To the Editor:
Bone marrow transplantation (BMT) is the only curative treatment for children with (severe) combined immunodeficiency [(S)CID].1 In the absence of HLA-identical siblings suitable as donors, alternative sources, such as HLA-identical marrow unrelated donors (MUD), should be used.2,3 Among the many factors that may influence the speed of T-cell repopulation, the source of the donor may be relevant. However, the majority of data available in the literature on the recovery of the T-cell number and function after BMT deal with transplantation from HLA-identical siblings and only one report described the immunological reconstitution in a series of children, mainly affected by malignancies, receiving T-cell–depleted BMT either from MUD or HLA-haploidentical family donors.4
Therefore, we have studied the reconstitution of T-cell number and function after BMT from MUD in eight children affected by (S)CID. Main clinical data are presented in Table 1. No patient developed chronic graft-versus-host disease (GVHD). Phenotypic analysis and proliferative response were evaluated every 1 to 2 months until month +6, then every 6 to 12 months. In vitro T-cell depletion with Campath-1M5 was performed only in patient GRMA transplanted from an MUD mismatched for one locus HLA-A. Immunological reconstitution in this patient was similar to that observed in other children; data concerning this patient are presented here pooled together with the others, and compared with those from 10 healthy children of similar age, studied as controls.
Patients’ Features
| Patient . | Age (mo)/ Sex . | Diagnosis . | Conditioning . | GVHD Prophylaxis . | Acute GVHD . | Acute GVHD Treatment . | Follow-up (mo) . |
|---|---|---|---|---|---|---|---|
| GRMA | 12/M | AR-SCID T−B+ | BuCy | CsA (7 mo) | I | PDN, ATG | A +50-150 |
| MOGI | 8/M | Omenn’s syndrome | BuCy, VP16 | CsA (9 mo), ATG | II | PDN | A +68 |
| RICR | 10/F | Omenn’s sydnrome | BuCy | CsA (5 mo) | I | PDN, ATG | A +48 |
| SAAL | 1/M | XL-SCID T−B+ | ATG | CsA (7 mo), MTX | 0 | None | A +60-150 |
| SPCH | 48/F | CID | BuCy, thiotepa | CsA (6 mo) | I | PDN, ATG | A +29 |
| SURO | 34/F | HLA II deficiency | BuCy | CsA, MTX, ATG | IV | PDN | D +4 |
| VAMI | 7/M | XL-SCID T−B+ | BuCy | CsA (12 mo) | III | PDN | A +41 |
| TEDA | 7/M | SCID T−B− | BuCy | CsA (7 mo) | I (skin) | PDN | A +29 |
| Patient . | Age (mo)/ Sex . | Diagnosis . | Conditioning . | GVHD Prophylaxis . | Acute GVHD . | Acute GVHD Treatment . | Follow-up (mo) . |
|---|---|---|---|---|---|---|---|
| GRMA | 12/M | AR-SCID T−B+ | BuCy | CsA (7 mo) | I | PDN, ATG | A +50-150 |
| MOGI | 8/M | Omenn’s syndrome | BuCy, VP16 | CsA (9 mo), ATG | II | PDN | A +68 |
| RICR | 10/F | Omenn’s sydnrome | BuCy | CsA (5 mo) | I | PDN, ATG | A +48 |
| SAAL | 1/M | XL-SCID T−B+ | ATG | CsA (7 mo), MTX | 0 | None | A +60-150 |
| SPCH | 48/F | CID | BuCy, thiotepa | CsA (6 mo) | I | PDN, ATG | A +29 |
| SURO | 34/F | HLA II deficiency | BuCy | CsA, MTX, ATG | IV | PDN | D +4 |
| VAMI | 7/M | XL-SCID T−B+ | BuCy | CsA (12 mo) | III | PDN | A +41 |
| TEDA | 7/M | SCID T−B− | BuCy | CsA (7 mo) | I (skin) | PDN | A +29 |
Abbreviations: AR, autosomal recessive; XL, X-linked A; BuCy, busulphan + cyclophosphamide; ATG, antithymocyte globulin; CsA, cyclosporine A; MTX, methotrexate; PDN, methylprednisolone; A, alive; D, dead.
Still receiving intravenous Ig.
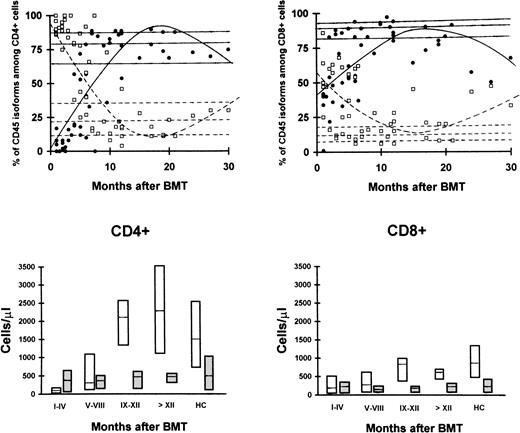
In the first months after BMT, the striking predominance of CD4+ cells coexpressed the CD45R0 molecule, associated with a primed/activated phenotype, whereas naive CD4+CD45RA+ cells were initially rare. On the other hand, CD45RA+ and CD45R0+ subsets were almost equivalent among CD8+ cells (Fig1). The absolute number of CD45RA+ cells progressively increased, reaching normal levels at month +8 after BMT, whereas that of CD45R0+ cells remained fairly constant (Fig 1). These changes led to a normalization of the proportion of these subsets within 1 year (Fig 1).
Proportions (top panels) and absolute numbers (bottom panels) of lymphocyte subsets identified by CD45 isoform expression among CD4+ (left) and CD8+ (right) cells after BMT from MUD. Progressive appearance of CD45RA+cells (•) and reciprocal decrease of the proportion of cells with CD45R0+ phenotype (□). Lines represent the result of regression analyses for CD45RA+ (—) and CD45R0+ cells (---). The absolute number of CD45RA+ cells (white) increases progressively whereas that of CD45R0+ cells remains fairly constant (gray). Boxes represent 25th-75th percentile and horizontal lines within boxes represent the median. HC, healthy controls.
Proportions (top panels) and absolute numbers (bottom panels) of lymphocyte subsets identified by CD45 isoform expression among CD4+ (left) and CD8+ (right) cells after BMT from MUD. Progressive appearance of CD45RA+cells (•) and reciprocal decrease of the proportion of cells with CD45R0+ phenotype (□). Lines represent the result of regression analyses for CD45RA+ (—) and CD45R0+ cells (---). The absolute number of CD45RA+ cells (white) increases progressively whereas that of CD45R0+ cells remains fairly constant (gray). Boxes represent 25th-75th percentile and horizontal lines within boxes represent the median. HC, healthy controls.
A high proportion of activated T cells (CD3+HLA-DR+) was also observed in the first months after BMT (months 1-4 after BMT: median: 30% [25th-75th percentile: 12-42]v 3% [2-8] in healthy controls; P < .05), which progressively decreased (r = −.43;P < .02) with normalization of values after month +5.
Proliferative response to phytohemagglutinin (PHA) was reduced in the first months after BMT, and increased progressively (r = .68; P < .001), reaching normal values after month +8 (94,050 cpm [60,650 to 158,300] v 96,100 [59,150 to 122,600]; P = not significant [NS]). Similar data were observed in cultures stimulated with CD3 monoclonal antibody. The level of proliferative response was significantly correlated with the proportion of CD4+CD45RA+cells among lymphocytes (r = .61; P = .001), but not with that of CD8+CD45RA+ cells (r = .26; P = NS).
Taken together, these data suggest that the defective proliferative response observed in the first months after BMT is linked to the presence of primed/activated T cells and recovers in parallel with the regeneration of naive CD4+ T cells. Defective lymphocyte proliferation might be caused by increased cell death during the culture: in fact, peripheral blood lymphocyte regenerating after BMT are highly susceptible to spontaneous or “programmed” cell death,6 as a consequence of defective production of interleukin-2 (IL-2) and downregulation of Bcl-2,7 and to activation-induced cell death after restimulation with mitogens, strictly correlated with a high level of CD95/Fas expression.7 Therefore, the T-cell hyperactivated status accounts for their susceptibility to apoptosis and impaired ability to mount a proliferative response, contributing to the genesis of immunodeficiency that follows BMT.
However, our data show a fast regeneration of naive, normally functioning, CD4+ T cells after BMT from MUD and indicate that the lack of host/donor HLA diversity and the possibility to avoid the process of T-cell depletion in this setting allow a full T-cell reconstitution within 8 months from BMT in children affected by (S)CID. Moreover, these observations are in agreement with studies reporting that the ability to regenerate naive CD4+ T-cell number after allogeneic BMT or after intensive chemotherapy is optimal in children and inversely correlated with age.8,9 This can be explained by the essential role of the thymic-dependent pathway, still operating in the first years of postnatal life, but limited with advancing age, in the process of T-cell regeneration.9 10
Our observations are reflected clinically in a recent report of the European registry: overall survival of children with primary immunodeficiency after BMT from MUD approached that obtained with BMT from HLA-identical sibling,11 whereas it was much worse after BMT from an HLA-haploidentical family donor. Interestingly, in this latter group, the survival was very poor in patients with defective T-cell reconstitution but good in those who achieved a full T-cell reconstitution,11 thus confirming the prognostic relevance of obtaining normal T cells after BMT.
ACKNOWLEDGMENT
A.M. is the recipient of a grant from the Associazione Donatori Midollo Osseo.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal