C5a, a potent peptide chemoattractant, stimulates interleukin-8 (IL-8) secretion from peripheral blood mononuclear cells (PBMC). Experiments were conducted to understand the mechanisms for C5a-induced IL-8 production, which was 14-fold greater than that in unstimulated cells by 2 hours. IL-8 secretion was accompanied by accumulation of IL-8 mRNA in the cytosol and by nuclear expression of a κB DNA binding activity within 30 minutes. AP-1 but not NF-IL-6 DNA binding activity was also detected in C5a-stimulated PBMC; however, its delayed expression (maximal at 4 hours) suggested a less important role in the rapid production of IL-8. The correlation between C5a-induced κB binding activity and IL-8 gene expression was examined in the RAW264.7 macrophage cells using reporter genes directed by the κB sequence from IκB and IL-8 promoter regions. C5a-induced reporter gene expression was abolished by introducing mutations into the κB sites and by coexpression of a dominant negative IκB construct resistant to agonist-induced phosphorylation. Pertussis toxin, which ADP-ribosylates the Gi proteins known to couple to the C5a receptor, produced minimal inhibition of C5a-induced IL-8 expression and had little effect on C5a-induced calcium mobilization in RAW264.7 cells. These results suggest that NF-κB activation is required for C5a-induced IL-8 gene expression and that this response is mediated primarily through a pertussis toxin-insensitive pathway.
C5a IS A PROINFLAMMATORY peptide generated through activation of the complement system, which occurs in the course of trauma, immune complex formation, and bacterial infection.1,2 C5a is more potent than the other two activated complement anaphylatoxins, C3a and C4a, in activating peripheral blood leukocytes.3 The proinflammatory effects of C5a are mediated through binding of this peptide agonist to specific receptors expressed on the surface of peripheral blood monocytes and granulocytes4 (reviewed in Gerard and Gerard5). More recent studies indicate that the same receptor is also present in hepatocytes, astrocytes, and glial cells.6-9 Molecular cloning and characterization of C5a receptors have led to the determination of its primary structure as a G-protein–coupled receptor with 7 putative transmembrane domains.10-13 C5a receptor, together with the receptors for fMet-Leu-Phe, platelet-activating factor, leukotriene B4, and a growing number of chemokines, form a subgroup within the large family of rhodopsin-like G-protein–coupled receptors.14 Binding of C5a to its receptor in phagocytes induces chemotaxis, generation of superoxide anions, and release of degradative enzymes.2,5 C5a receptor appears to have a nonredundant function in innate immunity, because targeted deletion resulted in weakened mocosal host defense againstPseudomonas aeruginosa infection in the lung.15
Several studies demonstrate that C5a actively regulates local cytokine networks by stimulating the production of proinflammatory cytokines. It has been reported that recombinant C5a stimulates transcription of the interleukin-1 (IL-1) and tumor necrosis factor (TNF) genes.16 C5a can also enhance the effect of lipopolysaccharide (LPS) in its stimulation of IL-6 synthesis17 and acts synergestically with γ-interferon to stimulate IL-1 synthesis.18 In addition, C5a has been shown to induce P-selectin expression in endothelial cells.19 The role of C5a in regulating gene expression was further suggested by recent studies of C5a receptor knockout mice, which demonstrate reduced TNFα and IL-6 levels in peritoneal reverse passive Arthus reaction compared with the wild-type littermates.20 Although these activities are consistent with the proinflammatory functions of C5a, the mechanism for C5a stimulated cytokine generation remains incompletely understood. There have been conflicting reports regarding whether C5a is able to stimulate transcription and translation of cytokine gene21,22 or its effect is confined only to transcriptional activation.16
To better understand the role of C5a in regulating transcription and translation during inflammation, we investigated the ability of C5a to stimulate IL-8 synthesis at both protein and nucleic acid levels. IL-8 is a CXC chemotactic cytokine that has been extensively characterized both structurally and functionally. IL-8 plays an important role in acute inflammation through binding to and activation of the IL-8 receptors on neutrophils and mononuclear cells.23,24 IL-8 is also angiogenic25 and may stimulate its own biosynthesis through an autocrine mechanism (unpublished results). The genomic structure of IL-8 and the transcriptional elements regulating its expression have been characterized previously,26 permitting a detailed study of the effect of C5a on IL-8 gene expression. Using a combination of immunologic and recombinant DNA approaches, we investigated IL-8 gene expression and protein synthesis in peripheral blood mononuclear cells and in a macrophage-like cell line RAW264.7. Our results indicate that C5a induces activation of NF-κB, which is necessary for a rapid increase in IL-8 mRNA and protein levels in the stimulated cells.
MATERIALS AND METHODS
Materials.
Human C5a was obtained from two sources: natural C5a purified from human serum (kindly provided by Dr T.E. Hugli, The Scripps Research Institute, La Jolla, CA) and recombinant human C5a (purchased from Sigma Chemical Co, St Louis, MO). The recombinant C5a was tested negative for the presence of LPS with a Limulus amebocyte lysate assay that detects LPS at a concentration of 25 pg/mL. Human IL-8 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Biosource (Camarillo, CA). Pertussis toxin was obtained from Calbiochem (San Diego, CA). Actinomycin D, cyclohexamide, and pyrrolidine dithiocarbamate were purchased from Sigma. Oligonucleotides with the consensus sequences of NF-κB, AP-1, and NF-IL-6 binding sites were obtained from Promega (Madison, WI). Rabbit antibodies against p50 and p65, and their neutralizing peptides, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of peripheral blood mononuclear cells (PBMC).
Human PBMC were prepared from fresh, heparinized venous blood by Ficoll-Hypaque (Sigma) density gradient centrifugation. Platelets were separated from PBMC by washing with Hank’s balanced salt solution (GIBCO-BRL, Gaithersburg, MD) and by centrifugation through a fetal bovine serum (FBS) cushion gradient that allows penetration of mononuclear cells but not platelets. The PBMC pellet was resuspended in RPMI 1640 culture medium (Irvine Scientific, Santa Ana, CA) and cultured in round-bottom culture tubes for induction of IL-8 synthesis at 37°C in a humidified atmosphere containing 5% CO2. All of the solutions used in these studies were either subjected to ultrafiltration for elimination of microbial products or treated with an endotoxin removal kit (Associates of Cape Cord, Inc, Woods Hole, MA).
Determination of immunoreactive IL-8 by ELISA.
Approximately 0.5 × 106 PBMC were cultured in 1 mL RPMI 1640 medium and stimulated with C5a for various time periods. The supernatants were collected and assayed for IL-8 secretion by ELISA according to instructions from the manufacturer (Biosource).
RNA isolation and Northern blot analysis.
PBMC (0.5 × 106) were preincubated with or without various inhibitors or bacterial toxins for 1 to 4 hours, followed by C5a stimulation for 2 hours. Cells were harvested, and pellets were resuspended in 1 mL TRI-reagent (Molecular Research Center, Inc, Cincinnati, OH) for total RNA isolation. For Northern blot, 10 μg of RNA was subjected to electrophoresis in a gel containing 1.2% agarose and 6.6% formaldehyde as denaturing agent. The RNA was then transferred to nylon membrane and fixed by short-wave UV radiation. The Northern blot was prehybridized for 1 hour in the ExpressHyb solution (Clontech, Palo Alto, CA) and hybridized with a [α-32P]dCTP-labeled IL-8 DNA fragment at 1 × 106 cpm/mL. Hybridization was performed at 65°C for 3 hours in the ExpressHyb solution. The blot was washed twice in 0.5× standard sodium citrate solution with 0.1% sodium dodecyl sulfate at 65°C for 15 minutes. The blot was then stripped and rehybridized with a [α-32P]dCTP-labeled β-actin cDNA for equal loading control.
Preparation of nuclear extracts.
Freshly isolated PBMC were placed at a density of 5 × 106 in round-bottom culture tubes and stimulated with C5a. After stimulation, cells were washed 3 times with ice-cold phosphate-buffered saline (PBS), harvested, and resuspended in 0.4 mL of buffer A (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol, and 0.5 mmol/L phenolmethylsulfonyl fluoride (PMSF). After 10 minute, 23 μL of 10 % Nonidet P-40 was added and mixed for 2 seconds. Nuclei were separated from cytosol by centrifugation at 13,000g for 10 seconds and resuspended in 50 μL of buffer B (20 mmol/L HEPES, pH 7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, and 0.1 mmol/L PMSF). After 30 minutes at 4°C, lysates were separated by centrifugation at 13,000g for 30 minutes, and supernatant containing nuclear proteins was aliquoted and stored at −80°C. The protein concentration of extracts was measured using the BCA protein dye reagent (Pierce, Rockford, IL) with bovine serum albumin as standard.
Electrophoretic mobility shift assays (EMSA).
EMSA were performed by incubating 2.5 μg of nuclear extract in 12 μL of binding buffer [5 mmol/L HEPES, pH 7.9, 5 mmol/L MgCl2, 50 mmol/L KCl, 0.5 mmol/L dithiothreitol, 0.4 mg/mL poly(dI-dC) (Pharmacia Biotech, Piscataway, NJ), 0.1 mg/mL sonicated double-stranded salmon sperm DNA, and 10% glycerol] for 10 minutes at room temperature. 32P-labeled oligonucleotide probe (30,000 cpm) was then added, and the reaction mixture was incubated for another 10 minutes at room temperature. For reactions involving competitive oligonucleotides, the unlabeld competitor, at 100-fold molar excess, and the labeled probe were premixed before addition to the reaction mixture. For supershift assays, the reaction mixture minus the probe was incubated with 2 μL of specific antibodies to NF-κB proteins for 30 minutes at room temperature. The 32P-labeled oligonucleotide probe was then added and incubation was continued for 15 minutes. The samples were analyzed on 6% acrylamide gels. The gels were pre-electrophoresed at 12 V/cm for 1.5 hours at room temperature and 0.5 hours in a cold room, and the electrophoresis was continued at the same voltage for another 2 hours at room temperature. Gel contents were transferred to Whatman chromatography paper (Markson Lab Sales, Hillsboro, OR), dried, and exposed to a PhosphorImage screen (Molecular Dynamics, Sunnyvale, CA) for 3 hours.
Cell culture and transfection.
The murine RAW264.7 macrophage cell line was maintained in RPMI 1640 culture medium containing 2 mmol/L L-glutamine, 100 IU/mL penicillin, 50 μg/mL streptomycin, and 10% fetal bovine serum at 37°C in a humidified 5% CO2 environment. RAW264.7 cells were cotransfected, by electroporation, with reporter gene plasmid DNA and the control plasmid pCMVβ. For each transfection, a total of 10 μg supercoiled DNA was added to 2 × 107 cells resuspended in 0.2 mL of fresh growth medium in an electroporation cuvette with 0.4-cm gap and incubated at room temperature for 5 minutes. Electroporation was performed using a Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA), with voltage and capacitance settings of 300 V (750 V/cm) and 960 μF, respectively. The cells were collected immediately after electroporation, seeded in 2 mL growth medium, and cultured overnight in a 6-well plate until starvation and stimulation.
Chloramphenicol acetyltransferase (CAT) assay.
Eight micrograms of each CAT reporter construct DNA and 2 μg of the control plasmid pCMVβ were cotransfected into 2 × 107 RAW264.7 cells by electroporation. In experiments with a dominant negative IκBα construct (kindly provided by Dr D. Van Antwerp and I. Verma, The Salk Institute, La Jolla, CA), 4 μg of the plasmid DNA was cotransfected with a CAT reporter. Sixteen hours after transfection, the cells were washed with serum-free medium, replaced with starvation medium (RPMI 1640 medium containing 2 mmol/L L-glutamine, 100 IU/mL penicillin, 50 μg/mL streptomycin, and 0.5% fetal bovine serum), and cultured for 4 hours. Cells were then stimulated with C5a (100 nmol/L) for 2 to 6 hours in the starvation medium and collected in 100 μL of 0.25 mol/L Tris (pH 7.8) followed by 3 cycles of freeze-thaw. CAT activities were measured in crude cellular extracts using [14C]chloramphenicol (Amersham, Arlington Heights, IL) as substrate and thin-layer chromatography to separate the native form from acetylated form. Relative transfection efficiency was determined by normalizing the CAT activity to β-galactosidase activity measured in cell extracts from the cotransfected pCMVβ.
Calcium mobilization assay.
Cells were treated with or without pertussis toxin (100 ng/mL, or as indicated in the figures) for 16 hours, harvested with trypsin-free cell dissociation buffer (GIBCO-BRL), and loaded with Indo-1 (5 μmol/L; Molecular Probes, Eugene, OR) by incubation at 37°C for 30 minutes with gentle rocking. Agonist-induced calcium mobilization was measured in an SLM 8000C spectrofluorometer (SLM-Aminco, Urbana, IL) detecting at 400 and 490 nm, with an excitation wavelength of 340 nm.
RESULTS
C5a stimulates synthesis of immunoreactive IL-8 from PBMC.
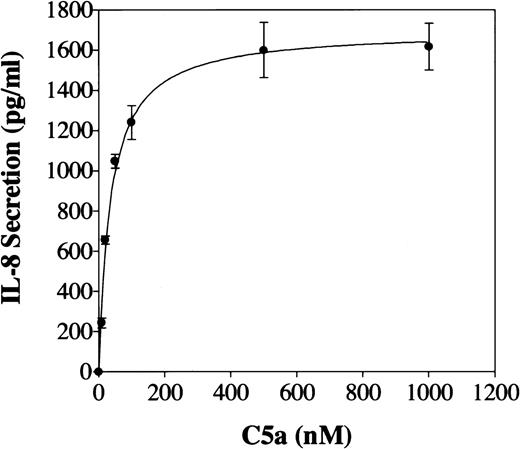
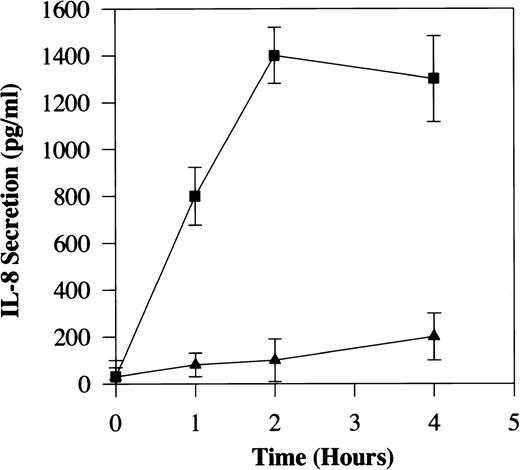
Freshly prepared human PBMC were stimulated with C5a at various concentrations and IL-8 secreted into the supernatant was measured by ELISA. Figure 1 shows that unstimulated PBMC did not produce detectable IL-8, whereas cells incubated with 100 nmol/L of recombinant human C5a produced up to 1,200 pg/mL of IL-8. C5a induction of IL-8 secretion was dose-dependent, detectable with 12.5 nmol/L of C5a and peaking at an agonist concentration of 500 nmol/L. PBMC from at least 6 different donors gave similar results (Fig 1). Natural C5a, purified from human serum, gave nearly identical results (data not shown). The IL-8 secreted into the culture medium increased rapidly in the first 2 hours of C5a stimulation, reaching 14-fold above the level of an unstimulated culture (Fig 2). This rapid increase in IL-8 secretion suggests a direct effect of C5a on IL-8 production. IL-8 level in unstimulated culture remained low (≤100 pg/mL) during the time period of investigation. These results are consistent with a previous report indicating C5a induction of IL-8 production in monocytes.22
C5a stimulation of immunoreactive IL-8 secretion from PBMC. PBMC were incubated with different concentrations (from 10 to 1,000 nmol/L) of recombinant human C5a. After 2 hours, cells were removed and immunoreactive IL-8 in the medium was determined by ELISA. For each stimulation, 0.5 × 106 freshly prepared PBMC were used. Data presented are the mean ± SE from 6 individual donors.
C5a stimulation of immunoreactive IL-8 secretion from PBMC. PBMC were incubated with different concentrations (from 10 to 1,000 nmol/L) of recombinant human C5a. After 2 hours, cells were removed and immunoreactive IL-8 in the medium was determined by ELISA. For each stimulation, 0.5 × 106 freshly prepared PBMC were used. Data presented are the mean ± SE from 6 individual donors.
Time course of C5a-induced IL-8 secretion from PBMC. Approximately 0.5 × 106 freshly prepared PBMC were incubated in the presence (▪) or the absence (▴) of 200 nmol/L of human C5a for different lengths of time (0 to 4 hours). The cells were then removed and the supernatants were taken for measurement of secreted IL-8 by ELISA. Results shown are the mean ± SE from three separate measurements, each in duplicates.
Time course of C5a-induced IL-8 secretion from PBMC. Approximately 0.5 × 106 freshly prepared PBMC were incubated in the presence (▪) or the absence (▴) of 200 nmol/L of human C5a for different lengths of time (0 to 4 hours). The cells were then removed and the supernatants were taken for measurement of secreted IL-8 by ELISA. Results shown are the mean ± SE from three separate measurements, each in duplicates.
C5a stimulates transcription of the IL-8 gene.
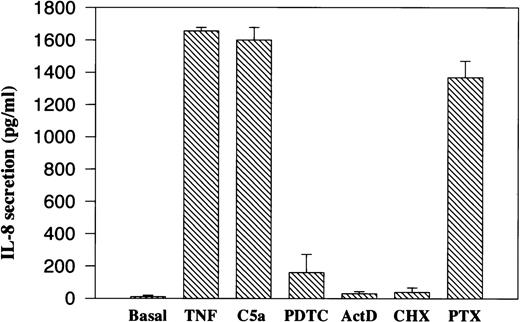
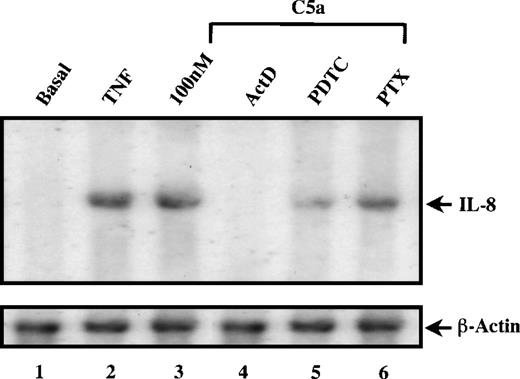
To investigate whether the induction of IL-8 by C5a is at the transcriptional level, PBMC were treated with various transcription and translation inhibitors for 1 hour before C5a stimulation. After 2 hours of incubation with C5a, the supernatants were collected and assayed for IL-8 by ELISA. As shown in Fig 3, C5a-stimulated IL-8 secretion was blocked almost completely by the translation inhibitor cyclohexamide (CHX) and by the transcription inhibitor actinomycin D (ActD). These data suggest that C5a-mediated production of IL-8 from PBMC is the result of de novo protein synthesis and not the release of a preformed protein product. This was further confirmed by Northern blot analysis using total RNA isolated from PBMC after 2 hours of C5a stimulation in the absence or presence of the above-noted inhibitors. As shown in Fig 4, C5a stimulated cytosolic accumulation of IL-8 mRNA (lane 3 vlane 1). Both IL-8 protein secretion and mRNA accumulation were not only inhibited by ActD, but also reduced by pyrrolidine dithiocarbamate (PDTC), a potent inhibitor of the transcription factor NF-κB (Fig 4, lanes 4 and 5). This result suggests the involvement of nuclear factor κB (NF-κB) in C5a-induced transcriptional activation of IL-8 gene.
Effect of transcription and translation inhibitors and pertussis toxin on C5a induced IL-8 secretion. Freshly prepared PBMC (0.5 × 106) were preincubated with different inhibitors for 1 hour and with pertussis toxin (PTX) for 4 hours. The cells were then stimulated with 200 nmol/L of recombinant human C5a for 2 hours. The supernatants were taken for measurement of secreted IL-8 by ELISA. The final concentration of PDTC was 10 μmol/L, the transcription inhibitor ActD was added to 5 μg/mL, and the final concentration of CHX was 10 μg/mL. PTX was added to 1 μg/mL. TNF (40 ng/mL) was used as a positive control. Data were from three separate experiments, each with duplicate measurements.
Effect of transcription and translation inhibitors and pertussis toxin on C5a induced IL-8 secretion. Freshly prepared PBMC (0.5 × 106) were preincubated with different inhibitors for 1 hour and with pertussis toxin (PTX) for 4 hours. The cells were then stimulated with 200 nmol/L of recombinant human C5a for 2 hours. The supernatants were taken for measurement of secreted IL-8 by ELISA. The final concentration of PDTC was 10 μmol/L, the transcription inhibitor ActD was added to 5 μg/mL, and the final concentration of CHX was 10 μg/mL. PTX was added to 1 μg/mL. TNF (40 ng/mL) was used as a positive control. Data were from three separate experiments, each with duplicate measurements.
C5a-induced increase in cytosolic IL-8 mRNA. Freshly prepared PBMC (0.5 × 106) were preincubated with either the transcription inhibitor actinomycin D, the NF-κB inhibitor PDTC, or PTX for 1 hour (lanes 4, 5, and 6), as specified on top of each lane. The concentrations of the inhibitors used in this study were the same as indicated in Fig 3. The cells were then stimulated with 200 nmol/L of human C5a for 2 hours or with 40 ng/mL of TNF (lane 2) as a positive control. Total RNA was extracted from the cell pellets, separated in formaldehyde/agarose gel (10 μg/lane) and subjected to Northern blot analysis using a 32P-labeled IL-8 cDNA fragment as probe. The autoradiograph shows specific bands of IL-8 transcript. The same blot was stripped and reprobed with32P-labeled β-actin for equal loading control.
C5a-induced increase in cytosolic IL-8 mRNA. Freshly prepared PBMC (0.5 × 106) were preincubated with either the transcription inhibitor actinomycin D, the NF-κB inhibitor PDTC, or PTX for 1 hour (lanes 4, 5, and 6), as specified on top of each lane. The concentrations of the inhibitors used in this study were the same as indicated in Fig 3. The cells were then stimulated with 200 nmol/L of human C5a for 2 hours or with 40 ng/mL of TNF (lane 2) as a positive control. Total RNA was extracted from the cell pellets, separated in formaldehyde/agarose gel (10 μg/lane) and subjected to Northern blot analysis using a 32P-labeled IL-8 cDNA fragment as probe. The autoradiograph shows specific bands of IL-8 transcript. The same blot was stripped and reprobed with32P-labeled β-actin for equal loading control.
C5a-induced nuclear expression of a κB DNA binding activity is primarily responsible for the early stage transcriptional activation of IL-8 gene.
The IL-8 gene consists of 4 exons and 3 introns with a single TATA box and a CAT box. The 5′-flanking region contains potential binding sites for known transcription factors AP-1, AP-2, NF-IL-6, and NF-κB.27 The sequence from −94 to −81 on IL-8 gene promoter shows similarity with potential binding site for NF-IL-6 (CAGTTGCAAATCGT), and the sequence from −82 to −70 (GTGGAATTTCCTC) is homologous to the consensus binding element for NF-κB, as in the genes for Ig κ light chain, SV40 and HIV (GGGGACTTTCC).27,28 NF-κB has been shown to play an important role in the transcription of genes for many inflammatory factors,29 including the IL-8 gene.28 It was therefore important to determine whether C5a is capable of activating this transcription factor as well as other factors known to cooperate with NF-κB in IL-8 gene expression, including AP-1 and NF-IL-6.
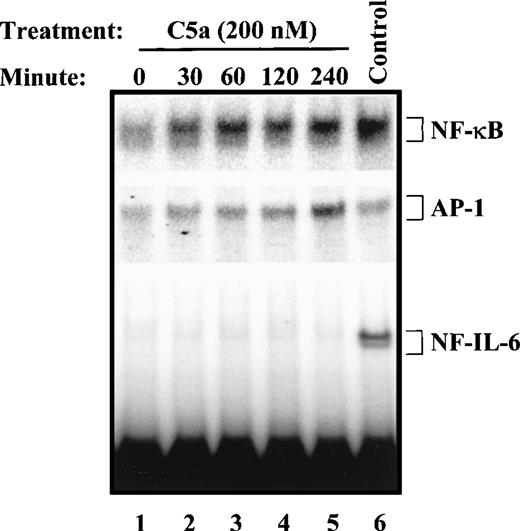
PBMC were stimulated with C5a or with the control agonists TNFα and LPS, and nuclear extracts were prepared for gel mobility shift assays using 32P-labeled probes for NF-κB, AP-1, and NF-IL-6. As shown in Fig 5, exposure of PBMC to C5a resulted in a marked increase in both κB and AP-1 binding activities. However, the expression of these binding activities followed different time courses. The κB binding activity was strongly expressed within 30 minutes after C5a stimulation and reached its peak in 60 minutes. However, the AP-1 binding activity was apparent 4 hours after C5a stimulation (Fig 5, middle panel). C5a did not stimulate NF-IL-6 binding activity for up to 4 hours, whereas LPS induced a strong NF-IL-6 binding activity in these cells (Fig 5, lower panel). Because of the rapid increase in both IL-8 mRNA and protein levels in the first 2 hours after C5a stimulation (Fig 2), the above-noted results suggest that C5a-induced NF-κB is most relevant to the early stage IL-8 gene expression in PBMC.
C5a-induced transcription factor activation in PBMC. For each sample, 5 × 106 freshly prepared PBMC were incubated with 200 nmol/L of human C5a at 37°C for different lengths of time (0 to 4 hours). The cells were then harvested and nuclear extracts were prepared as described in Materials and Methods. Nuclear extract (2.5 μg) from each time point was incubated with one of the three different probes: 32P-labeled, double-stranded oligonucleotides containing binding sites for κB, AP-1, and NF-IL-6. The binding mixture was subjected to 5% acrylamide gel electrophoresis. The binding activities for NF-κB (top panel), AP-1 (middle panel), or NF-IL-6 (lower panel) were measured by EMSA. The DNA-protein complexes are marked with brackets. TNF (40 ng/mL)-stimulated cells were used as positive controls for NF-κB and AP-1 activation, and LPS (5 μg/mL; lane 6) was used as a positive control for NF-IL-6 binding activity.
C5a-induced transcription factor activation in PBMC. For each sample, 5 × 106 freshly prepared PBMC were incubated with 200 nmol/L of human C5a at 37°C for different lengths of time (0 to 4 hours). The cells were then harvested and nuclear extracts were prepared as described in Materials and Methods. Nuclear extract (2.5 μg) from each time point was incubated with one of the three different probes: 32P-labeled, double-stranded oligonucleotides containing binding sites for κB, AP-1, and NF-IL-6. The binding mixture was subjected to 5% acrylamide gel electrophoresis. The binding activities for NF-κB (top panel), AP-1 (middle panel), or NF-IL-6 (lower panel) were measured by EMSA. The DNA-protein complexes are marked with brackets. TNF (40 ng/mL)-stimulated cells were used as positive controls for NF-κB and AP-1 activation, and LPS (5 μg/mL; lane 6) was used as a positive control for NF-IL-6 binding activity.
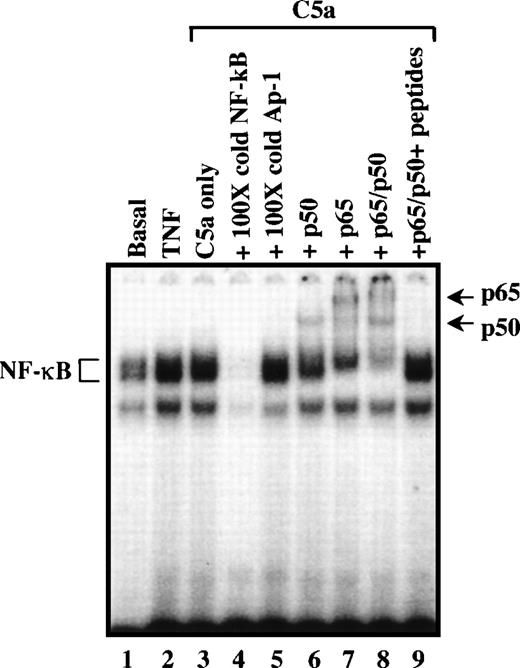
To test the specificity of C5a-induced κB binding activity, an excess amount of unlabeled κB oligonucleotide was used in gel mobility shift assays. Unlabeled κB oligonucleotide, at 100-fold molar excess, successfully competed with the labeled probe, whereas a 100-fold molar excess of the AP-1 oligonucleotide had no effect on κB binding activity (Fig 6, lanes 4 and 5). The two protein components of the prototypic NF-κB, p65 (RelA) and p50 (NFKB1), were found to be the constituents in supershift assays by inclusion of anti-p50 and anti-p65 polyclonal antibodies (Fig 6, lanes 6 through 8). Antibody-induced supershift was blocked by incubation with the p50 and p65 peptides, to which the antibodies were generated, indicating specificity of the antibodies for p50 and p65 (lane 9). These results demonstrated that C5a induces activation of NF-κB containing the p50 and p65 subunits.
Specificity of C5a-induced κB binding activity. Nuclear extracts prepared from unstimulated PBMC (basal; lane 1), PBMC stimulated with TNF (40 ng/mL for 2 hours; lane 2), and C5a (200 nmol/L for 2 hours; lanes 3 through 9) were used for analysis of κB binding activity by EMSA, in the absence (lane 3) or presence (lanes 4 and 5) of competitive unlabeled (cold) oligonucleotide probes. Lane 4 contains an oligonucleotide with the κB consensus sequence at 100-fold molar excess; lane 5 has an olinonucleotide with AP-1 consensus sequence at 100-fold molar excess. The κB DNA-protein complexes are marked with brackets. Lanes 6 through 9, nuclear extracts from cells stimulated with C5a were incubated in the presence of Ab (2 μg/sample) against NF-κB/Rel proteins: lane 6, anti-p50; lane 7, anti-p65; and lane 8, anti-p50 plus anti-p65. Lane 9 contains anti-p50 and anti-p65 plus the neutralizing peptides used for antibody production. Samples were analyzed by EMSA, using 6% acrylamide gel and32P-labeled double-stranded oligonucleotide containing the NF-κB binding site consensus sequence. The gel supershift bands induced by the anti-p50 and anti-p65 antibodies were indicated with arrows.
Specificity of C5a-induced κB binding activity. Nuclear extracts prepared from unstimulated PBMC (basal; lane 1), PBMC stimulated with TNF (40 ng/mL for 2 hours; lane 2), and C5a (200 nmol/L for 2 hours; lanes 3 through 9) were used for analysis of κB binding activity by EMSA, in the absence (lane 3) or presence (lanes 4 and 5) of competitive unlabeled (cold) oligonucleotide probes. Lane 4 contains an oligonucleotide with the κB consensus sequence at 100-fold molar excess; lane 5 has an olinonucleotide with AP-1 consensus sequence at 100-fold molar excess. The κB DNA-protein complexes are marked with brackets. Lanes 6 through 9, nuclear extracts from cells stimulated with C5a were incubated in the presence of Ab (2 μg/sample) against NF-κB/Rel proteins: lane 6, anti-p50; lane 7, anti-p65; and lane 8, anti-p50 plus anti-p65. Lane 9 contains anti-p50 and anti-p65 plus the neutralizing peptides used for antibody production. Samples were analyzed by EMSA, using 6% acrylamide gel and32P-labeled double-stranded oligonucleotide containing the NF-κB binding site consensus sequence. The gel supershift bands induced by the anti-p50 and anti-p65 antibodies were indicated with arrows.
C5a induction of NF-κB activation and IL-8 gene transcription in transfected cells.
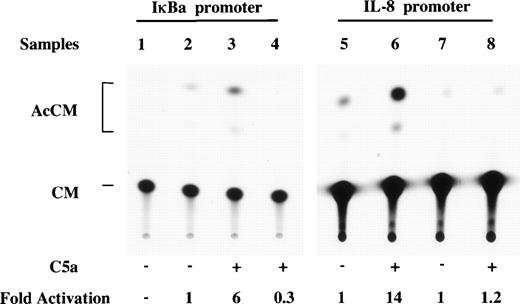
To establish a correlation between C5a-induced κB binding activity and IL-8 gene transcription, κB-CAT (chloramphenical acetyltransferase) reporter gene constructs were transiently transfected into the murine macrophage-like cell line RAW264.7, which expresses C5a receptor. The CAT activities were determined 48 hours after transfection. As shown in Fig 7, C5a induced a sixfold increase in CAT activity in the cells transfected with the wild-type IκBα promoter-CAT construct, p0.2kb(WT)CAT (lane 3 v lane 2), which contains one copy of a κB site (GGAAATTCCC).30 There was no increase in CAT activity in cells transfected with a mutant IκBα construct [p0.2kb(M)CAT; CGAAATTAAT; Fig 7, lane 4].
Correlation between C5a-induced κB binding activity and IL-8 gene transcription. The murine RAW264.7 macrophage cells were transfected with 8 μg plasmid DNA for either the IκB promoter-CAT reporters (lanes 1 through 4) or the p-272CAT reporters (lanes 5 through 8), together with 2 μg of the control plasmid pCMVβ DNA to monitor transfection efficiency. Relative CAT activities were determined in these cells after 2 hours of stimulation with C5a by normalizing the CAT activities to the β-galactosidase activities. The acetylated forms of CAT (AcCM) are marked with brackets. The net fold induction of relative CAT activities by C5a is shown at the bottom. Lane 1, no reporter plasmid was transfected. The p0.2kb(WT)CAT plasmid was used in the absence (lane 2) or presence (lane 3) of human C5a. Lane 4 was a sample with p0.2kb(M)CAT, which contains a mutated κB site in the IκB promoter. In lanes 5 through 8, CAT reporter gene plasmids driven by the IL-8 promoter were used in the absence (lanes 5 and 7) or the presence (lanes 6 and 8) of C5a. Lanes 5 and 6 contain the p-272CAT construct. A reporter with the κB binding sequence removed p-94(Δ 78-71) CAT was used in lanes 7 and 8. The thin-layer chromatography data shown here is representative of three CAT assays with similar results.
Correlation between C5a-induced κB binding activity and IL-8 gene transcription. The murine RAW264.7 macrophage cells were transfected with 8 μg plasmid DNA for either the IκB promoter-CAT reporters (lanes 1 through 4) or the p-272CAT reporters (lanes 5 through 8), together with 2 μg of the control plasmid pCMVβ DNA to monitor transfection efficiency. Relative CAT activities were determined in these cells after 2 hours of stimulation with C5a by normalizing the CAT activities to the β-galactosidase activities. The acetylated forms of CAT (AcCM) are marked with brackets. The net fold induction of relative CAT activities by C5a is shown at the bottom. Lane 1, no reporter plasmid was transfected. The p0.2kb(WT)CAT plasmid was used in the absence (lane 2) or presence (lane 3) of human C5a. Lane 4 was a sample with p0.2kb(M)CAT, which contains a mutated κB site in the IκB promoter. In lanes 5 through 8, CAT reporter gene plasmids driven by the IL-8 promoter were used in the absence (lanes 5 and 7) or the presence (lanes 6 and 8) of C5a. Lanes 5 and 6 contain the p-272CAT construct. A reporter with the κB binding sequence removed p-94(Δ 78-71) CAT was used in lanes 7 and 8. The thin-layer chromatography data shown here is representative of three CAT assays with similar results.
The IL-8 promoter-driven CAT constructs were next used to examine the direct effect of C5a on IL-8 gene expression. C5a induced a 14-fold increase in CAT activity in cells transfected with an IL-8 promoter reporter p-272CAT, which contains 272 bp upstream from the transcription initiation site (Fig 7, lane 6 v lane 5). The reporter construct harbors NF-κB, NF-IL-6, and AP-1 sites.28 To determine whether NF-κB plays a crucial function in C5a-induced IL-8 gene expression, another reporter construct, p-94(Δ78-71)CAT was used. In this construct, a critical part of the κB binding sequence (underlined in GTGGAATTTCCTC) was removed, resulting in a plasmid containing all other DNA binding sites except a functional κB site. Cells transfected with this construct did not show C5a inducible CAT activity (Fig 7, lane 8 v lane 7). Therefore, the κB site in the 5′ flanking region of IL-8 gene is indispensable for C5a-stimulated IL-8 gene transcription.
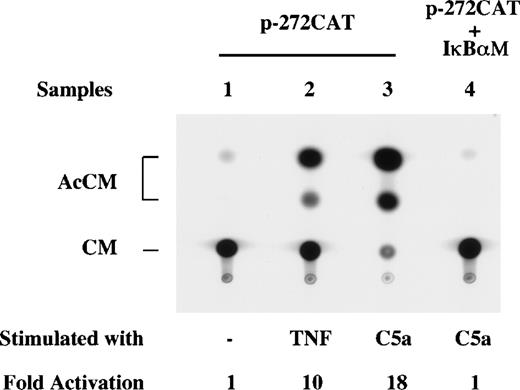
NF-κB activation results from phosphorylation and degradation of the IκB proteins.31-33 Prevention of IκBα phosphorylation leads to inhibition of NF-κB activation.34 To establish a function of C5a-induced NF-κB activation in IL-8 transcription, RAW264.7 cells were cotransfected with the p-272CAT reporter and a dominant negative form of IκBα (IκBαM), in which Ser32 and Ser36 were mutated to block agonist-induced IκBα phosphorylation and subsequent NF-κB activation.34 Coexpression of IκBαM completely blocked C5a-induced expression of the p-272CAT gene (Fig 8, lane 4), indicating involvement of IκBα phosphorylation and NF-κB activation in C5a-stimulated IL-8 gene expression. Additional evidence supporting a critical role of NF-κB activation in C5a-induced IL-8 gene transcription was obtained by using the NF-κB inhibitor PDTC, which effectively blocked C5a-stimulated CAT activity using the p-272CAT construct (Fig 9, lane 5). Similarly, the transcription inhibitor ActD and the translation inhibitor CHX also abolished C5a induction of CAT activity (Fig 9, lanes 3 and 4).
Inhibition of C5a-induced CAT activity by IκBM. RAW264.7 cells were cotransfected with the dominant negative IκBM plasmid (4 μg; lane 4) and the p-272CAT reporter plasmid, using transfection conditions described above and in Materials and Methods. After serum starvation, the cells were either unstimulated (lane 1) or stimulated with TNF (40 ng/mL, lane 1) or C5a (100 nmol/L, lanes 3 and 4) for 4 hours before the assay.
Inhibition of C5a-induced CAT activity by IκBM. RAW264.7 cells were cotransfected with the dominant negative IκBM plasmid (4 μg; lane 4) and the p-272CAT reporter plasmid, using transfection conditions described above and in Materials and Methods. After serum starvation, the cells were either unstimulated (lane 1) or stimulated with TNF (40 ng/mL, lane 1) or C5a (100 nmol/L, lanes 3 and 4) for 4 hours before the assay.
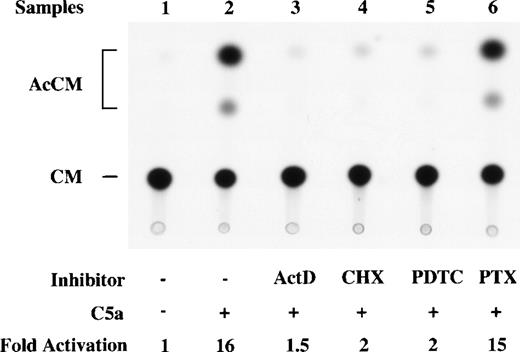
Effect of inhibitors and pertussis toxin on C5a-induced IL-8 promoter-directed CAT activities. RAW264.7 cells were transfected with an IL-8 promoter-CAT construct, p-272CAT, and stimulated with 200 nmol/L of C5a after 1 hour of treatment with the transcription inhibitor actinomycin D (ActD) or the translation inhibitor cycloheximide (CHX). PTX (1 μg/mL) was added 2 hours before C5a stimulation and was present during the course of C5a stimulation. The inhibitors and PTX and relative net fold induction of CAT activities by C5a are specified at the bottom of the figure.
Effect of inhibitors and pertussis toxin on C5a-induced IL-8 promoter-directed CAT activities. RAW264.7 cells were transfected with an IL-8 promoter-CAT construct, p-272CAT, and stimulated with 200 nmol/L of C5a after 1 hour of treatment with the transcription inhibitor actinomycin D (ActD) or the translation inhibitor cycloheximide (CHX). PTX (1 μg/mL) was added 2 hours before C5a stimulation and was present during the course of C5a stimulation. The inhibitors and PTX and relative net fold induction of CAT activities by C5a are specified at the bottom of the figure.
Involvement of pertussis toxin-sensitive and insensitive pathways in C5a stimulation of IL-8 gene expression.
The chemotactic function of C5a, like that of other chemoattractants, is known to be mediated through coupling of its 7-transmembrane domain receptor with the Gi class of G protein α-subunits that are sensitive to pertussis toxin (PTX) treatment.35 In addition to Gi proteins, C5a receptor can also couple to PTX-insensitive G protein α-subunits including Gα16 in certain types of cells.36-38 The involvement of Gi proteins in C5a-induced IL-8 gene expression was examined in cells treated with PTX, which ADP-ribosylates and inhibits the activities of the Gi and Go proteins. Results from both ELISA and Northern blotting indicated only a partial blockade (∼20%) of IL-8 secretion and IL-8 mRNA accumulation in the toxin-treated cells (Figs 3 and 4). In control experiments, the same pertussis toxin treatment was sufficient to block C5a-induced calcium mobilization and MAP kinase activation in neutrophils (data not shown). Consistent with the results from ELISA and Northern blot experiments shown above, PTX had a very small inhibitory effect on C5a-induced CAT activity (Fig 9, lane 6 compared with lane 2).
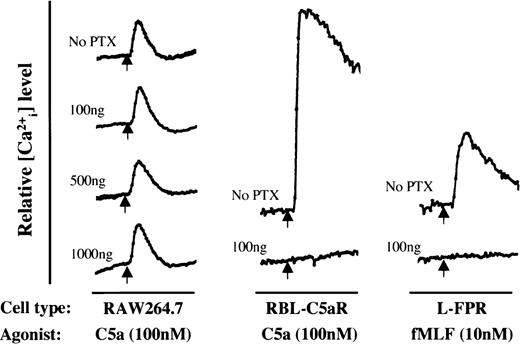
These findings suggest that C5a-induced IL-8 gene expression is mediated primarily through Gα proteins insensitive to PTX treatment. Alternatively, a pathway independent of G protein activation may be involved in activating NF-κB. To examine these possibilities, calcium mobilization assay was adopted, because C5a-induced increase of intracellular Ca2+ level is known to follow G protein and phospholipase C activation. Figure 10shows the results of experiments using three different types of cells. In RAW264.7 cells, 16 hours of PTX treatment at doses up to 1,000 ng/mL produced no inhibitory effect on C5a-induced Ca2+ flux. In the rat basophilic leukemia cell line RBL-2H3 stably transfected to overexpress the human C5a receptor, PTX completely blocked agonist-induced Ca2+ flux. Similar PTX-sensitive data were obtained using mouse L-cell fibroblasts expressing the human fMet-Leu-Phe receptor, FPR, which is known for its coupling to PTX-sensitive G proteins for Ca2+mobilization.39 These results support the notion that C5a receptor can couple to a G protein other than Gi for the activation of signaling pathways leading to IL-8 gene expression. C5a receptor may also selectively couple to either PTX-sensitive or PTX-insensitive G proteins depending on cell types.
Effects of pertussis toxin (PTX) on calcium mobilization in three different cells. The cells were treated for 16 hours with PTX at the concentrations indicated next to each tracing (in nanograms per milliliter). Agonist-induced calcium mobilization was then measured using indo-1 AM as a fluorescent calcium indicator. Relative intracellular calcium levels are shown. The arrows mark the time points when agonist was applied. Each horizontal bar represents approximately 50 seconds.
Effects of pertussis toxin (PTX) on calcium mobilization in three different cells. The cells were treated for 16 hours with PTX at the concentrations indicated next to each tracing (in nanograms per milliliter). Agonist-induced calcium mobilization was then measured using indo-1 AM as a fluorescent calcium indicator. Relative intracellular calcium levels are shown. The arrows mark the time points when agonist was applied. Each horizontal bar represents approximately 50 seconds.
DISCUSSION
The anaphylatoxin C5a has been previously shown to induce the synthesis and secretion of a number of proinflammatory cytokines including IL-1β and IL-8.16,18,22 However, how C5a regulates the biosynthesis of these cytokines remains unclear. Results from this work provide direct evidence for C5a activation of NF-κB in PBMC and in a transfected macrophage cell line. Thus, NF-κB activation may be an important mediator of C5a-induced transcriptional activation of these cytokines genes, which all contain κB-binding sequence in their promoter regions.29 This notion is supported by the finding that C5a stimulates IL-8 secretion, which peaks at 2 hours after agonist stimulation and follows NF-κB activation, which reaches maximum within 1 hour (Figs 2 and 5). Further attesting to the role of NF-κB in IL-8 gene expression, the antioxidant inhibitor of NF-κB, PDTC, markedly reduces C5a-induced IL-8 secretion and IL-8 message accumulation. The effective inhibition of NF-κB activation by a dominant negative IκBα mutant suggests a direct role of C5a stimulation of IκBα phosphorylation, which leads to NF-κB activation and IL-8 expression. Although C5a also stimulates AP-1 binding activity, the time course of AP-1 activation differs significantly from that of NF-κB and cannot account for the early phase (within 2 hours) upregulation of IL-8 at the protein and mRNA levels. The current work does not rule out the possibility that AP-1 activation contributes to IL-8 gene expression in the late phase, as was reported in several previous studies.40-42 The observed 14-fold increase in CAT activity with the p-272CAT construct compared with a sixfold increase using the 0.2-kb (WT)CAT construct supports a synergistic function of AP-1 in IL-8 gene expression with prolonged C5a stimulation, as was the condition used in CAT assays. Previously published studies demonstrate that NF-IL-6 can act synergistically with NF-κB to enhance IL-8 transcription in a cell type-dependent manner, but NF-IL6 alone is insufficient for IL-8 gene expression.40 However, in this study, C5a did not induce detectable NF-IL6 activation; therefore, the function of NF-IL-6 in IL-8 expression may be negligible. Our results suggest a critical role of NF-κB activation in C5a-stimulated IL-8 gene expression, because a reporter construct with a defective κB site did not respond to C5a stimulation despite the presence of the AP-1 and NF-IL-6 sites (Fig 7).
The actions of C5a are mediated by the G-protein–coupled C5a receptor expressed in granulocytes, monocytes, and several other types of cells. The C5a receptor is known to couple to Gi proteins that are sensitive to pertussis toxin-mediated ADP-ribosylation and G protein inactivation. In reported studies, PTX treatment abolishes C5a mediated functions such as chemotaxis, degranulation, and superoxide generation (reviewed in Gerard and Gerard5). A surprising finding from this study is the lack of complete inhibition by PTX of C5a-stimulated NF-κB activation and IL-8 secretion. Using calcium mobilization as an assay for G-protein–coupled response, we demonstrated that C5a receptor can couple to either PTX-sensitive or PTX-insensitive G proteins depending on cell types. The two likely candidates for PTX-insensitive G proteins are Gαq and Gα16, being both present in mononuclear cells and insensitive to PTX treatment. Gα16 has been shown in a previous report to couple to C5a receptor in transiently transfected COS-7 and 293 cells,37 38 making it a more likely candidate for the G protein that transduces C5a-induced signaling leading to transcription activation. This study provides an example of coupling of a chemoattractant receptor to PTX-insensitive G protein α-subunit(s) that effectively mediates a cellular response. Based on our data, we cannot rule out the possibility that C5a induces IL-8 gene expression without activating G proteins in the RAW264.7 cells. It will be important to further investigate the possible presence of a G-protein–independent pathway and to understand the structural basis for C5a receptor coupling to multiple Gα proteins that leads to different cellular responses.
Activation of NF-κB and other transcription factors for immediate-early genes provides an effective means for chemoattractant stimulated upregulation of proinflammatory cytokines. Results from the current study suggest that, in addition to a synergistic role of C5a in LPS-induced cytokine production,16 17 this peptide agonist can directly induce NF-κB activation that leads to biosynthesis of IL-8. A rapid induction of IL-8 biosynthesis after C5a stimulation supports a direct action of this chemoattractant on IL-8 gene expression. Because chemoattractants are molecules that leukocytes encounter at an early stage of inflammation, their effect on cytokine gene expression may influence the course of inflammation. By induction of IL-8 expression, a chemoattractant such as C5a and fMet-Leu-Phe can stimulate the generation of a potent chemotactic cytokine that, in turn, may attract additional phagocytes and lymphocytes to the site of inflammation. Recent studies conducted in our laboratory demonstrate that IL-8 produced by mononuclear cells may also regulate the secretion of other cytokines, including IL-8 itself. IL-8 binding to the IL-8 receptors can lead to transcription activation and an autocrine regulation of IL-8 biosynthesis (unpublished result). Additional efforts will be required to understand the mechanisms by which chemoattractants induce transcription activation, especially the signaling pathways leading from receptor stimulation to IκB kinase activation and nuclear translocation of the NF-κB proteins.
ACKNOWLEDGMENT
The authors thank Drs Dan Van Antwerp and Inder Verma for providing the IκBαM plasmid. The blood drawing service was provided by Scripps’ General Clinical Research Center, supported by National Institutes of Health Grant No. MO1RR00833. This is manuscript 11564-IMM from The Scripps Research Institute.
Supported by US Public Health Services Grant No. AI40176 and an Arthritis Foundation Biomedical Science Grant (to R.D.Y.). M.H.H. and D.D.B. are recipients of Arthritis Foundation Postdoctoral Fellowships. This work was performed during the tenure of an Established Investigatorship (to R.D.Y.) from the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Richard D. Ye, MD, PhD, Department of Pharmacology, MC868, University of Illinois at Chicago, 835 S Wolcott Ave, Chicago, IL 60612; e-mail: yer@uic.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal