We measured the urinary excretion of Isoprostane F2-III and Isoprostane-F2-VI, two markers of in vivo lipid peroxidation, and the circulating levels of the prothrombin fragment F1+2, a marker of thrombin generation, in 18 antiphospholipid antibodies-positive patients, in 18 antiphospholipid antibodies-negative patients with systemic lupus erythematosus, and in 20 healthy subjects. Furthermore, 12 patients positive for antiphospholipid antibodies were treated with (n = 7) or without (n = 5) antioxidant vitamins (vitamin E at 900 IU/d and vitamin C at 2,000 mg/d) for 4 weeks. Compared with antiphospholipid antibodies-negative patients, antiphospholipid antibodies-positive patients had higher urinary values of Isoprostane-F2-III (P = .0001), Isoprostane-F2-VI (P = .006), and plasma levels of the prothrombin fragment F1+2 (P= .0001). In antiphospholipid-positive patients, F1+2 significantly correlated with Isoprostane-F2-III (Rho = .56,P = .017) and Isoprostane-F2-VI (Rho = .61, P = .008). After 4 weeks of supplementation with antioxidant vitamins, we found a significant decrease in F1+2 levels (P < .005) concomitantly with a significant reduction of both Isoprostane-F2-III (P = .007) and Isoprostane-F2-VI (P < .005). No change of these variables was observed in patients not receiving antioxidant treatment. This study suggests that lipid peroxidation might contribute to the activation of clotting system in patients positive for antiphospholipid antibodies.
THE ANTIPHOSPHOLIPID syndrome (APS) identifies patients with circulating antiphospholipid antibodies (aPL) and episodes of venous and/or arterial thrombosis.1 Even if aPL have been found prevalently in patients with autoimmune diseases,1 aPL may be observed in other clinical conditions such as atherosclerosis. Thus, a significant association between anticardiolipin antibodies and myocardial infarction has been reported.2 3
Several mechanisms have been proposed for explaining the pathophysiological events that may potentially account for thrombosis in aPL-positive patients. The majority of these studies focused on the possibility that these antibodies per se induce thrombosis by affecting the activity of several cell lines such as endothelial cell, monocyte, and platelets or interfering with the clotting system.4-6Experiments in animal models gave support to this hypothesis, because in a mouse model, the injection of human monoclonal anticardiolipin antibody was associated with a thrombogenic effect.7However, the demonstration that aPL per se are thrombogenic in the human syndrome is still lacking.
In the present study we explore an alternative possibility, which is based on two previous findings. It has been reported that aPL-positive patients have an ongoing prothrombotic state, as indicated by high circulating levels of the prothrombin fragment F1+2, a marker of thrombin generation in vivo.8,9 Furthermore, following the study of Horkko et al,10 which reported that antibodies against cardiolipin bind exclusively to peroxidized phospholipids, our group has demonstrated that, in patients positive for aPL, there is a close association between lipid peroxidation and aPL.11
To study lipid peroxidation, we measured two distinct isoprostanes deriving from arachidonic acid oxidation (Fig 1), namely 8-iso-Prostaglandin-F2α and Isoprostane-F2α-I, now known as Isoprostane-F2α-III and Isoprostane-F2α-VI.12Isoprostane-F2α-III was used as a marker of lipid peroxidation, because it is elevated in clinical settings associated with in vivo oxidant stress13,14 and is generated during low density lipoprotein (LDL) oxidation in vitro in coincidence with lipid peroxides formation.15,16Isoprostane-F2α-III may also be generated as a by-product of COX enzyme,16,17 but this pathway appears to have a trivial contribution on the overall biosynthesis of the compound, as reflected by its excretion in urine even in syndromes of COX activation.14 Furthermore, we found a significant increase of a distinct isoprostane, Isoprostane-F2α-VI, formation of which in vivo and in vitro is totally independent of COX activity.18 Urinary levels of the isoprostanes were highly correlated, suggesting a common mechanism of formation.11
The aim of the present study was to investigate if there is a relationship between lipid peroxidation and clotting activation in patients with aPL.
MATERIALS AND METHODS
Study population.
Between October 1996 and March 1998, we studied 18 consecutive outpatients (16 women and 2 men; 19 to 53 years of age) considered positive for aPL, recruited in the Rheumatology and Thrombosis Units of the Institute of I Clinica Medica. In particular, 17 subjects showed positivity for anticardiolipin antibodies (aCL), with a titer ranging from 20 to 110 GPL or MPL; among these, 9 were also positive for lupus anticoagulant (LA). Only 1 patient was positive for LA but not for aCL. Eight of 18 aPL-positive patients were affected by primary antiphospholipid syndrome (PAPS),19 having a history of arterial and/or venous thrombosis in the previous 13 to 31 months: 5 had had an episode of arterial thrombosis (3 thromboembolic stroke, 1 myocardial infarction, and 1 retinal thrombosis), 2 had a deep venous thrombosis, and 1 had a deep venous thrombosis and recurrent fetal loss. The remaining 10 patients suffered from systemic lupus erythematosus (SLE), diagnosed in accordance with the criteria of the American College of Rheumatology, formerly the American Rheumatism Association20; among these subjects, 2 had had a thromboembolic stroke and 1 had a deep venous thrombosis in the previous 13 to 27 months.
In the same period, we also selected 18 patients (17 women and 1 man; 17 to 50 years of age) suffering from SLE but negative for aPL. Among these subjects, 5 experienced arterial and/or venous thrombosis in the previous 14 to 24 months: 3 had had an episode of arterial thrombosis (2 myocardial infarctions and 1 thromboembolic stroke) and 2 had a deep venous thrombosis.
At the time of the study, all patients with a previous episode of arterial thrombosis were treated with aspirin (100 mg/d). The 6 patients who had had an episode of venous thrombosis in the previous 16 to 27 months discontinued the anticoagulant treatment at least 6 months before the inclusion in the study.
Twenty healthy subjects (19 women and 1 man; 18 to 50 years of age) negative for aPL were also studied as controls.
The duration of disease in patients suffering from SLE averaged 8 ± 4 years (range, 1 to 16 years). Nineteen patients (8 positive and 11 negative for aPL) were being treated with corticosteroids (prednisone at 5 to 25 mg/d or methylprednisolone at 4 to 24 mg/d) and/or methotrexate (0.25 to 0.30 mg/kg intravenously once weekly). No patient with PAPS was on treatment with corticosteroids or methotrexate. Neither patients nor controls received vitamin supplementation 1 month before the study.
Serum levels of C3 and C4, C-reactive protein, and clottable fibrinogen, all acute-phase reactants, were measured as previously described.6 No patient had had active infections, trauma, surgery, liver diseases, or alcohol or acetaminophen abuse in the previous 3 months.
Among healthy subjects, none had cardiovascular risk factors, but 3 were smokers (6 cigarettes per day).
Study design.
In a first study, a cross-sectional comparison of the two isoprostane levels and prothrombin fragment F1+2 (F1+2) between patients and controls was performed. In the same day, a blood sample to measure the clotting parameter and 12 hours of urine collection to measure isoprostanes were taken from patients who had fasted for at least 12 hours. In a second study, we sought to investigate if antioxidant treatment affected the circulating levels of F1+2 as well as the urinary level of Isoprostane-F2α-III and Isoprostane-F2α-VI. To this purpose, 12 aPL-positive patients who had at least one isoprostane higher than the cut-off point, ie, mean + 2 SD of controls, were randomly treated with (group A, n = 7) or without (group B, n = 5) antioxidant supplementation (vitamin E at 900 IU/d, vitamin C at 2,000 mg/d). Isoprostanes and F1+2 were measured before and after 4 weeks of treatment. Three patients of group A and 2 of group B were also treated with corticosteroids (prednisone at 5 to 25 mg/d or methylprednisolone at 4 to 24 mg/d). The remaining patients, 4 of group A and 3 of group B, had PAPS.
Laboratory tests.
After overnight fasting and supine rest for at least 10 minutes, blood samples were taken into tubes containing 3.8% trisodium citrate and centrifuged at 5,000g for 10 minutes to obtain plasma. The plasma was used immediately for measurement of fibrinogen. Blood samples were also taken to measure plasma F1+2, vitamin E, vitamin C, serum anticardiolipin antibodies, C-reactive protein, the complement components C3 and C4, and tumor necrosis factor α (TNFα).
Plasma levels of prothrombin fragment F1+2 were assayed by an enzyme immunoassay based on the sandwich principle (Enzygnost F1+2; Behringwerke, Marburg, Germany; reference value, 0.6 ± 0.2 nmol/L; range, 0.3 to 1.2 nmol/L). Intra-assay and interassay coefficients of variation were 8% and 9%, respectively.6
Plasma vitamin E and vitamin C were assayed by high performance liquid chromatography with UV detection21 and electrochemical detection,22 respectively.
Serum TNFα was assayed in duplicate by an enzyme immunoassay (Biokine TNFα test kit; T Cell Diagnostics Inc, Cambridge, MA). The detection limit was calculated to be 10 pg/mL. Intra-assay and interassay coefficients of variation were 8% and 9%, respectively. Among 25 healthy subjects, 2 showed detectable TNFα serum levels (median <10 pg/mL; range, <10 to 34 pg/mL).
LA was measured in platelet-poor plasma centrifuged twice at 5,000g using four different coagulation tests: activated partial thromboplastin time (aPTT), kaolin clotting time (KCT), dilute Russel’s viper venom time (dRVVT), and dilute aPTT, as previously described.23 Patients were considered positive for LA if they had at least two abnormal (prolonged) clotting tests, which returned to normal values after adding 0.05 mmol/L phosphatidylcholine-phosphatidylserine liposomes (confirmatory test).23 An enzyme-linked immunoadsorbent assay, validated in an international workshop, was used for measurement of aCL. IgG or IgM aCL were considered positive when the serum concentration was greater than 10 GPL or 10 MPL units, respectively.24Patients were considered positive for aPL if LA and/or aCL were detected in two separate occasions at least 2 months apart.
Urinary Isoprostane-F2α-III and Isoprostane-F2α-VI were measured by GC/MS assayed as previously described.7 18 The internal standards used were [18O2]Isoprostane-F2α-III and [2H4]Isoprostane-F2α-VI. The intra-assay and interassay variability in urine obtained from healthy volunteers is ±3% and ±4% for Isoprostane-F2α-III and ±4% and ± 5% for Isoprostane-F2α-VI, respectively.
Statistical analysis.
Statistical analysis was performed by χ2 statistic or Fisher’s exact test (if n < 5) for independence and by appropriatet-test. When necessary, appropriate nonparametric tests were used. Correlation analysis was performed by Spearman test. Data were presented as the mean ± SD. Median and range are given for TNF, Isoprostane-F2α-III, and Isoprostane-F2α-VI, because they show appreciably skewed distribution. Only P values less than .05 were regarded as statistically significant. All calculations were made with the computer program STAT-View II (Abacus Concepts, Berkley, CA).25
RESULTS
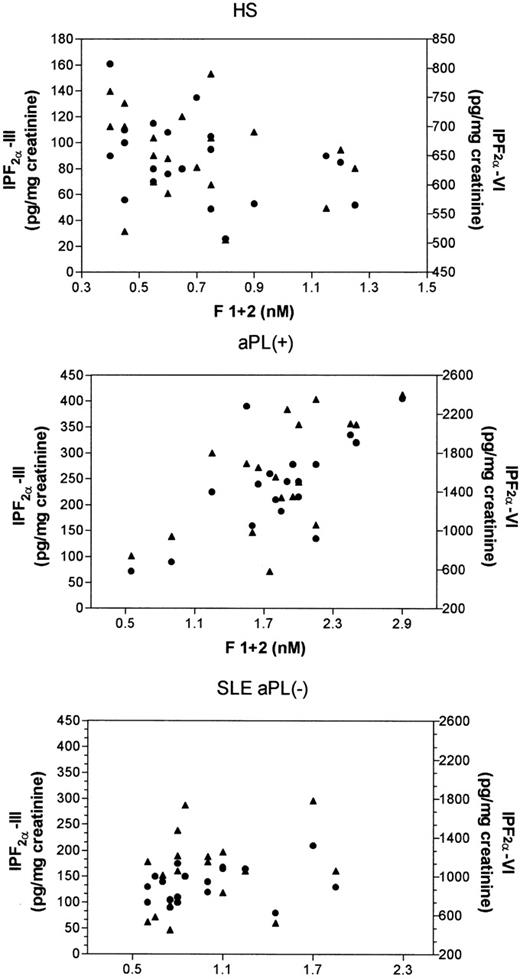
Table 1 reports on clinical and laboratory characteristics of aPL-positive patients and SLE patients who were negative for aPL. No significant differences in urinary Isoprostane-F2α-III and Isoprostane-F2α-VI were noticed as a function of sex, age, or cardiovascular risk factors, such as hypertension, dyslipidemia, or smoking. They did not show differences in renal function or acute-phase reactant proteins, such as C-reactive protein, C3, C4, and fibrinogen (not shown). Conversely, aPL-positive patients had higher values of Isoprostane-F2α-III (P = .0001), Isoprostane-F2α-VI (P = .006), and prothrombin fragment F1+2 (P = .0001) than SLE patients negative for aPL (Table 1 and Fig 2). Similar findings were observed when aPL-positive patients were compared with healthy subjects (Fig 2).
Clinical and Laboratory Characteristics of Patients Positive for aPL and of SLE Patients Negative for aPL
| . | aPL-Negative Patients (n = 18) . | aPL-Positive Patients (n = 18) . | P Value . |
|---|---|---|---|
| Age (yrs) | |||
| Mean ± SD | 37 ± 8 | 38 ± 11 | NS |
| Range | 23-50 | 17-53 | |
| Male sex (n) (%) | 1 (6) | 2 (11) | NS |
| Diabetes mellitus (n) (%) | 3 (17) | 4 (22) | NS |
| Hypertension (n) (%) | 4 (22) | 5 (28) | NS |
| Smoking (n) (%) | 5 (28) | 4 (22) | NS |
| F1 + 2 (nmol/L) | |||
| Mean | 1.02 | 1.83 | .0001 |
| SD | 0.38 | 0.56 | |
| IPF2α-III (pg/mg creatinine) | |||
| Median | 135 | 242 | .0001 |
| Range | 80-210 | 72-405 | |
| IPF2α-VI (pg/mg creatinine) | |||
| Median | 1,057 | 1,600 | .006 |
| Range | 449-1,780 | 580-2,400 |
| . | aPL-Negative Patients (n = 18) . | aPL-Positive Patients (n = 18) . | P Value . |
|---|---|---|---|
| Age (yrs) | |||
| Mean ± SD | 37 ± 8 | 38 ± 11 | NS |
| Range | 23-50 | 17-53 | |
| Male sex (n) (%) | 1 (6) | 2 (11) | NS |
| Diabetes mellitus (n) (%) | 3 (17) | 4 (22) | NS |
| Hypertension (n) (%) | 4 (22) | 5 (28) | NS |
| Smoking (n) (%) | 5 (28) | 4 (22) | NS |
| F1 + 2 (nmol/L) | |||
| Mean | 1.02 | 1.83 | .0001 |
| SD | 0.38 | 0.56 | |
| IPF2α-III (pg/mg creatinine) | |||
| Median | 135 | 242 | .0001 |
| Range | 80-210 | 72-405 | |
| IPF2α-VI (pg/mg creatinine) | |||
| Median | 1,057 | 1,600 | .006 |
| Range | 449-1,780 | 580-2,400 |
Statistical analysis was performed by Mann-Whitney-U test.
Abbreviation: NS, not significant.
Urinary levels of Isoprostane-F2-III (upper panel) and Isoprostane-F2-VI (middle panel) and plasma prothrombin fragment F1+2 (lower panel) in patients positive for antiphospholipid antibodies (aPL+), in patients with systemic lupus erythematosus negative for the antiphospholipid antibodies (SLE aPL−), and in healthy subjects (HS). Statistical analysis was performed by Mann-Whitney-U test.
Urinary levels of Isoprostane-F2-III (upper panel) and Isoprostane-F2-VI (middle panel) and plasma prothrombin fragment F1+2 (lower panel) in patients positive for antiphospholipid antibodies (aPL+), in patients with systemic lupus erythematosus negative for the antiphospholipid antibodies (SLE aPL−), and in healthy subjects (HS). Statistical analysis was performed by Mann-Whitney-U test.
SLE patients negative for aPL had similar values of prothrombin fragment F1+2 (1.02 ± 0.38 nmol/L v 0.69 ± 0.26 nmol/L,P > .05), but higher levels of Isoprostane-F2α-III (median, 135 pg/mg [range, 80 to 210 pg/mg] v 87 pg/mg [range, 26 to 161 pg/mg] creatinine;P = .002) and Isoprostane-F2α-VI (median, 1,057 pg/mg [range, 449 to 1780] v 655 pg/mg [range, 505 to 690 pg/mg] creatinine; P = .003) compared with controls (Fig2).
Patients with PAPS (n = 8) and aPL-positive SLE patients (n = 10) showed similar values for Isoprostane-F2α-III (median, 215 pg/mg [range, 90 to 390 pg/mg] v 245 pg/mg [range, 72 to 405 pg/mg] creatinine; P > .05), Isoprostane-F2α-VI (median, 1,350 pg/mg [range, 940 to 1,690 pg/mg] v 1,800 pg/mg [range, 580 to 2,400 pg/mg] creatinine; P > .05) and F1+2 (mean ± SD, 1.65 ± 0.37 nmol/L v ± 0.64 nmol/L; P > .05).
A further analysis was performed in all patients to assess the possible effect of the history of thrombosis on lipid peroxidation and clotting activation parameters. In aPL-positive patients, no difference in isoprostanes and F1+2 was observed between subjects with and without previous thrombosis; the same finding was observed in aPL-negative patients (Table 2). In aPL-positive patients, F1+2 correlated significantly with Isoprostane-F2α-VI (Rho = .61 P = .008) and Isoprostane F2α-III (Rho = .56, P = .017) levels, whereas in SLE patients negative for aPL, the correlation was not statistically significant (F1+2 vIsoprostane-F2α-VI, Rho = .20, P = .40; F1+2v Isoprostane-F2α-III, Rho = .22, P = .36; Fig 3). No correlation between either isoprostane and F1+2 was observed in healthy subjects.
Lipid Peroxidation and Clotting Activation Indexes in aPL-Positive and aPL-Negative Patients With and Without Previous Thrombosis
| . | aPL-Positive Patients . | aPL-Negative Patients . | ||||
|---|---|---|---|---|---|---|
| With Thrombosis (n = 11) . | . | Without Thrombosis (n = 7) . | With Thrombosis (n = 5) . | . | Without Thrombosis (n = 13) . | |
| IPF2α-III (pg/mg creatinine) | ||||||
| Median | 232 | NS | 245 | 110 | NS | 145 |
| Range | 90-405 | 72-335 | 80-165 | 100-210 | ||
| IPF2α-VI (pg/mg creatinine) | ||||||
| Median | 1,575 | NS | 1,820 | 806 | NS | 1,150 |
| Range | 940-2,400 | 580-2,245 | 520-1,150 | 449-1,780 | ||
| F 1 + 2 (nmol/L) | ||||||
| Mean | 1.78 | NS | 1.89 | 0.93 | NS | 1.07 |
| SD | 0.54 | 0.61 | 0.39 | 0.37 | ||
| . | aPL-Positive Patients . | aPL-Negative Patients . | ||||
|---|---|---|---|---|---|---|
| With Thrombosis (n = 11) . | . | Without Thrombosis (n = 7) . | With Thrombosis (n = 5) . | . | Without Thrombosis (n = 13) . | |
| IPF2α-III (pg/mg creatinine) | ||||||
| Median | 232 | NS | 245 | 110 | NS | 145 |
| Range | 90-405 | 72-335 | 80-165 | 100-210 | ||
| IPF2α-VI (pg/mg creatinine) | ||||||
| Median | 1,575 | NS | 1,820 | 806 | NS | 1,150 |
| Range | 940-2,400 | 580-2,245 | 520-1,150 | 449-1,780 | ||
| F 1 + 2 (nmol/L) | ||||||
| Mean | 1.78 | NS | 1.89 | 0.93 | NS | 1.07 |
| SD | 0.54 | 0.61 | 0.39 | 0.37 | ||
Statistical analysis was performed by Mann-Whitney-U test.
Abbreviation: NS, not significant.
Correlation analysis (Spearman test) of prothrombin fragment F1+2 versus Isoprostane-F2-III (•) and versus Isoprostane-F2-VI (▴) in healthy subjects (HS), in patients positive for antiphospholipid antibodies (aPL+), and in patients with systemic lupus erythematosus negative for antiphospholipid antibodies (SLE aPL−).
Correlation analysis (Spearman test) of prothrombin fragment F1+2 versus Isoprostane-F2-III (•) and versus Isoprostane-F2-VI (▴) in healthy subjects (HS), in patients positive for antiphospholipid antibodies (aPL+), and in patients with systemic lupus erythematosus negative for antiphospholipid antibodies (SLE aPL−).
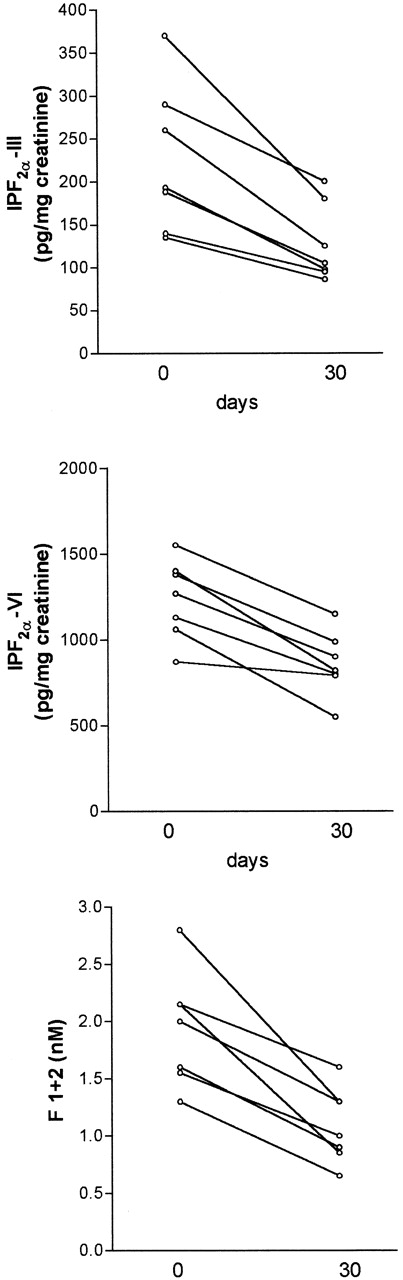
To assess if the antioxidant treatment affected the entity of lipid peroxidation and the plasma levels of prothrombin fragment F1+2, 12 patients were treated with (group A, 6 women and 1 man; 22 to 49 years of age) or without (group B, 5 women; 17 to 48 years of age) antioxidant vitamins for 4 weeks. Baseline levels of isoprostanes and F1+2 did not significantly differ between the two groups (P > .05). There was no difference in sex, age, and standard treatment between the two groups; in particular, 3 of 7 patients in the group A and 2 of 5 patients in the group B were on treatment with corticosteroids. Patients of group A showed a significant decrease of Isoprostane-F2α-III (median, 190 [range, 116 to 370]v 105 [range, 86 to 200]; P = .007), Isoprostane-F2α-VI (median, 1,200 [range, 870 to 1,780]v 845 [range, 550 to 1,390]; P < .005), and prothrombin fragment F1+2 (mean ± SD, 1.88 ± 0.43 v1.17 ± 0.22; P < .005; Fig 4). Conversely, no difference was observed in the group B between baseline and 4-week values of Isoprostane-F2α-III (median, 150 [range, 60 to 405]v 175 [range, 120 to 320]; P > .05), Isoprostane-F2α-VI (median, 1,350 [range, 580 to 2,245] v 1,280 [range, 1,380 to 2,000]; P > .05), and F1+2 (1.67 ± 0.91 v 1.90 ± 0.66 nmol/L;P > .05). During the follow-up, the aPL positivity, fibrinogen, and TNF did not significantly change in both groups (Table 3). To assess the compliance to antioxidant supplementation, vitamin E and C plasma levels were measured before and after 4 weeks of treatment. Both vitamin E (mean ± SD, from 15.3 ± 5.3 to 38.3 ± 11.3 μmol/L;P < .05) and vitamin C (mean ± SD, from 17.8 ± 11.5 to 22.9 ± 9.4 μmol/L; P < .05) significantly increased at the end of follow-up.
Decrease of urinary Isoprostane-F2-III (upper panel, P = .007) and Isoprostane-F2-VI (middle panel, P < .005) and plasma prothrombin fragment F1+2 (lower panel, P < .005) in patients positive for antiphospholipid antibodies after 4 weeks of combination therapy with vitamin E and vitamin C. Statistical analysis was performed by pairedt-test.
Decrease of urinary Isoprostane-F2-III (upper panel, P = .007) and Isoprostane-F2-VI (middle panel, P < .005) and plasma prothrombin fragment F1+2 (lower panel, P < .005) in patients positive for antiphospholipid antibodies after 4 weeks of combination therapy with vitamin E and vitamin C. Statistical analysis was performed by pairedt-test.
Laboratory Variables Before and After Treatment With (Group A) or Without (Group B) Antioxidant Vitamins in Patients Positive for aPL
| . | Group A (n = 7) . | Group B (n = 5) . | ||||
|---|---|---|---|---|---|---|
| Before . | After . | P . | Before . | After . | P . | |
| aPL positivity (n) | 7 | 5 | NS | 5 | 5 | NS |
| Fibrinogen (mg/dL) (mean ± SD) | 242 ± 71 | 245 ± 67 | NS | 242 ± 51 | 236 ± 66 | NS |
| TNF (pg/mL) | ||||||
| Median | 169 | 145 | NS | 178 | 189 | NS |
| Range | 42-286 | 61-272 | 102-224 | 62-286 | ||
| . | Group A (n = 7) . | Group B (n = 5) . | ||||
|---|---|---|---|---|---|---|
| Before . | After . | P . | Before . | After . | P . | |
| aPL positivity (n) | 7 | 5 | NS | 5 | 5 | NS |
| Fibrinogen (mg/dL) (mean ± SD) | 242 ± 71 | 245 ± 67 | NS | 242 ± 51 | 236 ± 66 | NS |
| TNF (pg/mL) | ||||||
| Median | 169 | 145 | NS | 178 | 189 | NS |
| Range | 42-286 | 61-272 | 102-224 | 62-286 | ||
Statistical analysis was performed by Mann-Whitney-U test.
Abbreviation: NS, not significant.
DISCUSSION
The mechanism accounting for the formation of antibodies against phospholipid in patients with primary or secondary antiphospholipid syndrome is still unclear.1
These autoantibodies are so named because they bind in vitro to phospholipids, but the exact nature of the epitope(s) recognized remain uncertain. Recently, Horkko et al26 have reported that at least some aPL recognize neoepitopes of protein-phospholipid complexes generated through a free radical mechanism: oxidation of phospholipids generates breakdown products, such as aldehydes, which form covalent adducts with aminoacidic residues of the associated protein. Whether these neoepitopes of oxidized phospholipids have biological activity linked to the thrombogenic mechanism remains to be investigated, but it is plausible that these oxidation-generated epitopes occur in vivo and possibly trigger synthesis of autoantibodies. This hypothesis is corroborated by recent evidence that SLE patients with aPL have enhanced lipid peroxidation in vivo, as documented by high urinary excretion of isoprostanes, which is highly correlated to anticardiolipin antibody titer.11 The suggestion that aPL positivity and lipid peroxidation are related is further supported by the results of the current study, which reports that the urinary excretion of isoprostanes is also elevated in patients with PAPS.
In the present study, we tested whether lipid peroxidation and clotting activation coexist in aPL-positive patients. We demonstrated that, in aPL-positive patients, F2 Isoprostanes and F1+2 plasma levels were significantly correlated, suggesting that in vivo lipid peroxidation and clotting activation are associated.
It is noteworthy that, in patients without aPL, the circulating levels of prothrombin fragment F1+2 were within normal range and did not correlate with F2 Isoprostanes. This further reinforces the suggestion that, in patients with aPL, there is a relationship between lipid peroxidation and clotting activation. The history of thrombosis did not influence such behavior, because the increase of isoprostanes and F1+2 was observed essentially in aPL-positive patients and was similar in patients with and without previous thrombosis.
To further explore such an association, we investigated whether natural antioxidants such as vitamin E and vitamin C could modulate the increase in lipid peroxidation and the activation of clotting system. We observed that, after vitamins supplementation, concomitantly with the decrease in urinary levels of both F2-isoprostanes, the circulating levels of the prothrombin fragment F1+2 were significantly reduced.
We have previously shown that, in SLE, lipid peroxidation and chronic inflammation coexist, particularly in cases of aPL positivity.11 In the present study we did not address the question as to whether a similar mechanism may account for enhanced lipid peroxidation in PAPS; therefore, further study is necessary to explore this issue. However, after antioxidant treatment, no changes in markers of acute inflammation were detected, suggesting that decrease of lipid peroxidation was not attributable to changes of disease activity.
Several lines of evidence suggest that oxygen free radicals contribute to cell activation.27 Actually, antioxidants have been reported to inhibit lipopolysaccharide-induced transcriptional and posttranscriptional activation expression of macrophage tissue factor,28,29 a protein that stimulates the extrinsic coagulation pathway by activating factor X to Xa.30Furthermore, human monocytes exposed to copper-induced oxidant stress had an enhanced expression of tissue factor, which was again inhibited by antioxidants.31 These data lead to hypothesize that the enhanced lipid peroxidation observed in aPL-positive patients could be an important mechanism leading to clotting activation. It is interesting to note that, in another clinical model associated with thrombosis, such as diabetes mellitus, low antioxidant capacity was inversely correlated with F1+2 plasma levels.32
In conclusion, this study is the first demonstration that, in patients with aPL, there is a relationship between in vivo lipid peroxidation and clotting activation. These data may open a new avenue to understand the pathogenesis of thrombosis in this clinical setting and to develop new therapeutic strategies for preventing thrombosis in these patients.
ACKNOWLEDGMENT
The authors are grateful to Dr Michael Maldonato for useful suggestions.
Supported in part by Finanziamento Progetti di Ricerca 40% (to F.V.), University “La Sapienza,” Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Francesco Violi, MD, Institute of Clinical Medicine I, University La Sapienza, 00185 Rome, Italy; e-mail:violi@uniroma1.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal