To the Editor:
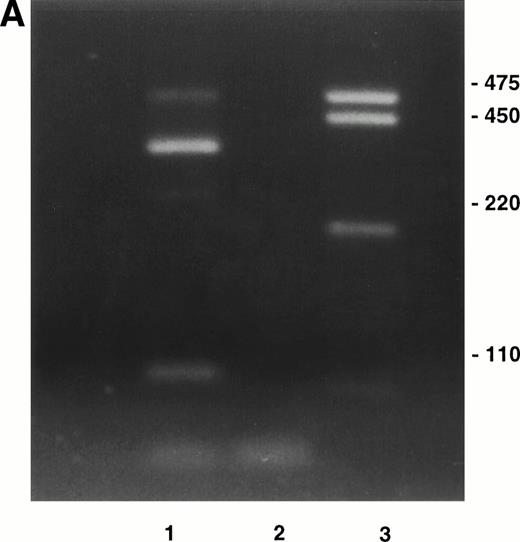
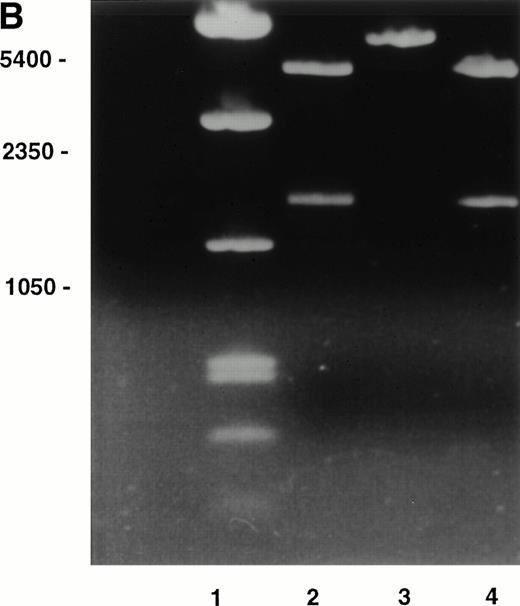
The distribution of fibronectin in the bone marrow stromal extracellular matrix will influence adhesion and localization of hematopoietic stem and progenitor cells that bind to its two major cell-binding domains via α4β1 and α5β1 integrins. The α4β1 integrin may have a role in adhesion of more primitive hematopoietic progenitor cells; both human long-term culture-initiating cells and colony-forming unit (CFU)-mix progenitors adhere to the fibronectin COOH-terminal heparin-binding domain through α4β1 integrin1 and primitive murine CFU-spleen (CFU-S) day 12 colony-forming cells adhere to the CS1-containing fibronectin fragment.2 Important differential splicing occurs at the type-III connecting segment (IIICS) in human fibronectin that codes for five potential RNA variants (Table1). The importance of the IIICS domain in hematopoiesis lies in its possession of two α4β1 integrin binding sites, represented by synthetic peptides called CS1 and CS5, which lie in separate, independently spliced regions so that either one, both, or neither site may be expressed.3,4 A third weaker site recognized by α4β1 is present in the adjacent heparin-binding domain, Hep II.5 As the interaction between α4β1 integrin and fibronectin will depend on the products of alternative splicing of the IIICS domain, eg, only two of the possible five products contain the CS1 sequence that binds α4β1 with highest affinity, we set out to determine expression of the IIICS variants at the RNA level in marrow stromal cells grown in long-term culture using reverse transcriptase-polymerase chain reaction (RT-PCR). The identity of the resulting PCR products was confirmed by restriction enzyme digestion and DNA sequencing. Normal marrow was cultured in standard long-term culture conditions to produce a confluent stromal layer. Three days before harvesting, the stromas were irradiated with 1,500 cGy of gamma radiation from a 137Caesium source to destroy hematopoietic cells. Total RNA was extracted from the adherent stromal cell layer and mRNA isolated. First-strand cDNA was generated by reverse transcription using avian RT primed with oligo-dT and the cDNA was amplified by primers complementary to sequences on each side of the IIICS region (5′-GAATAATCAGAAGAGCGAGCC and 3′-ACTCAGAAGTGTCCTGGAATG). Four distinct bands were seen consistent with expression of variants V120 (expected band size = 464 bp), V89 or V95 (expected band sizes = 371 or 389, respectively), V64 (expected band size = 296), and V0 (expected band size = 104) (Fig1A). The control with no RT confirmed that the products originated from expressed stromal RNA. The PCR products were cloned using the TA cloning system (Invitrogen, San Diego, CA). Seven PCR inserts were all of one size consistent with either V89 or V95, two were the size expected for V64, and one insert corresponded to the largest V120 variant. PCR inserts from selected clones were identified by restriction enzyme analysis with BglII, which cuts the IIICS-A sequence (containing CS1) but not the IIICS-B or IIICS-C sequences. BglII also cuts the pCRTM II vector resulting in two bands of sizes 1,155 bp and 3,148 bp with the IIICS-A/IIICS-B sequence (V89 variant) (as shown in Fig 1B) lanes 2 and 4. Lane 3 contains the IIICS-B sequence alone (V64 variant). None of the clones contained the V95 variant. Sequencing the inserts using the original IIICS primers confirmed the identity of V89 and V64. The identity of the largest and smallest variants (V120 and V0) was not confirmed by restriction enzyme analysis or sequencing, but they were clearly present in the original RT-PCR (Fig 1A). A summary of the variants detected in stroma is shown in Table 1.
Fibronectin IIICS Variants Showing Possible Combinations of CS1 and CS5-Containing Sequences
| IIICS-A CSI . | IIICS-B . | IIICS-C CS5 . | Variant . | RT-PCR (bp) . | Variant Expressed in Stroma . |
|---|---|---|---|---|---|
| √ | √ | √ | V120 | 464 | √ |
| — | √ | √ | V95 | 389 | — |
| √ | √ | — | V89 | 371 | √ |
| — | √ | — | V64 | 296 | √ |
| — | — | — | V0 | 104 | √ |
| IIICS-A CSI . | IIICS-B . | IIICS-C CS5 . | Variant . | RT-PCR (bp) . | Variant Expressed in Stroma . |
|---|---|---|---|---|---|
| √ | √ | √ | V120 | 464 | √ |
| — | √ | √ | V95 | 389 | — |
| √ | √ | — | V89 | 371 | √ |
| — | √ | — | V64 | 296 | √ |
| — | — | — | V0 | 104 | √ |
The independently spliced segments are referred to as IIICS-A, -B, and -C. The numbers assigned to the variants refer to the number of amino acid residues in the IIICS. The expected size of the variants by RT-PCR is shown together with those expressed in bone marrow stroma.
(A) RT-PCR amplification of marrow stromal RNA using primers complementary to sequences on each side of the alternatively spliced IIICS region of fibronectin. Ten microliters of PCR products was run out on a TBE/2% agarose gel. Lane 1, reaction with RT; lane 2, control without reverse transcriptase; lane 3, DNA marker. The four bands in lane 1 are consistent with variants V120 (464 bp), V95 or 89 (389 or 371 bp), V64 (296 bp), and V0 (104 bp). (B) Restriction enzyme digests of selected clones prepared from TA cloning RT-PCR products of IIICS region of fibronectin. Three microliters of digested products was run on a TBE/1% agarose gel.BglII cuts the IIICS region when the CS1 sequence is present. A second BglII site in the pCRTM II vector results in 1,155- and 3,148-bp products in the presence of a CS1/IIICS-B insert (V89) as shown in lanes 2 and 4. The band in lane 3 is consistent with a IIICS-B insert (V64) within the linearized vector (4,124 bp).
(A) RT-PCR amplification of marrow stromal RNA using primers complementary to sequences on each side of the alternatively spliced IIICS region of fibronectin. Ten microliters of PCR products was run out on a TBE/2% agarose gel. Lane 1, reaction with RT; lane 2, control without reverse transcriptase; lane 3, DNA marker. The four bands in lane 1 are consistent with variants V120 (464 bp), V95 or 89 (389 or 371 bp), V64 (296 bp), and V0 (104 bp). (B) Restriction enzyme digests of selected clones prepared from TA cloning RT-PCR products of IIICS region of fibronectin. Three microliters of digested products was run on a TBE/1% agarose gel.BglII cuts the IIICS region when the CS1 sequence is present. A second BglII site in the pCRTM II vector results in 1,155- and 3,148-bp products in the presence of a CS1/IIICS-B insert (V89) as shown in lanes 2 and 4. The band in lane 3 is consistent with a IIICS-B insert (V64) within the linearized vector (4,124 bp).
A previous study has described differences between IIICS variants in a variety of human normal and tumor cell types, though not marrow stroma.6 Although the CS1 sequence is present in a murine bone marrow–derived stromal cell line,2 we show here for the first time that normal human bone marrow stroma grown in long-term culture expresses four of the IIICS variants: V120, V89, V64, and V0. The V95 variant was not present. Expression of two CS1-containing variants together with two variants that do not have this sequence provides a mechanism for controlling α4β1integrin receptor-mediated interactions of hematopoietic progenitor cells at the level of mRNA splicing. It is also probable that protein modification in situ, which may change domain structures, is another potential level of control. Finally, the precise localization of the variant proteins within the marrow extracellular matrix would allow for fine control of progenitor and stem cell interactions with resulting changes in adhesion, migration, and growth.
ACKNOWLEDGEMENT
K.P.S. is a clinical research fellow of the Cancer Research Campaign.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal