimulated cells can be identified by virtue of having divided and formed clusters. Although these cells are committed to the myeloid lineage at the molecular level, they remain phenotypically undifferentiated.
To analyze the HOX gene expression in this limited amount of material, we used an RT-PCR amplification protocol. mRNA and cDNA from these cell clusters were generated using commercially available reagents (see Materials and Methods). PCR amplification of cDNA was performed with degenerate primers designed to recognize conserved sequences found in members of the HOX family. The 3′ primer (see Materials and Methods) is directed against the WFQNRRMK amino acid coding sequence, a highly conserved region overlapping the end of the α-helix 3 of the homeodomain. The 5′ primer (see Materials and Methods) was designed to recognize the cDNA code for the conserved pentapeptide IYPWM sequence located upstream of the homeodomain of many of the HOX genes.17 Because there is an intron situated between the 5′ primer recognition sequence and the homeodomain containing the 3′ primer recognition sequence, any genomic DNA contamination of the sample would be indicated by the presence of products larger than the expected 210-246 bp. A mock cDNA reaction control containing cell cluster mRNA but no reverse transcriptase failed to generate a PCR product of the same size, thereby confirming that the product seen was not due to contamination with foreign DNA (data not shown).
The PCR reaction product was subcloned into the TA vector and sequenced. This screen showed the expression of three members of the HOX gene family, HOX A5, HOX B6, and HOX B7, in GM-CSF–stimulated human bone marrow. The 3′ degenerate primer also appeared to have a high degree of affinity for the myeloperoxidase (MPO) cDNA; four clones encoding its DNA were also found in the screen. The detection of MPO in our samples provided a fortuitous control, because MPO mRNA is only expressed early in granulocytic cell differentiation,18 thereby confirming that the harvested cells were of early myeloid cell lineage. The expression and function of HOX B6 and HOX B7 during hematopoiesis was previously the subject of study by our group and others.5,7,9 19 However, the role of HOX A5 during hematopoiesis has not been extensively studied. We therefore designed experiments to elucidate a function for the HOX A5 gene during blood cell development.
Expression of HOX A5 in GM-CSF–stimulated CD34+cells.
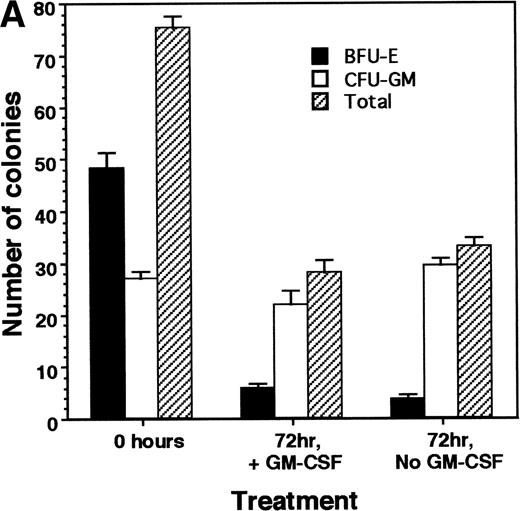
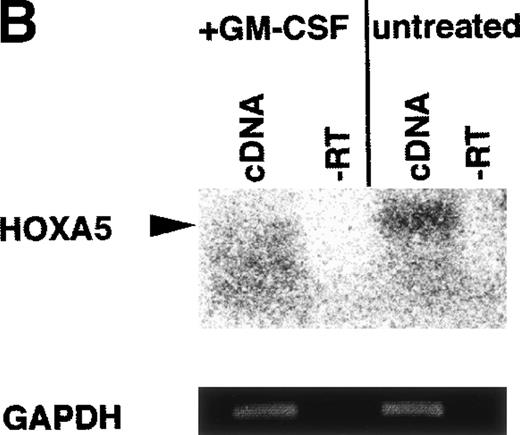
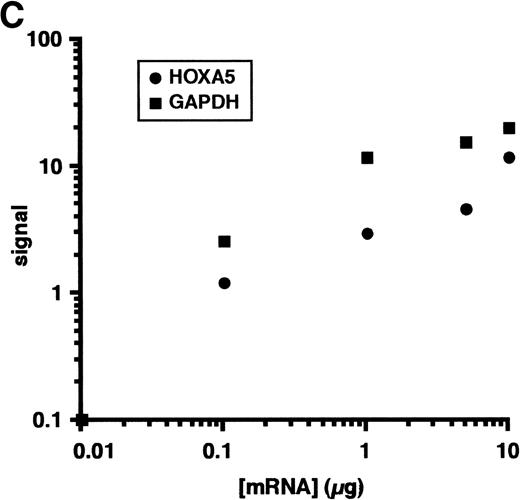
Our data indicate that HOX A5 expression can be detected in a subset of bone marrow cells that respond to GM-CSF stimulation, but it does not address whether GM-CSF stimulation plays any role in the observed expression of HOX A5. To investigate this, purified CD34+cells from the bone marrow of a healthy human donor were cultured in the presence or absence of 1 nmol/L GM-CSF in IMDM + 10% FCS for 72 hours. CD34 is a cell surface glycoprotein found on blood progenitor cells, including the pluripotent stem cell. Cells were then harvested, with a portion plated into colony assays to verify the viability and number of colony-forming cells and the remainder processed for analysis of HOX A5 expression. The colony assays (Fig 1A) showed that the presence or absence of GM-CSF in the culture medium during the 72-hour incubation did not make a significant difference in the viability or differentiation potentials of the two populations, because both showed the same pattern and number of BFU-E and CFU-GM formation. BFU-E and CFU-GM are defined as progenitor cells commited to generate a colony of mature erythroid or granulocyte/macrophage cells, respectively. To perform semiquantitative RT-PCR, PCR primers were designed to recognize sequences unique to the HOX A5 cDNA. The expected product from these primers also spans an intron, so contaminating genomic DNA would be recognized as a larger product. Amplification of cDNA from the GM-CSF–treated and untreated populations with the HOX A5 primers was followed by Southern blotting with a HOX A5-specific probe (Fig 1B), which detected HOX A5 message in CD34+ cells before treatment with GM-CSF. After treatment with GM-CSF, the level of HOX A5 expression in CD34+ cells decreased in two independent experiments. Amplification of the same cDNAs with the internal control GAPDH-specific primers, followed by densitometric analysis of an ethidium bromide-stained gel containing their PCR products, confirmed that the cDNAs from both populations were equal in concentration and free of genomic DNA contamination. Titration experiments show parallel linear increases in signal, in the range of 0.1 to 5 μg of input mRNA (Fig 1C).
(A) No significant difference is seen in the viability of CD34+ cells treated with or without GM-CSF for 72 hours, as measured by colony-forming ability. (B) GM-CSF treatment of CD34+ cells reduces expression of HOX A5 mRNA. cDNAs from GM-CSF–treated and untreated populations were amplified with GAPDH- and HOX A5-specific primers. GAPDH products are visualized by ethidium bromide staining, and HOX A5 products are visualized by Southern blotting with a HOX A5-specific32P-labeled probe. Controls containing mock cDNA reactions are labeled as “−RT.” The diffuse signal below the HOX A5 band is hybridization of the probe to the PCR primers. (C) Titration experiment demonstrating the parallel linear increase in signal relative to the concentration of input mRNA. Semiquantitative PCR was conducted as described in Materials and Methods.
(A) No significant difference is seen in the viability of CD34+ cells treated with or without GM-CSF for 72 hours, as measured by colony-forming ability. (B) GM-CSF treatment of CD34+ cells reduces expression of HOX A5 mRNA. cDNAs from GM-CSF–treated and untreated populations were amplified with GAPDH- and HOX A5-specific primers. GAPDH products are visualized by ethidium bromide staining, and HOX A5 products are visualized by Southern blotting with a HOX A5-specific32P-labeled probe. Controls containing mock cDNA reactions are labeled as “−RT.” The diffuse signal below the HOX A5 band is hybridization of the probe to the PCR primers. (C) Titration experiment demonstrating the parallel linear increase in signal relative to the concentration of input mRNA. Semiquantitative PCR was conducted as described in Materials and Methods.
These data are consistent with the findings of Sauvageau et al,20 who reported the presence of HOX A5 in primitive human CD34+, CD45RA−, CD71−, and CD38low bone marrow cells and a decrease in its expression in a more mature subpopulation enriched in granulopoietic clonogenic cells.
HOX A5 antisense ODN inhibit GM-CSF–stimulated colony formation.
Antisense ODN have proven to be a viable means by which to suppress the expression of a gene of interest, and they can thereby serve as valuable tools for the analysis of the biological function of a specific gene.21 We and others used antisense ODN in colony assays to study homeobox gene function during hematopoiesis.5,9,19 22 We used a similar approach to analyze HOX A5 function during myelopoiesis. Antisense ODN to HOX A5 were designed to specifically recognize the HOX A5 mRNA (sequence in the Materials and Methods). To confirm the activity of the ODN, K562 cells transduced to overexpress the full-length HOX A5 mRNA were treated with 15 μmol/L antisense, sense, or nonsense ODN directed against HOX A5 for 24 hours. HOX A5 mRNA expression of ODN-treated cells was measured by HOX A5-specific semiquantitative RT-PCR. Relative levels of total mRNA from each cell sample were measured by RT-PCR amplification of GAPDH. Figure 2 depicts the results of the densitometric scan of an ethidium bromide-stained agarose gel containing HOX A5 and GAPDH RT-PCR products from each of the ODN-treated cell samples. RT-PCR HOX A5 signal was normalized to GAPDH signal. Antisense ODN-treated K562-LA5SN cells had 40% to 50% of the level of expression found in untreated, sense, ODN- or nonsense ODN-treated cells, and similar results were obtained in a second independent experiment. Because these K562 cells have been engineered to overexpress the HOX A5 gene, the levels of HOX A5 expression are expected to be much higher than those found in normal bone marrow cells.
Antisense ODN to HOX A5 decrease levels of HOX A5 mRNA. K562 cells transduced to overexpress HOX A5 are treated with 15 μmol/L of antisense, sense, nonsense (scrambled antisense), or no ODN for 24 hours. Lanes 1 through 4 (+RT) contain cDNAs generated from each treatment and were subjected to PCR amplification with HOX A5- and GAPDH-specific primers. Lanes 6 through 10 (−RT) contain HOX A5 and GAPDH PCR products of mock cDNA reactions lacking reverse transcriptase. Ethidium bromide-stained gels were digitally scanned, and relative intensities of HOX A5 product bands compared with their corresponding GAPDH bands are denoted below the lanes.
Antisense ODN to HOX A5 decrease levels of HOX A5 mRNA. K562 cells transduced to overexpress HOX A5 are treated with 15 μmol/L of antisense, sense, nonsense (scrambled antisense), or no ODN for 24 hours. Lanes 1 through 4 (+RT) contain cDNAs generated from each treatment and were subjected to PCR amplification with HOX A5- and GAPDH-specific primers. Lanes 6 through 10 (−RT) contain HOX A5 and GAPDH PCR products of mock cDNA reactions lacking reverse transcriptase. Ethidium bromide-stained gels were digitally scanned, and relative intensities of HOX A5 product bands compared with their corresponding GAPDH bands are denoted below the lanes.
In a separate experiment, 5.4 × 105 human bone marrow CD34+ cells (78% positive) were cultured in X-vivo 10 defined medium in the presence of 15 μmol/L ODN for 2 days. mRNA from each well was isolated and used to conduct semiquantitative RT-PCR reactions for HOX A5 levels normalized to GAPDH. Similar to the results observed in K562 cells, the A5 signal in the antisense ODN-treated bone marrow cells was 51% of the signal generated from cells treated with nonsense ODN and 38% of the signal generated from diluent control-treated cells.
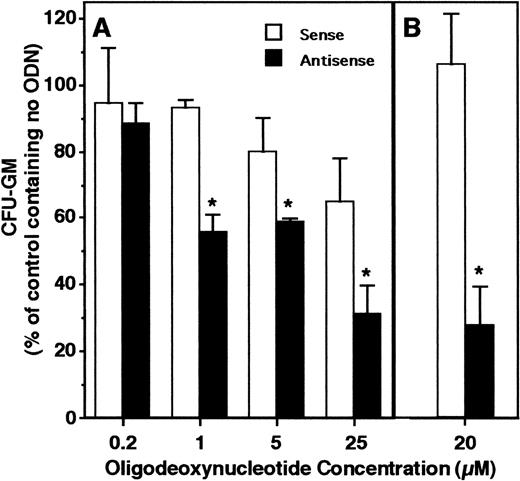
HOX A5 expression was detected in early myeloid progenitors harvested as clusters. Antisense ODN were used to determine whether the expression of HOX A5 plays a functional role in myeloid cell proliferation or maturation. Normal human bone marrow cells plated into GM-CSF–containing colony assays demonstrated a specific dose-dependent decrease in the generation of colonies in the presence of HOX A5 antisense ODN compared with control ODN (Fig 3A). In further experiments using a single 20 μmol/L dose of ODN, granulocytic-monocytic colony formation in the presence of HOX A5 antisense ODN was reduced nearly 80% (Fig3B). These results show that HOX A5 expression is required for the proliferation and maturation of CFU-GM, the bipotential progenitors of mature granulocytes and monocytes.
HOX A5 antisense ODN inhibit GM-CSF–stimulated colony formation. (A) Human bone marrow cells plated into colony assays containing 1 nmol/L human recombinant GM-CSF and increasing concentrations of ODN. (B) A separate experiment similar to (A) at a single dose of 20 μmol/L ODN. Data from both experiments are reported relative to the number of colonies formed in assays containing no ODN and are calculated from the mean of three assay wells.
HOX A5 antisense ODN inhibit GM-CSF–stimulated colony formation. (A) Human bone marrow cells plated into colony assays containing 1 nmol/L human recombinant GM-CSF and increasing concentrations of ODN. (B) A separate experiment similar to (A) at a single dose of 20 μmol/L ODN. Data from both experiments are reported relative to the number of colonies formed in assays containing no ODN and are calculated from the mean of three assay wells.
HOX A5 antisense ODN promotes the generation of BFU-E during ex vivo expansion.
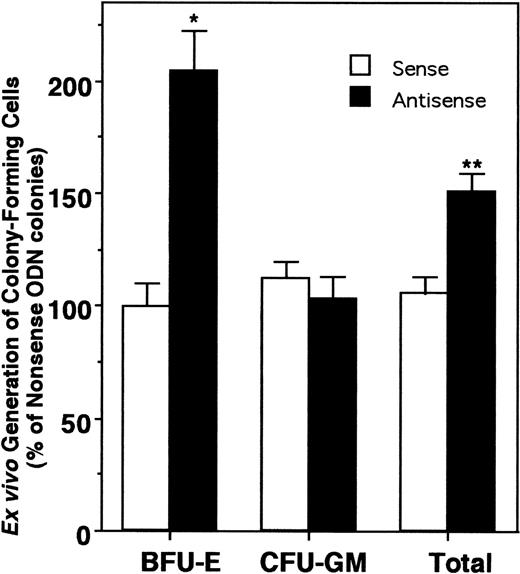
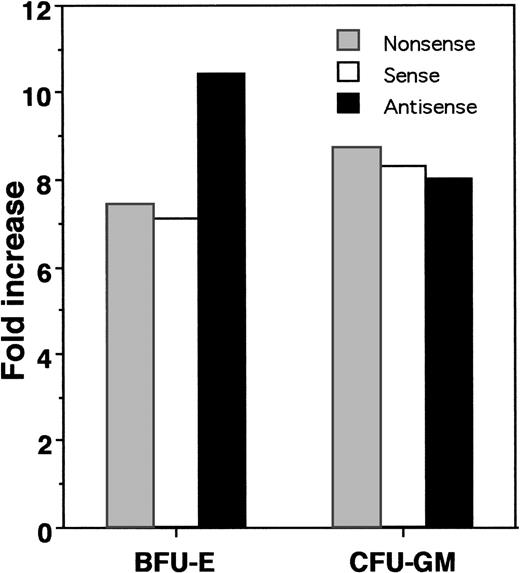
The GM-CSF colony assay results suggested an important biological role for HOX A5 in determining lineage specificity during myelopoiesis. We extended the analysis of the role of this gene by investigating its actions in earlier hematopoietic progenitors. Our laboratory has developed a system that supports the ex vivo expansion of CFU-GM and BFU-E progenitors over a 10- to 14-day period.13 In this ex vivo expansion system, CD34+ progenitor cells are cultured in serum-free medium (GIBCO HSCM) containing IL-3, IL-6, G-CSF, and SCF for a period of 10 days. CD34 is a cell surface glycoprotein found on early hematopoietic progenitors and is routinely used for selection of this cell population.23 During this culture, we observe a considerable increase in nucleated cell number, along with limited continuing generation of colony-forming progenitor cells. To gauge the effect of HOX A5 suppression on the expansion of progenitor cells from a pre–colony-forming cell, antisense, sense, or nonsense ODN to HOX A5 were included in the ex vivo expansion cultures. After 10 days in the presence of ODN, the expansion cultures were plated into colony assays containing IL-3, IL-6, G-CSF, SCF, and Epo without ODN to assay what effect the presence of the ODN had on the expanding progenitors. CD34+ cells cultured with HOX A5 antisense ODN during this ex vivo expansion protocol (Fig 4) displayed a dramatic and reproducible 100% increase in the number of BFU-E colonies but no significant change in the numbers of CFU-GM. This effect was specific for HOX A5 antisense ODN, because sense and nonsense ODN showed no effect on expansion of progenitors. This increase was apparent against a background of overall expansion of BFU-E (Fig 5), suggesting that the increase in the number of BFU-E seen at the end of the expansion is not merely the result of the increased survival of erythroid progenitors during expansion, but is due to an actual increase in their proliferation. Thus, blocking the expression of HOX A5 in early hematopoietic progenitors expands a population of cells with erythroid potential without affecting the generation of granulocytic/monocytic colony-forming cells. However, subsequent colony formation by these CFU-GM is blocked by HOX A5 antisense ODN (Fig 3).
Blocking HOX A5 expression increases the generation of BFU-E. Ex vivo expansion cultures of CD34+ cells with HOX A5 antisense, sense, or nonsense ODN were plated into colony assays containing IL-3, IL-6, SCF, and G-CSF and 2 U Epo (see Materials and Methods). Data are reported relative to the number of colonies generated from nonsense ODN-treated cells and are representative of the mean of three independent experiments, with each experiment containing three assay wells.
Blocking HOX A5 expression increases the generation of BFU-E. Ex vivo expansion cultures of CD34+ cells with HOX A5 antisense, sense, or nonsense ODN were plated into colony assays containing IL-3, IL-6, SCF, and G-CSF and 2 U Epo (see Materials and Methods). Data are reported relative to the number of colonies generated from nonsense ODN-treated cells and are representative of the mean of three independent experiments, with each experiment containing three assay wells.
HOX A5 antisense ODN potentiates the expansion of BFU-E during the ex vivo expansion of CD34+ cells. Fold increase in the total numbers of colony types formed after ex vivo expansion in the presence of HOX A5 antisense, sense, or nonsense ODN compared with colony types present in colony assays plated at start of expansion.
HOX A5 antisense ODN potentiates the expansion of BFU-E during the ex vivo expansion of CD34+ cells. Fold increase in the total numbers of colony types formed after ex vivo expansion in the presence of HOX A5 antisense, sense, or nonsense ODN compared with colony types present in colony assays plated at start of expansion.
Enforced expression of HOX A5 inhibits K562 erythroid differentiation.
The multipotential cell line, K562, displays primitive erythroid features and can be induced by sodium butyrate to differentiate into cells expressing more mature erythroid markers such as heme.24,25 Treatment of K562 cells with TPA has been demonstrated to increase their expression of monocytic and megakaryoblastic markers.26 To assess the effect of overexpression of HOX A5 on hematopoietic differentiation, K562 cells were transiently transfected with a vector expressing HOX A5 tagged with the FLAG epitope (pFAT). This vector also expresses murine Thy 1.2 as a cell surface marker for selection of transfected cells via the Miltenyi MiniMac system. Control K562 cells were transfected with the vector expressing murine Thy 1.2 alone (pCMV-thy-1). Purification of transfected cells via murine Thy 1.2 expression provided populations containing at least 90% pFAT or pCMV-thy-1–transfected cells, as determined by FACS analysis of Thy 1.2 staining (data not shown). Western blot analysis using an anti-FLAG antibody confirmed the high expression of the transfected FLAG-tagged HOX A5 (Fig 6) at the expected molecular weight of 36.4 kD.
Expression of FLAG-tagged HOX A5 in K562 cells. K562 cells were transfected with FLAG-tagged HOX A5 (pFAT), empty vector (pCMV-thy-1), or no vector (mock). Cells were purified via cell surface expression of the transfection marker, murine Thy 1.2. Lanes labeled “FT1” and “FT2” represent cell extracts from the flow-through fraction of the two affinity columns used to select Thy 1.2-positive cells; lanes labeled “Thy 1.2+” are cell extracts from the Thy 1.2-positive eluates from the columns; and the lane labeled “FLAG-BAP” is FLAG-tagged bacterial alkaline phosphatase (Eastman Kodak) used as a positve control for the anti-FLAG Western antibody.
Expression of FLAG-tagged HOX A5 in K562 cells. K562 cells were transfected with FLAG-tagged HOX A5 (pFAT), empty vector (pCMV-thy-1), or no vector (mock). Cells were purified via cell surface expression of the transfection marker, murine Thy 1.2. Lanes labeled “FT1” and “FT2” represent cell extracts from the flow-through fraction of the two affinity columns used to select Thy 1.2-positive cells; lanes labeled “Thy 1.2+” are cell extracts from the Thy 1.2-positive eluates from the columns; and the lane labeled “FLAG-BAP” is FLAG-tagged bacterial alkaline phosphatase (Eastman Kodak) used as a positve control for the anti-FLAG Western antibody.
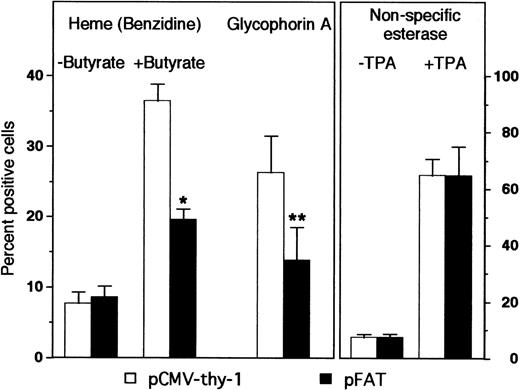
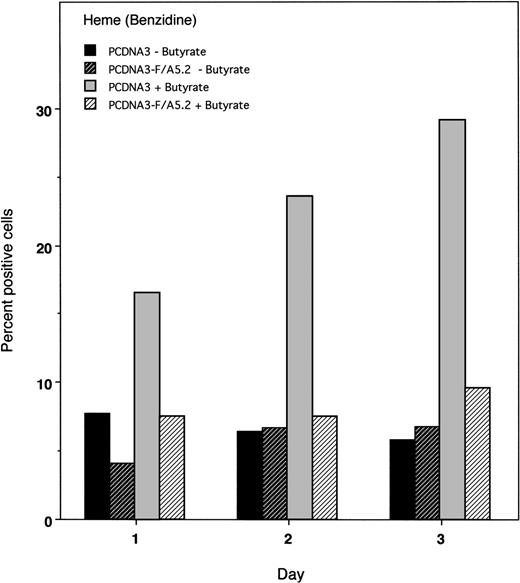
Overexpression of HOX A5 had no effect on TPA-induced monocytic differentiation compared with control cells, as assayed by staining for nonspecific esterases (α-napthyl acetate; Fig 7). This could be due to the fact that TPA is a sufficiently strong inducer of monocyte differentiation such that no further enhancement is seen with the addition of HOX A5. Alternatively, other as yet unspecified factors may be limiting in the cells that fail to differentiate. However, K562 cells overexpressing HOX A5 exhibited only half the level of butyrate-induced erythroid differentiation seen in pCMV-thy-1–transfected cells (Fig 7). Additionally, a second marker of erythroid phenotype, glycophorin A, was found to be significantly diminished in K562 cells overexpressing HOX A5 (Fig 7). A time course analysis shows that, even in transiently transfected K562 cells, HOX A5 expression limits erythyroid differentiation for 72 hours (Fig 8).
HOX A5 overexpression inhibits the butyrate-induced differentiation of K562 cells. K562 cells were transfected with FLAG epitope-tagged HOX A5 (pFAT) or the empty vector (pCMV-thy-1). Erythroid differentiation was measured by heme content and cell surface expression of glycophorin A 72 hours posttransfection. Heme content was determined by direct benzidine staining of cells grown with or without butyrate; the data represent a mean of seven experiments. Glycophorin A was measured by FACS analysis and scored as the percentage of transfected cells displaying specific fluorescence intensity above background fluorescence levels; the data represent the mean of three experiments. Monocytic differentiation was measured by nonspecific esterase positivity of cells treated for 72 hours with or without 8 nmol/L TPA, and stained cytospun cells were scored by light microscopy; the data represent the mean of two experiments. *P < .001; **P < .05.
HOX A5 overexpression inhibits the butyrate-induced differentiation of K562 cells. K562 cells were transfected with FLAG epitope-tagged HOX A5 (pFAT) or the empty vector (pCMV-thy-1). Erythroid differentiation was measured by heme content and cell surface expression of glycophorin A 72 hours posttransfection. Heme content was determined by direct benzidine staining of cells grown with or without butyrate; the data represent a mean of seven experiments. Glycophorin A was measured by FACS analysis and scored as the percentage of transfected cells displaying specific fluorescence intensity above background fluorescence levels; the data represent the mean of three experiments. Monocytic differentiation was measured by nonspecific esterase positivity of cells treated for 72 hours with or without 8 nmol/L TPA, and stained cytospun cells were scored by light microscopy; the data represent the mean of two experiments. *P < .001; **P < .05.
Time course of butyrate-induced erythroid differentiation. K562 cells were transiently cotransfected with pCDNA3-F/A5.2 or the empty vector and pCMV-thy-1, as described in Materials and Methods. Aliquots of cells were harvested at 24-hour intervals, and heme content of cells without or with butyrate was measured by benzidene staining.
Time course of butyrate-induced erythroid differentiation. K562 cells were transiently cotransfected with pCDNA3-F/A5.2 or the empty vector and pCMV-thy-1, as described in Materials and Methods. Aliquots of cells were harvested at 24-hour intervals, and heme content of cells without or with butyrate was measured by benzidene staining.
DISCUSSION
In recent years, members of the homeobox family of genes have been identified as important mediators of blood cell differentiation. The expression patterns of these genes have been studied extensively in cell lines of leukemic origin and, to a lesser degree, in normal hematopoietic cells (reviewed in Lawrence and Largman3). Many of the studies of HOX gene function have been performed in cell lines of leukemic origin, in which expression of HOX genes may reflect the cell line’s transformed phenotype.3,10 Our work is unique, in that it examines HOX gene expression at a fixed and tightly defined moment in normal human myelopoiesis. Our results indicate that at least three HOX genes, HOX A5, HOX B6, and HOX B7, are present in normal human bone marrow progenitor cells early after a proliferative response to GM-CSF. The function of HOX B7 during blood cell development was the focus of one of our previous studies. We and others demonstrated that HOX B7 expression is required for GM-CSF–stimulated colony formation from both human and murine bone marrow5,9and that downregulation of the gene is required for granulocytic differentiation of HL-60 cells.5
Published reports indicate that HOX A5 expression appears to be restricted to cells of myelomonocytic lineage and absent in cells of erythroid lineage. Magli et al27 reported HOX A5 expression is present in HL-60 and U937 cells, which preferentially differentiate along the granulocytic-monocytic pathway, but is absent in the erythroleukemic cell lines, K562 and OCIM2. Vieille et al28confirmed these findings in the same cells. Sauvageau et al20 showed that the level of HOX A5 message in a subpopulation of human CD34+ cells with erythroid potential is lower than that found in more primitive cells or CD34+cells displaying granulopoietic potential. The work presented here complements these previous findings and defines a functional role for HOX A5 in blood cell development. The increased generation of erythroid progenitors seen when HOX A5 expression was reduced in the ex vivo expansion assay suggests that HOX A5 may act as a repressor of the generation or proliferation of erythroid progenitor cells. The decrease in the erythroid phenotype of HOX A5-overexpressing K562 cells suggests that the differentiation of an erythroid progenitor cell is also repressed by HOX A5. Therefore, abrogation of HOX A5 expression would allow for increased generation of erythroid progenitors and their subsequent maturation. In another role, HOX A5 expression is present and appears to be required for generation of mature progeny from CFU-GM but not for production of the CFU-GM itself.
Our finding that HOX A5 expression appears to diminish in CD34+ cells after GM-CSF stimulation supports a hypothesis proposed by Lawrence et al29 that there is temporal regulation of HOX gene expression during hematopoiesis, with high expression of the widest range of HOX genes associated with uncommitted progenitor cells and downregulation of the expression of certain HOX genes being associated with differentiation. Their examination of human CD34+ progenitor cell subpopulations showed numerous HOX genes expressed at a high level in the most immature cells and that this level of expression was diminished in lineage-committed cells. Other investigations have shown that HOX gene overexpression can lead to creation of hematopoietic cells with high proliferative activity or leukemic phenotypes while displaying a decrease in differentiation.6 30 During the ex vivo expansion assay, expression of HOX A5 may help to maintain progenitor cells in an uncommitted state. The removal of HOX A5 expression may have favored the proliferation of a population of multipotential cells of a more erythroid-committed subtype. Thus, understanding the means by which HOX gene expression is suppressed may prove to be as important as understanding how it is activated.
There is clear evidence that HOX genes regulate the expression of other HOX genes in a self-referential pattern. Being transcription factors, individual HOX genes likely bind directly to the promoter elements of other HOX genes and regulate their transcription. Lobe31demonstrated that exogenous expression of murine HOX A5 activated the expression of numerous endogenous HOX genes, and HOX A5 has been shown to bind to its own promoter.16 Second, translational regulation of a HOX gene by another homeodomain protein could occur by a mechanism recently described in which the homeodomain protein bicoid binds the mRNA and acts as a translational repressor of the homeodomain protein caudal in Drosophila embryos.32,33 This interaction is postulated to be responsible for establishment of the posterior-to-anterior concentration gradient of caudal seen duringDrosophila embryogenesis, and this gradient contributes to the patterning of structures in the embryo. Finally, members of the murine Pbx family of homeodomain proteins have been demonstrated to heterodimerize with members of the murine HOX family, including HOX A5,34 and this heterodimerization affects the DNA binding and transcriptional regulatory function of the HOX gene. Thus, regulated function of HOX genes could arise through competition for heterodimerizing partners among the different HOX genes.
Whether HOX gene expression is directly influenced by cytokine stimulation during hematopoiesis remains an open question. It has been established that, during embryogenesis and in cell lines, polypeptide growth factors and morphogens such as retinoic acid can regulate the expression of homeobox genes.2 It is also possible that cytokines may play no direct role in homeobox gene expression. According to the stochastic model of hematopoiesis, lineage commitment is a process that occurs randomly, and the role of a specific cytokine is to prevent apoptosis and promote the proliferation of those cells that express the receptor for that ligand, not to define what that lineage may be (reviewed in Gordon and Amos35 and Ogawa36). The observation that HOX genes are expressed at a high level in very immature blood cells suggests that there may be a high degree of competition between the HOX genes for DNA binding sites and other molecular interactions. Disturbing this competitive balance by a random change in the expression of specific HOX genes may provide the underlying cause of lineage commitment.
We have defined an important role for the HOX A5 gene as a regulator of myeloid cell proliferation and differentiation. Although we demonstrated that manipulation of a single HOX gene can elicit profound effects on these processes, it is likely that many different HOX genes work together to fine-tune the output of blood cells produced. Additionally, very little is known about the downstream effectors of HOX genes. A more complete understanding of HOX gene function during hematopoiesis awaits investigation of these issues.
ACKNOWLEDGMENT
The authors thank Dr Gay Crooks for providing the LA5SN vector; Dr Michael Lill, Libby Walker, David Samuels, Maureen Lynch, and Negoita Neagos for their help; and Wendy Aft for preparation of the manuscript.
Supported by National Institutes of Health (NIH) SCOR Grant No. HL54850 (J.C.G.), NIH Grant No. R01 CA40163 (J.C.G.), and US Public Health Service Award No. CA09056 (J.F.F.) from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Judith C. Gasson, PhD, Director, UCLA Jonsson Comprehensive Cancer Center, 8-684 Factor Bldg, Box 951781, Los Angeles, CA 90095-1781.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal