Abstract
A novel gene, jumonji was identified by a mouse gene trap strategy. The jumonji gene encodes a protein containing a putative DNA binding domain. The mice homozygous for jumonjigene with a BALB/cA genetic background show hypoplasia of the fetal liver and embryonic lethality, suggesting impaired hematopoiesis. In the peripheral blood of jumonji mutant embryos, the number of fetal liver–derived definitive erythrocytes, but not yolk sac–derived primitive erythrocytes, showed a marked reduction, suggesting thatjumonji mutants die of anemia. The defects of definitive erythrocytes in jumonji mutants seemed to be caused by a decrease in the numbers of multiple hematopoietic progenitors including colony-forming unit-spleen (CFU-S) in the fetal liver. However, hematopoietic stem cells (HSCs) in the fetal liver of jumonjimutants could reconstitute the hematopoietic system of lethally irradiated recipients. In the fetal liver, the jumonji gene is expressed in fibroblastic cells and endothelial cells, but not in Lin−/c-Kit+/Sca-1+ cells known to include HSCs. These results suggest that an environmental defect induce the impaired hematopoiesis in the fetal liver ofjumonji mutant embryos.
THE JUMONJI (jmj) gene and jmj mutant mice were obtained by a mouse gene trap strategy.1 The mouse jmj gene maps at chromosome 13.2 The jmj gene encodes a protein that is partially homologous to the human retinoblastoma-binding protein RBP-2, and to a putative protein encoded by human gene XE169, which escapes X-chromosome inactivation.1 In addition, the Jmj protein contains nuclear localization signals, and a region homologous to the AT-rich interaction domain (ARID) identified in the DNA binding protein Dead Ringer in Drosophila, in transcription factor Bright in mouse, and in SWI1 in yeast,1,3-6 implying the possibility that Jmj protein is a transcription factor. The human jmj gene has also been identified and maps at 6p24-6p23.7Interestingly, the mouse jmj gene was independently isolated by a gene trap method.8
The jmj homozygous mutant (jmjtrap/trap) mice fail to express normal jmj mRNA.1 The phenotypes of jmjtrap/trap mice are dependent on the genetic background.9 Thejmjtrap/trap mice were originally established with a BALB/cA and 129/Ola mixed genetic background.1 With this mixed background, approximately half of thejmjtrap/trap embryos showed neural tube defects, and all jmjtrap/trap embryos progressively died between 10.5 days postcoitum (dpc) and 15.5 dpc.1 Thejmjtrap/trap embryos with a BALB/cA, C57BL/6J, or DBA/2J background, however, displayed no neural tube defects, and almost all jmjtrap/trap embryos died around 15.5 dpc with hemorrhage and edema.9 In addition, severe hypoplasia of the fetal liver, thymus, and spleen was observed injmjtrap/trap embryos, and in the fetal liver ofjmjtrap/trap embryos with a BALB/cA background, a decrease in total cell number, an increase in megakaryocytes, and excessive cell death have been reported.9 On the other hand, jmj heterozygous mutant (jmj+/trap) mice showed no apparent abnormalities.1,9 The expression ofjmj gene can be monitored by the β-galactosidase (β-gal) activities in jmj+/trapembryos.1,4,9 In the fetal liver, the jmj gene is expressed in megakaryocytes, unidentified hematopoietic cells, fibroblastic cells, and endothelial cells.9 Because hypoplasia of the fetal liver in jmjtrap/trapembryos suggests that hematopoiesis is defective, we further characterized the fetal liver of jmjtrap/trapembryos with a BALB/cA background. We found abnormalities in the colony-forming unit-spleen (CFU-S) progenitors in the fetal liver ofjmjtrap/trap embryos. Our studies suggest that thejmj gene plays a pivotal role in definitive hematopoiesis during mouse embryogenesis.
MATERIALS AND METHODS
Mice.
The jmjtrap/trap mice were originally generated with a 129/Ola and BALB/cA mixed genetic background.1 Thejmjtrap/trap embryos used in this study were generated by intercross of F16-17 jmj+/trap mice with a BALB/cA genetic background. The presence of a vaginal plug was regarded as 0.5 dpc. All embryos were stained with X-gal (Calbiochem-Novabiochem Co, La Jolla, CA), which indicates genotype, and the genotype was confirmed by polymerase chain reaction (PCR) using genomic DNA isolated from the yolk sacs.1 All embryos that stained strongly with X-gal were homozygotes. All animals were cared for by supervised trained technicians in our animal center.
Cytochemical analysis.
To determine the numbers of definitive and primitive erythrocytes in the peripheral blood, peripheral blood was collected directly from the umbilical cords of 14.5 dpc and 15.5 dpc embryos through a fine glass needle. The total number of recovered cells per volume of the peripheral blood was scored. The ratio of definitive to primitive erythrocytes was determined by staining peripheral blood smears with May-Grünwald-Giemsa stain (Merck, Darmstadt, Germany).
The numbers of myeloperoxidase positive (MPO+) cells and nonspecific esterase positive (NSE+) cells were determined in single cell preparations from 12.5 dpc fetal livers obtained by passing a fetal liver sample through into the 26-gauge needle. The cells were counted, cytocentrifuged, fixed, and stained with diaminobenzidine (Wako Pure Chemical Co, Osaka, Japan) for MPO+ or α-naphtylbutyrate (Muto Pure Chemicals Co, Tokyo, Japan) for NSE+ cells.10 More than 1,000 cells were counted in each individual sample. The number of positive cells per fetal liver was estimated from the percentage of positive cells in the samples.
Histochemical analysis.
For immunohistochemical analysis, the embryos were fixed overnight in 4% paraformaldehyde (Sigma Chemical Co, St Louis, MO) in Dulbecco’s phosphate-buffered saline (PBS, Nissui Pharmaceutical Co, Tokyo, Japan) at 4°C. The fixative was then replaced in 30% sucrose at 4°C, embedded in optical cutting temperature (OCT) compound (Miles Inc, Elkhart, IN) and stored at −80°C. The frozen specimens were cut into 7-μm sections in cryostat, collected on poly-L-lysine–coated slides, and immediately dried. The sections were stained overnight with biotinylated rat anti–TER-119 antibody (PharMingen Inc, San Diego, CA) or rabbit anti-albumin antibody (Inter-Cell Technologies Inc, Hopewell, NJ) at 4°C. The sections were examined with a Vectastatin ABC Elite kit (Vector Laboratories Inc, Burlingame, CA). The number of TER-119 positive (TER-119+) or albumin positive (albumin+) cells per field (magnification × 500) was counted under a microscope.
Flow cytometrical analysis.
Single cell suspensions from 12.5 dpc to 14.5 dpc fetal livers were prepared as described above. The following monoclonal antibodies were used: anti-B220/CD45R, anti–c-Kit (ACK2), anti-CD4, anti-CD8, anti–Gr-1, anti–Thy-1.2, biotinylated anti–c-Kit, biotinylated anti–TER-119, fluorescein isothiocyanate (FITC)–anti-CD45, and FITC-anti–Sca-1 antibodies. ACK2 anti–c-Kit antibody was a kind gift from Dr S.-I. Nishikawa (Kyoto University, Kyoto, Japan). FITC–anti-CD45 antibody was purchased from Bethesda Research Laboratories (Gaithersburg, MD). Other antibodies were purchased from PharMingen Inc. Biotinylated anti–TER-119, ACK2 anti–c-Kit, and biotinylated anti–c-Kit antibodies were visualized by FITC-streptavidin (Vector Laboratories Inc), biotinylated antirat IgG antibody (Vector Laboratories, Inc) followed by FITC-streptavidin and allophycocyanin (APC)-streptavidin (PharMingen Inc), respectively. The percentage of TER-119+, CD45 positive (CD45+), or c-Kit positive (c-Kit+) in 104 fetal liver cells was determined by FACScan (Becton Dickinson, Mountain View, CA). The number of positive cells per fetal liver was estimated. The cells negative for lineage markers (anti-B220, anti-CD4, anti-CD8, anti–Gr-1, and anti–TER-119 antibodies) in the fetal liver of jmj+/trap embryos at 14.5 dpc were isolated by using Dynabeads M-450 anti-rat IgG antibody (Dynal, Inc, Lake Success, NY) and a Dynal MPC-1 magnet (Dynal, Inc). The lineage negative (Lin−) cells were stained with biotinylated anti–c-Kit and FITC–anti–Sca-1 antibodies. The Lin−, c-Kit+, and Sca-1 positive (Lin−/c-Kit+/Sca-1+) cells were isolated by FACSvantage (Becton Dickinson) and stained with X-gal.
In vitro colony-forming assay.
Single cell suspensions from the yolk sacs at 10.5 dpc were obtained by treatment with 0.1% collagenase (Sigma Chemical Co).11Single cell suspensions from fetal livers were obtained as described above. Cell numbers were determined from trypan blue dye exclusion, and the cells were suspended in α-minimum essential medium (MEM) (GIBCO Life Technologies Inc, Grand Island, NY) containing 30% fetal bovine serum (FBS) (JRH BioSciences, Lenexa, KS), 1.2% methylcellulose, 1% deionized bovine serum albumin (BSA), 0.1 mmol/L 2-mercaptoethanol (2-ME), and penicillin-streptomycin. The methylcellulose, BSA, 2-ME, and penicillin-streptomycin were purchased from Sigma Chemical Co. Cytokines were added as follows: erythropoietin (Epo, 2 U/mL; Boehringer Mannheim, Mannheim, Germany) for mature erythroid progenitors (colony-forming units-erythroid, [CFU-E]), interleukin-3 (IL-3, 10 ng/mL, Biosource International, Calmario, CA), stem cell factor (SCF, 50 ng/mL, Biosource International), and Epo for immature erythroid progenitors (burst-forming units-erythroid, [BFU-E]), IL-3, SCF, granulocyte-macrophage-colony stimulating factor (GM-CSF, 10 ng/mL; GIBCO Life Technologies, Inc) for myeloid progenitors (colony-forming units-granulocyte-macrophage [CFU-GM]). The cells were cultured in 24-well dishes (Corning Costar Co, Cambridge, MA), and the numbers of colonies were determined by phase contrast microscopy after 2 days for CFU-E or 7 days for BFU-E and CFU-GM. The numbers of colonies per fetal liver were estimated.
Spleen colony-forming assay.
The spleen colony-forming assay was performed as reported previously.12 Briefly, 5 × 106 fetal liver cells were suspended in 0.5 mL PBS and injected intravenously into lethally (950 rad) irradiated BALB/cA mice. After 8 days, the spleens were dissected out, fixed in Bouin’s solution (Sigma Chemical Co), and the number of CFU-S per fetal liver was estimated from the number of macroscopic colonies.
Reconstitution analysis.
The fetal liver cells obtained from 14.5 dpcjmj+/trap or jmjtrap/trapembryos were injected intravenously into lethally (950 rad) irradiated recipient mice (BALB/cA) with 2 × 105 bone marrow cells of wild-type mice at 6 weeks of age for immediate short-term radiation protection. Control irradiated mice, which were not transplanted, died between 10 and 14 days. After 1 month, the bone marrow, thymus, and spleen of recipient mice were recovered. Biotinylated antibodies, anti-B220, Gr-1, TER-119, and Thy-1.2 (all purchased from PharMingen Inc) were used for isolation of B lymphoid, myeloid, erythroid, and T-lymphoid cells, respectively. B220 positive (B220+) cells from spleen, Gr-1 positive (Gr-1+), or TER-119+ cells from bone marrow, and Thy-1.2 positive (Thy-1+) cells from thymus were sorted by using Dynabeads M-280 streptavidin (Dynal Inc) and a Dynal MPC-E-1 magnet (Dynal Inc). The genomic DNA was recovered from 2 × 106 cells, and the presence of donor-derived hematopoietic cells was determined by 30 cycles of PCR specific forjmjtrap allele.1
RESULTS
The number of definitive erythrocytes is remarkably reduced in the peripheral blood of jumonji mutant embryos.
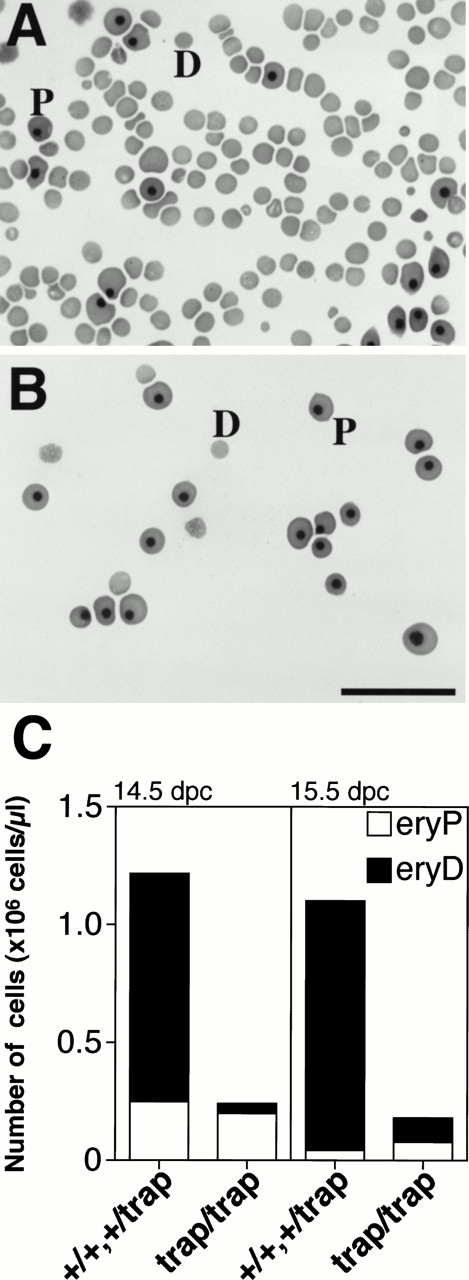
We previously reported hypoplasia of the fetal liver, thymus, spleen, and embryonic lethality around 15.5 dpc injmjtrap/trap embryos with a BALB/cA genetic background,9 suggesting that hematopoiesis is impaired injmjtrap/trap embryos. To clarify the defect in hematopoiesis in jmjtrap/trap embryos, we first examined the number of erythrocytes in the peripheral blood (Fig 1). In the peripheral blood from control littermates, the majority of cells, approximately 80%, is enucleated erythrocytes derived from definitive hematopoiesis in the fetal liver; in addition, a small number of nucleated, yolk sac–derived, primitive erythrocytes is observed at 14.5 dpc (Fig 1A). In contrast, only a few, approximately 17%, enucleated definitive erythrocytes were seen in the peripheral blood ofjmjtrap/trap embryos at 14.5 dpc (Fig 1B). Injmjtrap/trap embryos at 14.5 dpc and 15.5 dpc, the numbers of circulated definitive erythrocytes were 5% to 10% the level in control (Fig 1C). On the other hand, the number of primitive erythrocytes showed no significant decrease compared with controls (Fig1C). The numbers of total erythrocytes in the peripheral blood ofjmjtrap/trap embryos at 14.5 dpc and 15.5 dpc were 15% to 20% of controls (Fig 1C). These data strongly suggest that definitive, but not primitive, erythropoiesis is severely impaired injmjtrap/trap embryos, and embryonic lethality ofjmjtrap/trap embryos seems to be caused by anemia.
The marked reduction in the number of definitive erythrocytes in the peripheral blood ofjmjtrap/trap embryos. Representative photographs of erythroid cells from the peripheral blood of control (jmj+/+) embryos (A) andjmjtrap/trap embryos (B) at 14.5 dpc are shown. D and P indicate a definitive erythrocyte and a primitive erythrocyte, respectively. Scale bar, 50 μm. (C) The numbers of erythrocytes in the peripheral blood of 14.5 dpc and 15.5 dpc embryos (genotypes shown below). eryD and eryP indicate definitive erythrocytes and primitive erythrocytes, respectively.
The marked reduction in the number of definitive erythrocytes in the peripheral blood ofjmjtrap/trap embryos. Representative photographs of erythroid cells from the peripheral blood of control (jmj+/+) embryos (A) andjmjtrap/trap embryos (B) at 14.5 dpc are shown. D and P indicate a definitive erythrocyte and a primitive erythrocyte, respectively. Scale bar, 50 μm. (C) The numbers of erythrocytes in the peripheral blood of 14.5 dpc and 15.5 dpc embryos (genotypes shown below). eryD and eryP indicate definitive erythrocytes and primitive erythrocytes, respectively.
The erythroid cells are impaired in the fetal liver of jumonjimutant embryos.
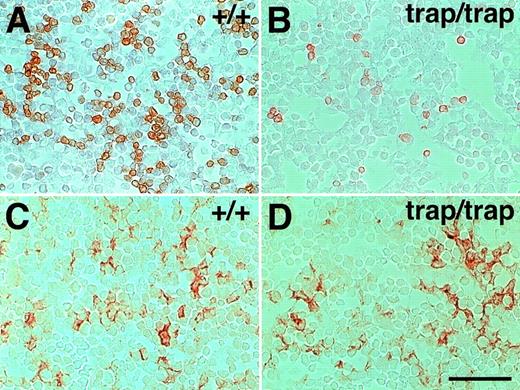
Definitive erythropoiesis in the fetal liver ofjmjtrap/trap embryos is suggested to be impaired as shown above, and hypoplasia of the fetal liver occurs injmjtrap/trap embryos.9 To know whether the erythropoiesis is impaired in the fetal liver ofjmjtrap/trap embryos, we stained the sections from the fetal livers with anti–TER-119 antibody, a specific marker of late erythroid cells.13 In control littermates, numerous TER-119+ cells were observed in the fetal livers at 12.5 dpc (Fig 2A). However, in the fetal liver of jmjtrap/trap embryos at 12.5 dpc, the number of TER-119+ cells was markedly reduced (Fig2B). The number of TER-119+ cells in the fetal liver was counted per field and that of jmjtrap/trap embryos was 19.6% of controls. We next compared the numbers of nonhematopoietic cells, hepatocytes, in the fetal liver ofjmjtrap/trap embryos and those of controls using an anti-albumin antibody, a marker of hepatocytes. It was important to determine whether the abnormality in the fetal liver ofjmjtrap/trap embryos occurs only in erythroid cells or not. In the fetal liver ofjmjtrap/trap embryos, the number of albumin+ cells was roughly comparable to control embryos (Fig 2C and D). The number of albumin+ cells in the fetal liver of jmjtrap/trap embryos per field was 84.8% of controls. These data suggest that the number of erythroid cells, but not hepatocytes, is preferentially reduced in the fetal liver ofjmjtrap/trap embryos.
A few late erythroid cells, but not hepatocytes, in the fetal liver of jmjtrap/trap embryos at 12.5 dpc. Representative photographs of transverse sections from the fetal liver of 12.5 dpc jmj+/+ embryos (A and C) andjmjtrap/trap embryos (B and D) are shown. The sections were stained with TER-119 antibody (A and B), or albumin antibody (C and D). The positive cells stain brown. Scale bar, 50 μm.
A few late erythroid cells, but not hepatocytes, in the fetal liver of jmjtrap/trap embryos at 12.5 dpc. Representative photographs of transverse sections from the fetal liver of 12.5 dpc jmj+/+ embryos (A and C) andjmjtrap/trap embryos (B and D) are shown. The sections were stained with TER-119 antibody (A and B), or albumin antibody (C and D). The positive cells stain brown. Scale bar, 50 μm.
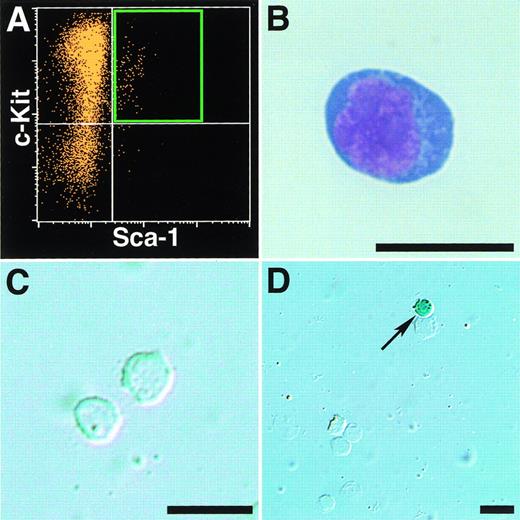
No expression of jmj gene in Lin−/c-Kit+/Sca-1+ cells in the fetal liver. The fetal liver cells ofjmj+/trap embryos at 14.5 dpc were used for sorting of Lin−/c-Kit+/Sca-1+cells. The sorting gate was shown (A). The Lin−/c-Kit+/Sca-1+ cells were stained with May-Grünwald-Giemsa (B) and X-gal (C). The X-gal staining of unfractionated fetal liver cells (D). The arrow indicates a β-gal+ cell. Scale bar, 20 μm.
No expression of jmj gene in Lin−/c-Kit+/Sca-1+ cells in the fetal liver. The fetal liver cells ofjmj+/trap embryos at 14.5 dpc were used for sorting of Lin−/c-Kit+/Sca-1+cells. The sorting gate was shown (A). The Lin−/c-Kit+/Sca-1+ cells were stained with May-Grünwald-Giemsa (B) and X-gal (C). The X-gal staining of unfractionated fetal liver cells (D). The arrow indicates a β-gal+ cell. Scale bar, 20 μm.
The numbers of differentiated erythroid, myeloid, and immature hematopoietic cells are less in the fetal liver of jumonjimutant embryos.
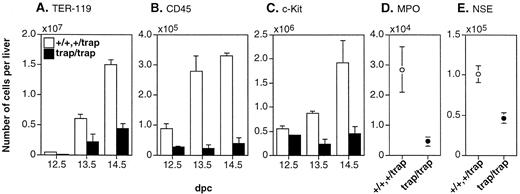
To know which lineages of hematopoietic cells are impaired in the fetal liver of jmjtrap/trap embryos, we investigated the number of cells representative of hematopoietic lineage, TER-119+ late erythroid cells, CD45+leukocytes,14 and c-Kit+ immature hematopoietic cells.15 16 The numbers of TER-119+, CD45+, or c-Kit+ cells in the fetal liver ofjmjtrap/trap embryos were lower than in control embryos at 12.5 dpc to 14.5 dpc (Fig 3A-C). The numbers of TER-119+ cells per 104 fetal liver cells in the jmjtrap/trap embryos were lower than in controls at 12.5 dpc, but comparable to those in controls at 13.5 dpc and 14.5 dpc.
The lower numbers of representative hematopoietic cells in the fetal livers of jmjtrap/trap embryos. The numbers of TER-119+ cells (A), CD45+ cells (B), and c-Kit+ cells (C) per fetal liver from 12.5 dpc to 14.5 dpc were estimated by flow cytometry. The numbers of MPO+ cells (D) and NSE+ cells (E) per fetal liver at 12.5 dpc were estimated by cytochemical analyses. (A through E) The open columns and circles indicate the average numbers of positive cells from jmj+/+ andjmj+/trap embryos, and the closed columns and circles indicate the average numbers fromjmjtrap/trap embryos. In all cases, more than three embryos were analyzed. The error bars indicate SEM.
The lower numbers of representative hematopoietic cells in the fetal livers of jmjtrap/trap embryos. The numbers of TER-119+ cells (A), CD45+ cells (B), and c-Kit+ cells (C) per fetal liver from 12.5 dpc to 14.5 dpc were estimated by flow cytometry. The numbers of MPO+ cells (D) and NSE+ cells (E) per fetal liver at 12.5 dpc were estimated by cytochemical analyses. (A through E) The open columns and circles indicate the average numbers of positive cells from jmj+/+ andjmj+/trap embryos, and the closed columns and circles indicate the average numbers fromjmjtrap/trap embryos. In all cases, more than three embryos were analyzed. The error bars indicate SEM.
CD45+ leukocytes include myeloid and lymphoid cells. The differentiated lymphoid cells represented as B220+ B lymphocytes or Thy-1 strongly positive T lymphocytes are undetectable in the fetal liver before 15.5 dpc.13 Therefore, we could not examine the number of lymphocytes. To assess the number of myeloid cells, we analyzed the number of MPO+ or NSE+cells in the fetal liver of jmjtrap/trap embryos at 12.5 dpc by cytochemical analysis. The activities of MPO and NSE have been reported to be restricted in myeloid lineage cells.17The number of MPO+ or NSE+ cells in the fetal liver of jmjtrap/trap embryos at 12.5 dpc was 16% or 46% of controls, respectively (Fig 3D and E). In megakaryocytic lineage cells in the fetal liver of jmjtrap/trapembryos, we previously showed an increased percentage of megakaryocytes.9 Taken together, the numbers of differentiated erythroid and myeloid cells, but not megakaryocytes, were lower in the fetal liver of jmjtrap/trapembryos.
The numbers of erythroid and myeloid progenitors of definitive hematopoiesis are low in the fetal liver of jumonji mutant embryos.
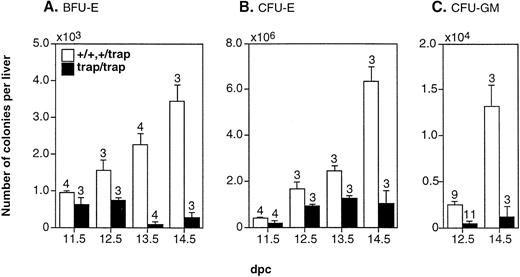
We next investigated the number of erythroid and myeloid-committed progenitors in the fetal liver of jmjtrap/trapembryos using an in vitro colony-forming assay to know why the numbers of differentiated erythroid and myeloid cells were less. The numbers of BFU-E, CFU-E, and CFU-GM colonies from the fetal liver ofjmjtrap/trap embryos were fewer compared with controls at all stages examined (Fig 4). Moreover, the numbers of BFU-E in the fetal livers, which gradually increase in control mice, showed no increase injmjtrap/trap embryos between 12.5 dpc and 14.5 dpc (Fig 4A). The number of CFU-GM in the fetal livers between 12.5 dpc and 14.5 dpc also increased significantly in controls, but not injmjtrap/trap embryos (Fig 4C). However, the gross morphologies of the erythroid and myeloid colonies fromjmjtrap/trap embryos were indistinguishable from those of controls (data not shown). These results strongly suggest that the lower numbers of differentiated erythroid and myeloid cells in the fetal livers of jmjtrap/trap embryos are caused by the reduction in the numbers of erythroid and myeloid progenitors.
The reduction in the numbers of hematopoietic progenitors in the fetal livers of jmjtrap/trap embryos. The numbers per fetal liver of BFU-E (A) and CFU-E colonies (B) in 11.5 dpc to 14.5 dpc embryos and CFU-GM colonies (C) in 12.5 dpc and 14.5 dpc embryos were determined. The open and closed columns indicate the average numbers of colonies from jmj+/+ andjmj+/trap embryos and fromjmjtrap/trap embryos, respectively. The numbers of fetal livers analyzed are shown above the columns and the error bars indicate SEM.
The reduction in the numbers of hematopoietic progenitors in the fetal livers of jmjtrap/trap embryos. The numbers per fetal liver of BFU-E (A) and CFU-E colonies (B) in 11.5 dpc to 14.5 dpc embryos and CFU-GM colonies (C) in 12.5 dpc and 14.5 dpc embryos were determined. The open and closed columns indicate the average numbers of colonies from jmj+/+ andjmj+/trap embryos and fromjmjtrap/trap embryos, respectively. The numbers of fetal livers analyzed are shown above the columns and the error bars indicate SEM.
Erythroid and myeloid progenitors of definitive hematopoiesis in the yolk sac are unaffected in jumonji mutant embryos.
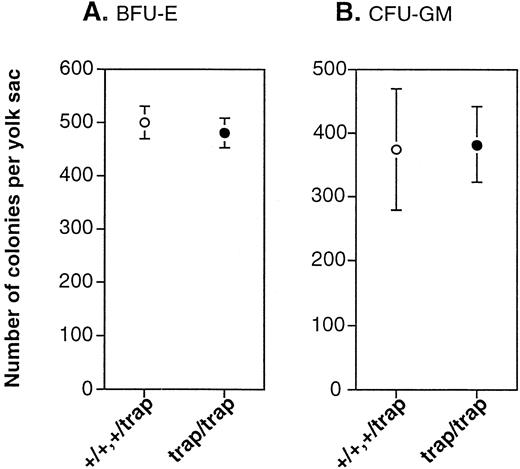
To understand why the numbers of BFU-E and CFU-GM progenitors in the fetal liver of jmjtrap/trap embryos were less, we first examined the numbers of BFU-E and CFU-GM in the yolk sacs at 10.5 dpc. Erythroid and myeloid progenitors of definitive hematopoiesis in the fetal liver of normal embryos seem to be derived from the colonization from the yolk sac18 and from the differentiation from hematopoietic stem cells (HSCs) in the fetal liver. The numbers of BFU-E and CFU-GM colonies from the yolk sac of jmjtrap/trap embryos were equivalent to those from controls at 10.5 dpc (Fig 5), suggesting the possibility that the numbers of erythroid and myeloid progenitors of definitive hematopoiesis injmjtrap/trap embryos are the same as in control embryos before colonization to the fetal liver.
Hematopoietic progenitors of definitive hematopoiesis in 10.5 dpc yolk sacs are unaffected in jmjtrap/trapembryos. (A) The number of BFU-E colonies per yolk sac.jmj+/+ and jmj+/trapembryos (n = 6) and jmjtrap/trapembryos (n = 6) were analyzed. (B) The number of CFU-GM colonies per yolk sac. jmj+/+ andjmj+/trap embryos (n = 4) andjmjtrap/trap embryos (n = 4) were analyzed. The open and closed circles represent the average numbers of colonies fromjmj+/+ and jmj+/trapembryos and from jmjtrap/trap embryos, respectively. The error bars indicate SEM.
Hematopoietic progenitors of definitive hematopoiesis in 10.5 dpc yolk sacs are unaffected in jmjtrap/trapembryos. (A) The number of BFU-E colonies per yolk sac.jmj+/+ and jmj+/trapembryos (n = 6) and jmjtrap/trapembryos (n = 6) were analyzed. (B) The number of CFU-GM colonies per yolk sac. jmj+/+ andjmj+/trap embryos (n = 4) andjmjtrap/trap embryos (n = 4) were analyzed. The open and closed circles represent the average numbers of colonies fromjmj+/+ and jmj+/trapembryos and from jmjtrap/trap embryos, respectively. The error bars indicate SEM.
The number of CFU-S progenitors is significantly reduced in the fetal liver of jumonji mutant embryos.
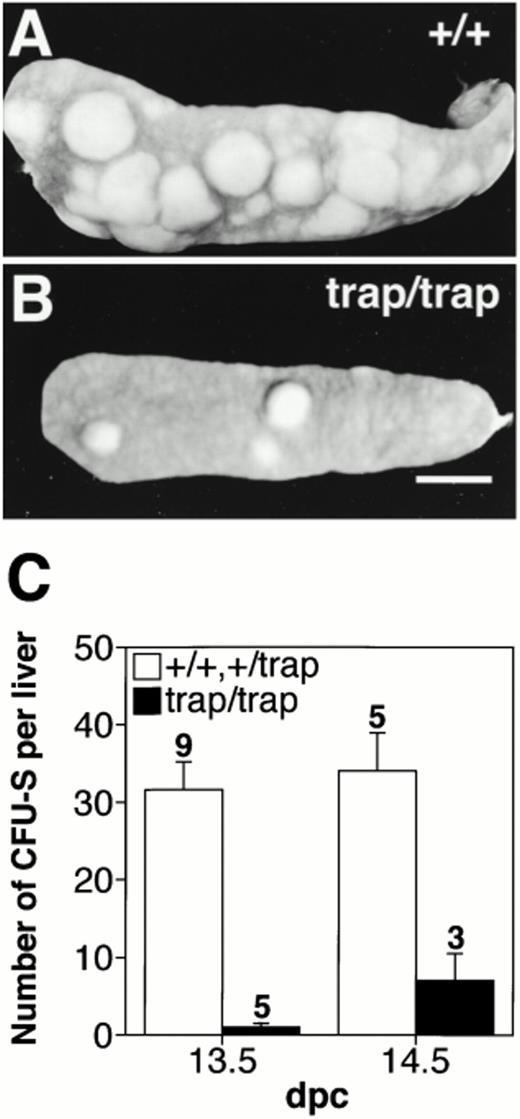
We next examined the number of CFU-S, erythroid and myeloid common progenitors to know why the numbers of erythroid and myeloid progenitors in the fetal liver of jmjtrap/trapembryos were less and failed to increase. When 5 × 106 cells from 13.5 dpc fetal livers were used for single injection, numerous colonies were observed from controls, but only a few colonies from jmjtrap/trap embryos (Fig 6A and B). As shown in Fig 6C, the average number ± standard error of mean (SEM) of CFU-S per fetal liver from control and jmjtrap/trap embryos at 13.5 dpc was 31.6 ± 3.6 (n = 9) and 1.0 ± 0.5 (n = 5), respectively (P < .001). In addition, the average number of CFU-S per 106 fetal liver cells of control andjmjtrap/trap embryos at 13.5 dpc was 5.0 and 0.4, respectively. These results strongly suggest that the decrease in the numbers of erythroid and myeloid progenitors in the fetal liver ofjmjtrap/trap embryos is caused by the lower number of CFU-S progenitors.
The significant reduction in the number of CFU-S progenitors in the fetal livers of jmjtrap/trapembryos. Representative photographs of CFU-S colonies from 5 × 106 cells from 13.5 dpc fetal livers ofjmj+/+ (A) and jmjtrap/trapembryos (B). Scale bar, 2 mm. (C) Number of CFU-S colonies per fetal liver. The open and closed columns indicate the average numbers of colonies from jmj+/+ andjmj+/trap embryos and fromjmjtrap/trap embryos, respectively. The number of experiments is shown above each column and the error bars indicate SEM.
The significant reduction in the number of CFU-S progenitors in the fetal livers of jmjtrap/trapembryos. Representative photographs of CFU-S colonies from 5 × 106 cells from 13.5 dpc fetal livers ofjmj+/+ (A) and jmjtrap/trapembryos (B). Scale bar, 2 mm. (C) Number of CFU-S colonies per fetal liver. The open and closed columns indicate the average numbers of colonies from jmj+/+ andjmj+/trap embryos and fromjmjtrap/trap embryos, respectively. The number of experiments is shown above each column and the error bars indicate SEM.
The HSCs in the fetal liver of jumonji mutant embryos can reconstitute the hematopoietic system of lethally irradiated recipient mice.
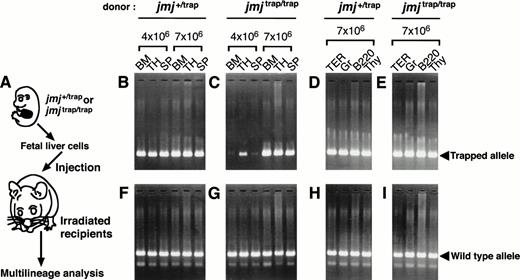
To know whether the hematopoietic defect ofjmjtrap/trap embryos is caused cell-autonomously or not, we examined reconstitution analysis using fetal liver cells. The strategy is shown in Fig 7A. Lethally irradiated recipients were transplanted with 4 × 106or 7 × 106 cells from 14.5 dpc fetal livers ofjmj+/trap embryos (0.4 or 0.7 embryo equivalent, respectively) or those of jmjtrap/trap embryos (1.2 or 2.1 embryo equivalent). After 1 month, fetal liver cells fromjmjtrap/trap embryos reconstituted hematopoietic cells in the bone marrow, thymus, and spleen of the recipients, as well as those from jmj+/trap embryos, when 7 × 106 cells were injected (Fig 7B and C). Moreover, donor-derived hematopoietic cells were detected in T lymphoid (Thy-1+ cells in thymus), B lymphoid (B220+cells in spleen), erythroid (TER-119+ cells in bone marrow), and myeloid (Gr-1+ cells in bone marrow) lineage cells of recipients transplanted with the fetal liver cells fromjmj+/trap or jmjtrap/trapembryos (Fig 7D and E). PCR products specific forjmjtrap allele were not detected in wild-type mice (data not shown). However, PCR products specific forjmjtrap/trap allele from the recipients transplanted with 4 × 106 fetal liver cells injmjtrap/trap embryos showed weak signals compared with those in jmj+/trap embryos (Fig 7B and C). On the other hand, PCR products of wild-type jmj allele in recipients transplanted with fetal liver cells ofjmjtrap/trap embryos was equivalent to those in controls (Fig 7F through I). Although these PCR analyses were semiquantitative, these results suggest that the number of HSCs may be reduced in the fetal liver of jmjtrap/trap embryos, but HSCs in the fetal liver of jmjtrap/trap embryos have a potential for differentiation into representative hematopoietic cell lineages.
Multilineage reconstitution of recipient mice transplanted with the fetal liver cells ofjmjtrap/trap embryos. Strategy of reconstitution analysis (A). Lethally irradiated recipient mice were injected with the fetal liver cells of jmjtrap/trap andjmj+/trap and after 1 month, the presence of donor-derived cells was determined by PCR. The representative results of genotype determined by PCR analysis specific forjmjtrap allele (B-E) and wild-type jmjallele (F through I). The recipients transplanted with the fetal liver cells of jmj+/trap embryos (B, D, F, and H) andjmjtrap/trap embryos (C, E, G, and I) were examined. BM, SP, and TH indicate bone marrow, spleen, and thymus, respectively. TER, Gr, B220, and Thy indicate TER+, Gr-1+, B220+, and Thy-1+cells, respectively. TER+ and Gr-1+ cells were isolated from the bone marrow. B220+ and Thy-1+ cells were from spleen and thymus, respectively.
Multilineage reconstitution of recipient mice transplanted with the fetal liver cells ofjmjtrap/trap embryos. Strategy of reconstitution analysis (A). Lethally irradiated recipient mice were injected with the fetal liver cells of jmjtrap/trap andjmj+/trap and after 1 month, the presence of donor-derived cells was determined by PCR. The representative results of genotype determined by PCR analysis specific forjmjtrap allele (B-E) and wild-type jmjallele (F through I). The recipients transplanted with the fetal liver cells of jmj+/trap embryos (B, D, F, and H) andjmjtrap/trap embryos (C, E, G, and I) were examined. BM, SP, and TH indicate bone marrow, spleen, and thymus, respectively. TER, Gr, B220, and Thy indicate TER+, Gr-1+, B220+, and Thy-1+cells, respectively. TER+ and Gr-1+ cells were isolated from the bone marrow. B220+ and Thy-1+ cells were from spleen and thymus, respectively.
No expression of jumonji gene in Lin−/c-Kit+/Sca-1+ cells in the fetal liver.
It is important to know whether the jmj gene is expressed in HSCs or not. In the fetal liver, we previously showed that thejmj gene is expressed in megakaryocytes, small unidentified cells, fibroblastic cells, and endothelial cells.9 In addition, the expression of jmj gene was also detected in a few CD34+ cells and a subset of GATA-1+ cells, but not in any TER-119+ cells and albumin+hepatocytes in the fetal liver (Kitajima et al, in preparation). The HSC activity is reported to be found in Lin−/c-Kit+/Sca-1+ cells in the fetal liver.19Lin−/c-Kit+/Sca-1+ cells in the fetal liver of jmj+/trap embryos at 14.5 dpc were then sorted (Fig 8A [see page 90]), and the expression ofjmj gene in the Lin−/c-Kit+/Sca-1+ cells was examined by X-gal staining, because the β-gal activities mimic faithfully the expression of jmj gene injmj+/trap embryos.1Lin−/c-Kit+/Sca-1+ cells (total of 197 cells) were examined under a microscope. In the Lin−/c-Kit+/Sca-1+ cells sorted, all observed cells were blastic cells (Fig 8B). However, in Lin−/c-Kit+/Sca-1+ cells ofjmj+/trap embryos, no β-gal activity was detected (Fig 8C). On the other hand, in unfractionated cells in the fetal liver, small hematopoietic cells were stained with X-gal (Fig 8D), as previously described.9 Thus, the jmj gene is not expressed in the hematopoietic stem cells in the fetal liver.
DISCUSSION
The reduction in the number of definitive erythrocytes in the peripheral blood of jumonji mutant embryos.
Fetal liver–derived erythrocytes begin to circulate in the peripheral blood at about 12.5 dpc and completely replace the primitive erythrocytes by 16.5 dpc. In the peripheral blood ofjmjtrap/trap embryos, the numbers of definitive, but not primitive, erythrocytes were 90% to 95% lower than in controls at 14.5 dpc and 15.5 dpc (Fig 1C). This result strongly suggests that definitive, but not primitive, hematopoiesis is severely impaired in jmjtrap/trap embryos.
In the peripheral blood of jmjtrap/trap embryos, the numbers of total erythrocytes were 15% to 20% of those in controls (Fig 1C). This result strongly suggests that embryonic lethality of jmjtrap/trap embryos is caused by anemia. The reduction in the numbers of circulated definitive erythrocytes is also reported in proto-oncogene c-myb or homeobox gene Hlx null mice.20,21 In c-mybnull embryos, hematocrit of peripheral blood was approximately 15% of controls between 14.5 dpc and 15.5 dpc.20 In Hlxnull embryos, the number of total erythrocytes in the peripheral blood was approximately 30% of that in control embryos at 14.5 dpc.21 These mutant mice die at about 15.5 dpc probably due to anemia.20 21 These studies support the notion that the embryonic mortality of jmjtrap/trap embryos around 15.5 dpc is due to anemia.
The defects in definitive hematopoiesis in the fetal liver ofjumonji mutant embryos.
In the fetal liver of jmjtrap/trap embryos, the numbers of TER-119+ late erythroid cells, CD45+leukocytes, c-Kit+ immature hematopoietic cells, and MPO+ or NSE+ myeloid cells were lower (Fig 3). On the other hand, hepatocytes appear not to be severely affected (Fig2C and D). It is suggested that hypoplasia of the fetal liver is caused by the reduction in the number of TER-119+ cells, because the vast majority of cells in the fetal liver after 13.5 dpc is erythroid lineage.15 The reduction in the numbers of differentiated erythroid and myeloid cells in the fetal liver ofjmjtrap/trap embryos raises a question concerning the ability of erythroid and myeloid progenitors injmjtrap/trap embryos to differentiate into mature cells. Although the numbers of differentiated erythroid and myeloid cells were lower, these cells were present in the fetal liver and peripheral blood of jmjtrap/trap embryos (Figs 1and 3), suggesting that erythroid and myeloid progenitors ofjmjtrap/trap embryos can pursue terminal differentiation.
In the fetal liver of jmjtrap/trap embryos, the number of BFU-E and CFU-GM failed to increase as in controls (Fig 4A and C). The significant reduction in the number of CFU-S in the fetal liver of jmjtrap/trap embryos (Fig 6C) suggests that the failure of BFU-E and CFU-GM progenitors is caused by a fewer supply of BFU-E and CFU-GM progenitors from CFU-S progenitors. The presence of BFU-E and CFU-GM in the fetal liver ofjmjtrap/trap embryos in the early stages of definitive hematopoiesis (11.5 dpc and 12.5 dpc, Fig 4A and C) can be explained by the colonization of erythroid and myeloid progenitors from the yolk sac.18
The function of the jumonji gene in definitive hematopoiesis.
In the fetal liver of jmjtrap/trap embryos at 13.5 dpc, the number of CFU-S progenitors was less than 5% that in controls (Fig 6). This result strongly suggests that hypoplasia of the fetal liver and embryonic lethality are caused by the significant reduction in the number of CFU-S progenitors in the fetal liver ofjmjtrap/trap embryos. On the other hand, in the fetal liver of jmjtrap/trap embryos, in vitro development of erythroid and myeloid progenitors are normal (data not shown), the HSCs reconstitute all hematopoietic lineages in irradiated recipients (Fig 7), and Lin−/c-Kit+/Sca-1+ cells, known to have HSC activity, do not express the jmj gene (Fig8). These results suggest that the reduced number of CFU-S progenitors in the fetal liver of jmjtrap/trap embryos is caused by an environmental defect.
The generations of CFU-S progenitors and long-term repopulating (LTR)-HSCs are reported to occur in the aorta-gonad-mesonephros (AGM) region at about 10.5 to 11.5 dpc.22-27 Moreover, the CFU-S progenitors and LTR-HSCs in the AGM region seem to migrate and colonize the fetal liver.25 26 The results of CFU-S, reconstitution, and expression analyses (Figs 6 through 8) suggest an impairment of the generation of CFU-S in the AGM region, colonization of CFU-S progenitors from the AGM region to the fetal liver, or hematopoietic environment, which supports expansion of CFU-S progenitors in the fetal liver.
First, the generation of HSCs and CFU-S progenitors in the AGM region of jmjtrap/trap embryos and the expression ofjmj gene in the AGM region remains to be further examined. Recently, it was reported that immature hematopoietic cells including multipotent hematopoietic progenitors can be emerged by in vitro culture of AGM cells.28 We examined the generation of hematopoietic cells in the AGM region by this culture system. In the culture of AGM cells of jmjtrap/trap embryos, immature hematopoietic cells were normally developed (K.K., unpublished data, June 1997). This in vitro culture system suggests that the AGM region of jmjtrap/trap embryos has a potential for the generation of hematopoietic cells. Next, injmjtrap/trap embryos, the fetal liver environment supporting hematopoiesis may not be severely impaired, because the number of CFU-S in the fetal liver of jmjtrap/trapembryos at 14.5 dpc increased to approximately sevenfold of that at 13.5 dpc (Fig 6). Finally, there is a possibility that the colonization and migration of CFU-S progenitors from the AGM region to the fetal liver may be affected by the defect of nonhematopoietic cells such as endothelial cells in the fetal liver and dorsal aorta ofjmjtrap/trap embryos. The morphologic analysis of endothelial cells in jmjtrap/trap embryos is currently in progress.
In summary, our results suggest that the jmj gene has an important role in the hematopoietic microenvironment. Further investigation of the jmj gene will contribute to our understanding of the development of the hematopoietic system in mammals.
ACKNOWLEDGMENT
The authors thank Dr Shin-Ichi Nishikawa (Kyoto University, Japan) for the gift of ACK2 antibody, Dr Hajime Takayama and Yoshiko Shirota (Mitsubishi Kasei Institute of Life Sciences, Tokyo, Japan) for supporting FACS analysis, CFU-S assay, and reconstitution experiments, Takashi Sekiguchi (Tokyo University, Japan) for cell sorting analysis, and Dr Toru Nakano (Osaka University, Japan) for discussion and critical review of the manuscript.
Supported in part by a Grant-in-Aid for Creative Basic Research from the Ministry of Education, Science, Sports, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Takashi Takeuchi, PhD, Mitsubishi Kasei Institute of Life Sciences, 11, Minamiooya, Machida, Tokyo 194-8511, Japan; e-mail: take@libra.ls.m-kagaku.co.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal