Abstract
Human T-cell lymphotropic virus type I (HTLV-I) is the causative agent of adult T-cell leukemia/lymphoma (ATL). ATL is an aggressive proliferation of mature activated T cells associated with a poor prognosis. The combination of the antiviral agents, zidovudine (AZT) and interferon (IFN), is a potent treatment of ATL. Recently, arsenic trioxide (As) was shown to be an effective treatment of acute promyelocytic leukemia (APL). We have tested the effects of the combination of As and IFN on cell proliferation, cell cycle phases distribution, and apoptosis in ATL-derived or control T-cell lines. A high synergistic effect between IFN and As was observed in ATL-derived cell lines in comparison to the control cell lines, with a dramatic inhibition of cell proliferation, G1 arrest, and induction of apoptosis. Similar results were obtained with fresh leukemia cells derived from an ATL patient. Although the mechanisms involved are unclear, these results could provide a rational basis for combined As and IFN treatments in ATL.
ADULT T-CELL leukemia/lymphoma (ATL) is an aggressive lymphoproliferative disorder associated with the human T-cell lymphotropic virus type I (HTLV-I).1 ATL-associated tumor cells are constitutively-activated CD4+ T cells with characteristic convoluted nuclei and basophilic cytoplasm.2ATL remains of very bad prognosis due to severe immunosuppression and to an intrinsic resistance of leukemia cells to high doses of chemotherapy. Combination chemotherapy regimens, in particular those designed for treatment of aggressive non-Hodgkin’s lymphoma or acute lymphoblastic leukemia have little effect in the treatment of ATL patients, with a median survival around 6 months in the acute form.3 Important advances in the treatment of ATL were reported in two independent phase II studies4-6 with the combination of an antiretroviral agent zidovudine (AZT) and interferon-α (IFN-α). This combination exhibits a high response rate in ATL patients, especially in the acute ATL type and prolongs survival. Unfortunately, most of the patients relapse, thereby necessitating the search for alternative or complementary therapies.
Arsenic trioxide (As) was shown to be an effective treatment for acute promyelocytic leukemia (APL). In a phase II study,7 15 of 16 APL patients resistant to all-trans retinoic acid (ATRA) and conventional chemotherapy achieved complete remission with intravenous infusion of As. In vitro studies on APL-derived cell line (NB4) showed that As induced apoptosis at micromolar concentration.7 The molecular mechanisms of As action in APL are not fully elucidated; however, we have shown that As induces the specific degradation of the PML/RARα fusion protein resulting from the t(15;17) translocation.8 Moreover, in non-APL cells, As accelerates the targeting of PML onto nuclear bodies (NB) and induces the degradation of the PML protein. Because NB/PML localization appears required for its antiproliferative effect, As-induced apoptosis could relate to As-targeting of PML. Interestingly, Tax, an HTLV-I transcriptional activator with transforming potential, appears to specifically delocalize a PML-associated protein Int-6, initially identified as an insertion target of mouse mammary tumor virus (MMTV). This prompted us to examine the effects of As with or without IFN on ATL cells.
Here we report a synergistic effect of the combination of As and IFN on cell proliferation, cell cycle phases distribution, and apoptosis in HTLV-I–infected cells. Our results support the use of As and IFN in ATL therapy.
MATERIALS AND METHODS
Reagents and Drugs
Recombinant IFN (Hoffman-La Roche, Basel, Switzerland) and As were prepared as stock solutions in normal saline. Working stocks were diluted in RPMI 1640 culture medium (GIBCO-BRL, Gaithersburg, MD), supplemented with 10% heat-inactivated fetal calf serum (FCS; GIBCO-BRL). The in vitro dose of 100 U/mL of IFN and 1 μmol/L of As corresponds to the pharmacological level in vivo.7 9 Two other drug concentrations of IFN were used (10 U/mL and 1,000 U/mL) to span 3 log magnitudes.
Cell Lines
HUT-102 and MT2 cell lines (a gift of A. Gessain, Institut Pasteur, Paris, France) are HTLV-I–infected CD4+ T cells derived from an ATL patient, which constitutively express the interleukin-2 (IL-2) receptor alpha chain (CD25) and are independent of IL-2 for growth. They constitutively express HTLV-I proteins and virus.10 11 HTLV-I− CD4+T-cell lines CEM and Jurkatt were used as controls. Cell lines were grown in RPMI-1640 medium containing 10% heat inactivated FCS and antibiotics. Cell growth was assessed by the incorporation of3H-thymidine, cell count (hemocytometer), and trypan blue dye exclusion protocols. Cell concentration of 1 × 105 cells/mL was chosen for seeding for all experiments.
Preparation of Peripheral Blood Mononuclear Cells (PBMC)
PBMC were extracted from diluted venous blood from a patient with acute ATL and from a healthy seronegative individual by Hypaque-Ficoll centrifugation (Lymphoprep, Nyegaard, Norway). Cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, antibiotics, and with 100 U/mL recombinant human IL-2 (rhIL-2). Cells were maintained at 37°C in a 5% CO2 humidified atmosphere.
Proliferation Assay
Cells were cultured in 96-well, flat-bottom microtiter plates (Nunc, Naperville, IL) in quadruplicates. Test drugs were added at the indicated concentrations at the initiation of culture. At different time points of culture (24 hours, 48 hours, and 72 hours),3H-thymidine (1 μCi/well; Amersham, Buckinghamshire, UK) was added for the last 4 hours. Cells were harvested on a PHD cell harvester (Cambridge Technology, Watertown, MA), and dried at 50°C overnight. Bio-HP liquid scintillation fluid (Fisher Scientific, Fair Lawn, NJ) was then added. DNA synthesis was quantified by measuring the cellular incorporation of 3H-thymidine using a Wallac 1409 liquid scintillation counter (Pharmacia-LKB, Uppsala, Sweden), and were expressed as counts per minute (CPM). Cell proliferation was expressed as percentage of corresponding control. Each experiment was performed in quadruplicate and repeated a minimum of three times.
DNA Content Analysis
Cells were obtained at different times after treatment, washed twice with cold phosphate-buffered saline (PBS), and fixed in cold (−20°C) 100% ethanol and kept overnight at 4°C. Subsequently, cells were rinsed with PBS, treated with Tris-HCl buffer (pH 7.4) containing 1% RNase, and stained with propidium iodide (PI) 100 μg/mL (final concentration). Distribution of cell cycle phases with different DNA contents was determined using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). In each sample, 10,000 ungated events were acquired. Analysis of cell cycle distribution (including apoptosis) was performed using Modfit software (Becton Dickinson).
Apoptosis Studies
Nuclear staining.
Cells under study were treated with test drugs, which were added at the indicated concentrations at the initiation of culture. Nuclei were then labeled by Hoechst 33342 (Polysciences, Warrington, PA) for 2 minutes at room temperature. Cells were then observed under fluorescence microscopy using ultraviolet (UV) filter pack. The total number of nuclei and the percentage of apoptotic nuclei was noted.
TUNEL assay.
The terminal deoxy-transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay was used to monitor the extent of DNA fragmentation due to apoptosis. The assay was performed according to the recommendations of the manufacturer (Boehringer, Mannheim, Germany). Fluorescein-conjugated dUTP incorporated in nucleotide polymers was detected and quantified using flow cytometry. Approximately 10,000 cells per sample were acquired and analyzed using Cell-Quest software (Becton Dickinson).
Annexin-V staining.
Cells under study were incubated with various test agents and apoptosis was assessed by Annexin-V binding. Briefly, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin-V according to the recommendations of the manufacturer (Boehringer), in the presence of 1 mmol/L CaCl2. Nuclei of cells were counterstained with PI and cells were either screened by flow cytometry (approximately 10,000 events) or by fluorescence microscopy. Apoptosis was estimated by the relative amount of FITC+-PI− cell populations. Under these conditions, FITC+ PI+cells correspond to either necrotic or postapoptotic cells.
Western Blot Analysis
Approximately 107 cells from various treatments were solubilized at 4°C in lysis buffer (0.01 mol/L Tris-Cl (pH 7.4), 0.5% sodium deoxycholate, 0.5% Triton X-100, 0.05% sodium dodecyl sulfate [SDS]). The samples were loaded onto a 12% SDS-polyacrylamide gel, subjected to electrophoresis, and transferred to nitrocellulose membrane that was subsequently stained with 0.2% Ponceau red to assure equal protein loading and transfer. After blocking of the membrane in 1% bovine serum albumin (BSA), the blots were reacted with 1:100 dilution of HTLV-I seropositive patients sera for 2 hours at 37°C. After washing, the blots were incubated with antihuman IgG (Fc-specific) conjugated to peroxidase. Binding of antibodies was detected by staining by chemoluminescence using the ECL detection kit (Amersham, Buckinghamshire, UK).
RESULTS
Effects of IFN, As, and Their Combination on Cell Proliferation
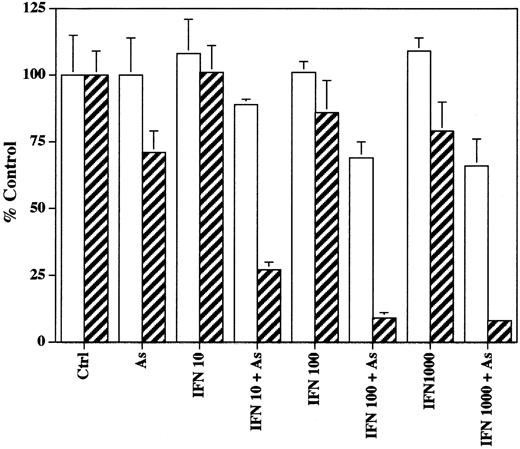
Cell proliferation was assessed by the ability of cells to incorporate3H-thymidine into their DNA. Results are expressed as percent of control and represent the mean of at least four independent experiments. Figure 1 represents the most striking differences between the HTLV-I+ cell line (HUT-102) and the HTLV-I− cell line (CEM) at 48 hours of treatment. The results observed with MT-2 and Jurkatt cells (data not shown) were similar to those obtained with HUT-102 and CEM cells, respectively. No major difference was observed for the basal incorporation in the different cell lines at the different time points (24, 48, and 72 hours). Several conclusions emerge from this study. First, HTLV-I–transformed cells appear to be much more sensitive to the effect of IFN than control cells in which growth suppression is minimal and only occurs at 3 days (data not shown). This is interesting in view of the fact that these patients clinically respond to IFN. Secondly, HTLV-I HUT-102 cells are considerably more sensitive to As than control cells (CEM) or MT2 cells at 3 days of treatment (incorporation rates as percent of control are: 26 ± 5; 83 ± 16; and 107 ± 9, respectively for HUT-102, CEM, and MT-2). Finally, a very strong synergy is found between IFN and As at any time and any dose of IFN used, in both HTLV-I–infected cells HUT-102 (Fig 1) and MT-2 (data not shown), contrasting with control cells CEM (Fig 1) and Jurkatt (data not shown). In control cells, a modest synergy was found at day 3 as in most cell lines.11a Indeed, a 50% reduction in 3H-thymidine incorporation into CEM cells was only observed at 72 hours of treatment for IFN doses over 100 U/mL in the presence of As (data not shown). Finally, note that in HTLV-I+ cells HUT-102 (Fig 1) and MT-2 (data not shown), As addition leads to an almost complete growth arrest for IFN doses over 100 U/mL (the therapeutic dose in patients).
Effects of IFN, As, and their combination on the3H-thymidine incorporation at 48 hours of leukemia cell lines CEM (□) and HUT-102 (▨). Incorporation rates that reflected both the number of cells and the individual DNA synthesis are expressed as percentage (±SD) of control and represent the mean of the results obtained in at least four independent experiments.
Effects of IFN, As, and their combination on the3H-thymidine incorporation at 48 hours of leukemia cell lines CEM (□) and HUT-102 (▨). Incorporation rates that reflected both the number of cells and the individual DNA synthesis are expressed as percentage (±SD) of control and represent the mean of the results obtained in at least four independent experiments.
Effects of IFN, As, and Their Combination on Cell Cycle
To investigate whether the inhibition of cell proliferation was due to cell cycle arrest or/and apoptosis, cell cycle analyses were performed under the same culture conditions as previously described. We first examined cellular DNA contents distribution by flow cytometry for the presence of a pre-G1 region phase. No major variation was observed between HUT-102 and CEM cells at different time points. Hence, Table 1 illustrates data collected at 72 hours of treatment. As, but not IFN alone, induced a significant increase in apoptosis in HUT-102 cells, but not in CEM cells. A clear synergy between the two agents was observed in either HTLV-I+ or HTLV-I− cells, consistent with our previous data in other cells.11a Note, however, that the synergy was much stronger in HUT-102 cells reaching 48% with modest doses of each agent.
Cell-Cycle Distribution
| Treatment 72 Hours . | Pre-G1 (Apoptosis) . | S + G2 + M (cycling cells) . | ||
|---|---|---|---|---|
| HUT . | CEM . | HUT . | CEM . | |
| Control | 5.9 | 7.9 | 49.4 | 51.3 |
| As | 19.4 | 8.5 | 35.7 | 54.3 |
| IFN 10 | 8.3 | 6 | 52.3 | 57.6 |
| IFN 10 + As | 45.8 | 18.4 | 37.7 | 53.6 |
| IFN 100 | 10 | 21.2 | 50.3 | 61.8 |
| IFN 100 + As | 4.5 | 31.5 | 43.6 | 54.5 |
| Treatment 72 Hours . | Pre-G1 (Apoptosis) . | S + G2 + M (cycling cells) . | ||
|---|---|---|---|---|
| HUT . | CEM . | HUT . | CEM . | |
| Control | 5.9 | 7.9 | 49.4 | 51.3 |
| As | 19.4 | 8.5 | 35.7 | 54.3 |
| IFN 10 | 8.3 | 6 | 52.3 | 57.6 |
| IFN 10 + As | 45.8 | 18.4 | 37.7 | 53.6 |
| IFN 100 | 10 | 21.2 | 50.3 | 61.8 |
| IFN 100 + As | 4.5 | 31.5 | 43.6 | 54.5 |
Effects of IFN, As, and their combination on the cell cycle distribution of leukemic cell lines HUT-102 and CEM. Histogram-related nuclear DNA contents was measured on flow cytometry and analyzed by Modfit software. The pre-G1 percentages represent apoptotic cells. Cycling cells, the sum of (S + G2 + M) phases, are a percentage of nonapoptotic cells.
Interestingly, As also specifically induced G1 arrest, because As alone sharply diminished the number of cycling living cells, in HTLV-I+ cells specifically. Such As-induced G1 arrest was apparently not enhanced by IFN. Together with induction of apoptosis, such cell cycle arrest likely accounts for the drastic effects of combined As and IFN on thymidine incorporation.
Apoptosis Studies
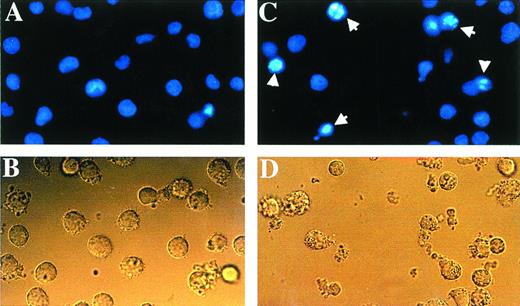
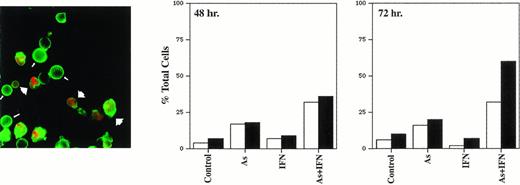
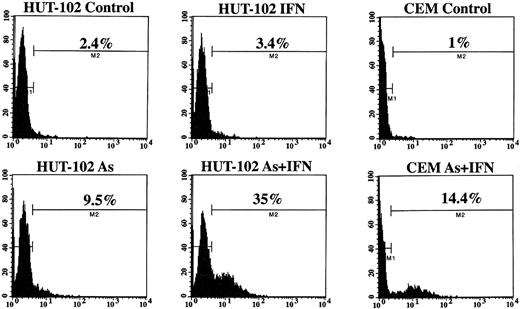
The cell-cycle distribution studies suggested that As induced apoptosis in HTLV-I–derived cell lines, a process enhanced by IFN treatment. Three established criteria were subsequently used to assess apoptosis in our system. First, HUT-102 cells were stained with Hoechst 33342. Figure 2 shows that the nuclei of HUT-102 cells treated with As and IFN underwent apoptosis, as shown by their characteristically condensed, fragmented, and intensely fluorescent nuclei (Fig 2C; arrowheads). Control cells displayed the typical morphology of normal nuclei (Fig 2A), as HUT-102 cells treated with As only (data not shown). Under light microscopy, cells treated with both As and IFN (Fig 2D) were less numerous and exhibited membrane blebbing when compared with control cells (Fig 2B). Second, the TUNEL assay shows DNA cleavage by labeling free 3′-OH ends. At 48 hours of culture, the percentage of TUNEL+ HUT-102, representing apoptotic cells, was 3% for the control cells, 7% with As, 3% with 1,000 U/mL, 16% with the combination of As and 1,000 U/mL of IFN. At 72 hours of culture (Fig 3), the percentage of TUNEL+ HUT-102, was 2% for the control cells, 9% with As, 3% with 1,000 U/mL of IFN, 35% with the combination of As and 1,000 U/mL of IFN. For the control cell line CEM, at 72 hours of culture (Fig 3), the percentage of TUNEL+ CEM cells was 1% for the control cells and 14% with the combination of As and 1,000 U/mL of IFN. Finally, cell death was also determined in the HUT-102 cell line using FITC-conjugated Annexin-V membrane staining and PI nuclear counterstaining. The fluorescence microscopy analysis of HUT-102 cells treated with the combination of As and IFN showed the presence of apoptotic cells (FITC+, PI−) with some necrotic or postapoptotic ones (FITC+, PI+) (Fig 4 [see page 281]). These different cell populations were quantified by flow cytometry analysis at different times of culture (48 hours and 72 hours). In Fig 4, the percentage of dead cells is relative to the total cell population, while the percentage of apoptotic cells is presented with regard to the PI−, ie, nondead cells. Again, As lead to a significant increase in both apoptotic or postapoptotic cells, and this was enhanced by IFN cotreatment at either 24 or 48 hours.
The combination of As and IFN induced morphologic changes characteristic of apoptosis when stained with Hoescht 33342. The nuclei of HUT-102 cells treated with the combination of As and IFN (C) underwent apoptosis, as shown by their characteristically shrunken and intensely-fluorescent nuclei (arrowheads). Control cells (A) displayed the typical morphology of nuclei, as did HUT-102 cells treated with As only (data not shown). Under light microscopy, cells treated with the combination of As and IFN (D) were less numerous and exhibited membrane blebbing when compared with control cells (B).
The combination of As and IFN induced morphologic changes characteristic of apoptosis when stained with Hoescht 33342. The nuclei of HUT-102 cells treated with the combination of As and IFN (C) underwent apoptosis, as shown by their characteristically shrunken and intensely-fluorescent nuclei (arrowheads). Control cells (A) displayed the typical morphology of nuclei, as did HUT-102 cells treated with As only (data not shown). Under light microscopy, cells treated with the combination of As and IFN (D) were less numerous and exhibited membrane blebbing when compared with control cells (B).
Annexin-V binding: HUT-102 cells were treated for 48 hours or 72 hours with As (1 mmol/L), IFN (1,000 U/mL), or their combination. Using FITC-conjugated Annexin-V membrane staining and PI nuclear counterstaining, the relative amount of apoptotic cells (FITC+ PI−) and dead cells either necrotic or postapoptotic (FITC+ PI+) was determined. The percentage of dead cells is relative to the total cell population, while the percentage of apoptotic cells is presented with regard to the PI−, ie, nondead cells. (□), Apoptotic; (▪), dead. Fluorescence microscopy analysis showed the presence of apoptotic cells (small arrows) and dead cells (large arrows).
Annexin-V binding: HUT-102 cells were treated for 48 hours or 72 hours with As (1 mmol/L), IFN (1,000 U/mL), or their combination. Using FITC-conjugated Annexin-V membrane staining and PI nuclear counterstaining, the relative amount of apoptotic cells (FITC+ PI−) and dead cells either necrotic or postapoptotic (FITC+ PI+) was determined. The percentage of dead cells is relative to the total cell population, while the percentage of apoptotic cells is presented with regard to the PI−, ie, nondead cells. (□), Apoptotic; (▪), dead. Fluorescence microscopy analysis showed the presence of apoptotic cells (small arrows) and dead cells (large arrows).
TUNEL analysis of the HTLV-I–infected HUT-102 cell line and control cell line CEM treated or not with IFN (1,000 U/mL) and/or As (1 mmol/L) for 72 hours. Histogram analysis was achieved by setting a region for control cells and defining the region for positive TUNEL to include 2% of the normal cells. Statistics of positive TUNEL region are displayed for each histogram.
TUNEL analysis of the HTLV-I–infected HUT-102 cell line and control cell line CEM treated or not with IFN (1,000 U/mL) and/or As (1 mmol/L) for 72 hours. Histogram analysis was achieved by setting a region for control cells and defining the region for positive TUNEL to include 2% of the normal cells. Statistics of positive TUNEL region are displayed for each histogram.
Ex Vivo Studies
The effect of the combination of As and IFN in vitro was substantiated by ex vivo experiments. PBMC from a patient with acute ATL and one healthy seronegative control were cultured ex vivo and immediately treated with As, IFN, and their combination. At 72 hours of culture, the percentage of ATL cells in the pre-G1 phase was 18% for the untreated cells, 42% with As, 42% with 100 U/mL IFN, and 62% with the combination of As and 100 U/mL of IFN. By contrast, in the control normal lymphocytes, at 72 hours of culture, the percentage of cells in the pre-G1 phase was 9% for the untreated cells, 17% with As, 7% with 100 U/mL, and 18% with the combination of As and 100 U/mL of IFN. Moreover, at 3 days in ATL cells treated with the combination of As and IFN had almost completely withdrawn from the cell cycle with (S + G2 + M) phases at 23% for the untreated cells, 12% with As, 30% with 100 U/mL of IFN, and 5% with the combination of As and 100 U/mL of IFN. By contrast, the cell-cycle distribution of normal lymphocytes was unaffected by this treatment.
Analysis of HTLV-I Protein Expression
Western blot analysis of HUT-102 proteins using anti–HTLV-I+ sera showed the typical profile of HTLV-I proteins as described previously.12 No quantitative or qualitative modification of the profile pattern was observed in cells treated with IFN, As, or their combination (data not shown).
DISCUSSION
Here we show that As and IFN induce apoptosis and cell-cycle arrest in HTLV-I–infected cells. The dramatic synergy between these two agents and the modest effect on HTLV-I− cell lines suggest that they may target a specific step in HTLV-I–triggered transformation and could provide an experimental basis for combined As/IFN ATL treatment.
Use of IFN alone or in combination with other drugs held promise for the treatment of several hematologic malignancies and solid tumors. Although clinical trials reported by us and others demonstrated the efficacy of the combination of zidovudine (AZT) and IFN in the treatment of ATL,4-6 the search for a more effective and lasting treatment is essential. APL, another hematologic malignancy, was reported to undergo complete clinical remission with As through induction of apoptosis.7 We have demonstrated that As specifically targets the PML protein through alteration of its localization and then induction of its degradation. Because (1) IFN, which exerts some beneficial effects in ATL, is a direct transcriptional activator of the PML gene; (2) Tax-triggered delocalization of the int-6 oncogene, a protein that is associated with PML nuclear bodies, was proposed to contribute to ATL leukemogenesis; and (3) we recently showed that IFN and arsenic cooperate to induce apoptosis, we thought it might be relevant to test the effect of IFN and As on ATL cells. Although, the molecular mechanism by which this combination exerts its effects is still under investigation, the drastic cooperation of these two agents in ATL is evident. In HTLV-I–infected cell lines, cell proliferation was impeded in a highly synergistic manner between As and IFN, in sharp contrast to control cell lines. The examination of nuclear DNA contents by flow cytometry showed that the combination of As and IFN induced both cell cycle alteration and apoptosis. As treatment suppressed cell cycling (S + G2 + M) in HUT-102 cells, but not CEM cells. By contrast the addition of IFN to As-treated freshly isolated ATL cells resulted in complete abolishing of the cell cycle (<5%) and massive induction of apoptosis.
The mechanism of action of As alone or in combination with IFN in ATL-derived cell lines and fresh ATL cells is still unknown. A significant induction of apoptosis was observed, but the extent of the inhibition of cell proliferation suggests the existence of mechanisms other than apoptosis. In APL cells, As induced apoptosis and downregulated bcl-2 gene expression at both mRNA and protein levels in NB4 cells, but did not influence bax, bcl-x, c-myc, and p53 gene expression.7 Preliminary results from our laboratories did not implicate bcl-2 in As and IFN combination effect on HUT-102 cells. Modification of PML localization could be an alternative pathway. PML is a phosphoprotein located within the nucleus where it displays a characteristic speckled staining.13-16 This typical PML staining pattern is due to the location of the protein into discrete PML oncogenic domains (PODs) or nuclear bodies of unknown function.17 In APL cells, the structure of PODs is destroyed, and anti-PML staining is distributed in hundreds of micropunctuates.13,14 Retinoic acid (RA) or As treatment corrects PML localization by inducing PML/RARα degradation (RA, As), as well as PML targeting onto the nuclear bodies (As).8Similarly, Tax was shown to delocalize int-6, a nuclear body component, although it did not directly affect PML.18 Supposing that PML directly or indirectly contacts int-6, As and IFN could greatly enhance expression of PML and its targeting onto nuclear bodies and could then correct int-6 localization, constituting a novel example of molecular targeted treatment.11a
In conclusion, we have shown high synergy of As and IFN treatment with regard to the inhibition of proliferation and DNA synthesis, modification of the cell cycle phases, and induction of apoptosis of ATL-derived cell lines and fresh ATL cells. Because the synergistic effectiveness of the combination treatment at pharmacologic doses was specific for HTLV-I–infected cells (both cell lines and fresh leukemia cells) and in light of the success of the AZT/IFN combination as a treatment for ATL, our results support the development of a phase II clinical study for the treatment of HTLV-I–associated ATL by the combination of arsenic and IFN.
ACKNOWLEDGMENT
The authors thank Drs F. Homaidan and G. Dbaibo for their critical reading of the report. The excellent technical assistance of the American University of Beirut Core Laboratory Facilities personnel is greatly appreciated.
Supported by grants from the American University of Beirut (Medical Practice Plan and University Research Board).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ali Bazarbachi, MD, PhD, Department of Internal Medicine, American University of Beirut, PO Box 113-6044, Beirut, Lebanon; email: bazarbac@aub.edu.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal