Abstract
COS cell transfection has been used to monitor the assembly and secretion of fibrinogen molecules, both those of the subclass containing the novel E chain and those of the more abundant subclass whose chains lack E’s globular C-terminus. That region, referred to as the EC domain, is closely related to the ends of β and γ chains of fibrinogen (βC and γC). Transfection of COS cells with E, β, and γ cDNAs alone results in secretion of the symmetrical molecule (Eβγ)2, also known as Fib420. Cotransfection with cDNA for the shorter chain yielded secretion of both (βγ)2 and (Eβγ)2 but no mixed molecules of the structure E(βγ)2. Exploiting the COS cells’ fidelity with regard to Fib420 production, identification was made of the highly conserved Asn667 as the sole site of N-linked glycosylation in the E chain. No evidence from Cys → Ser replacements was found for interchain disulfide bridges involving the four cysteines of the EC domain. However, for fibrinogen secretion, the E, β, and γ subunits do exhibit different requirements for integrity of the two intradomain disulfide bridges located at homologous positions in their respective C-termini, indicating dissimilar structural roles in the process of fibrinogen assembly.
© 1998 by The American Society of Hematology.
FIBRINOGEN, THE protein that forms the matrix of a blood clot, is a complex molecule composed of paired sets of three subunits (α, β, and γ), each encoded by a separate gene. A few years ago we identified a subclass of native fibrinogen molecules based on α subunit differences.1 Molecules of this subclass are characterized by their greater mass (∼420 kD) and lower plasma levels relative to the more abundant form (∼340 kD), and we have coined the terms Fib420 and Fib340 to distinguish between them. Whereas the novel Fib420molecules’ three subunits all end in globular domains that resemble each other, those of the more commonly known subclass Fib340 have truncated α subunits that lack such a domain.
Although Fib420 molecules represent only one of every 100 fibrinogen molecules in the blood of normal, healthy adults,2 counterparts have been found throughout the vertebrate kingdom.3 4 This implies an important function for the novel subclass that is distinct from that of the more abundant Fib340, but that is as yet undefined.
Discovery of Fib420 in our laboratory evolved from the seminal finding that the complete sequence of the human gene encoding the α subunit contains an additional exon (exon VI) undetected by previous investigators.5 6 Alternative splicing to include exon VI gives rise to the isoform of the α chain (αE) with a globular carboxy-terminal extension. It is the 236 residues encoded by exon VI (technically the αEC domain, but often referred to as the VI-domain) that are as similar to the sequences of the C-terminal domains of the fibrinogen β and γ chains (βC and γC) as the latter two are to each other (40% identity); the truncated C-terminus of the common α chain (αC) has a very different character.
Early investigations with transfected COS cells seemed to suggest the obligatory presence of common α chains for the assembly and secretion of recombinant αE-containing fibrinogen.6 The subsequent unexpected finding that two αE chains are incorporated per molecule of Fib4201 prompted the current investigation using batches of COS cells that more closely resemble hepatic cells in their overall efficiency of fibrinogen assembly. In this study, we show that such cells incorporate two αE subunits per molecule of fibrinogen, whether the common α isoform is present or not. Further use of the system to explore the role of specific residues in Fib420 assembly and secretion was undertaken based on an extensive series of studies showing an absolute requirement for particular sets of interchain and intrachain disulfide bonds in assembly and secretion of the more abundant Fib340,7-10 which has 29 disulfide bonds in all and no free cysteine residues.11 In this study, we examine the roles of the four additional cysteines and two potential glycosylation sites contributed by each of the αEC domains unique to Fib420.
MATERIALS AND METHODS
The original α, β, and γ cDNAs of human fibrinogen were generous gifts from Dominic Chung (University of Washington, Seattle). Construction of vectors containing full-length cDNAs for the αE, α, β, and γ fibrinogen subunits has been described.6,12 If not otherwise indicated, pED4-Neo vectors (Genetics Institute, Cambridge, MA) were used.13 Cys → Ser mutations in the α, β, and γ chains were described previously.7,9,10 The N-terminal and “αC”-domain mutations of αE were derivatized from the α mutants as done previously.6 The mutations Cys → Ser, Cys → Ala, and Asn → Gln in the αE chain’s VI-domain were generated by polymerase chain reaction (PCR)-directed mutagenesis according to standard procedures. As before, all constructs were verified by sequencing.
Transient transfections of COS-1 cells were performed by the calcium phosphate method used earlier.12 To achieve αE synthesis comparable to that of the other subunits, an excess of αE-cDNA was generally added, as indicated. Qualitative evaluation of fibrinogen production was made by labeling the cells for 2 hours with [35S]methionine in the presence of 15 μg/mL heparin and 30 KIU aprotinin, then immunoprecipitating fibrinogen from cell lysates or culture medium with rabbit antibodies either to whole human fibrinogen (DAKO, Carpinteria, CA) or to the VI-domain of αE.1,6 12 The intact fibrinogen species were separated by sodium dodecyl sulfate (SDS)/4%PAGE under nonreducing conditions; separation of the component subunits was achieved by SDS/7.5% PAGE under reducing conditions. Signals were detected by autoradiography. The results reported here were confirmed with several different passages of COS cells.
RESULTS
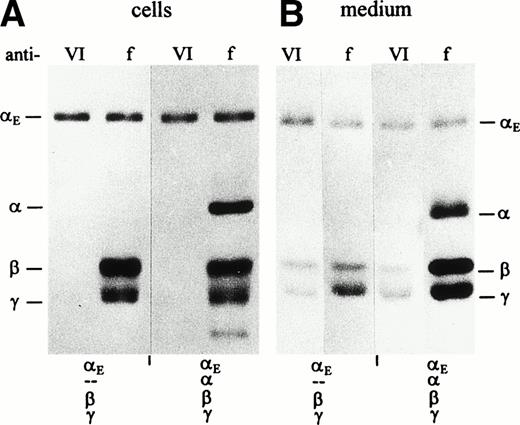
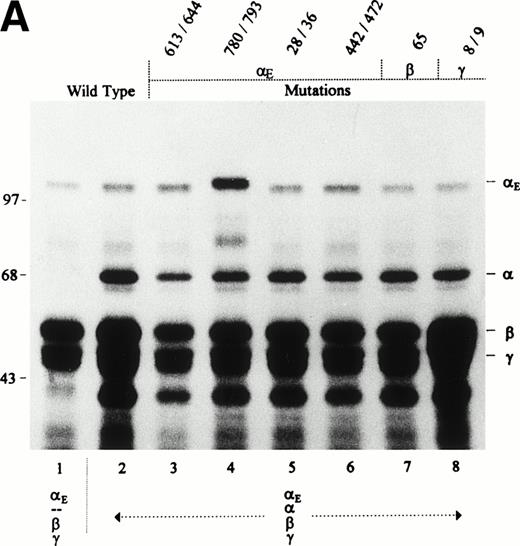
COS cells transfected simultaneously with either the αE/β/γ or the αE/α/β/γ sets of cDNAs synthesized all three or four fibrinogen subunits, respectively, as shown by SDS-PAGE analysis of cell lysates immunoprecipitated with antifibrinogen (Fig 1A). Predominantly αE chains were immunoprecipitated by anti-VI, indicating that most of the intracellular αE subunits are not assembled, ie, they exist as free polypeptides. Similar intracellular pools of free αE chains have been found in the human hepatocarcinoma cell line HepG2 (Grieninger et al, in preparation).
Synthesis and secretion of E- and -fibrinogen by transfected COS cells. Cells were transfected with stoichiometric proportions of pBC-vectors containing fibrinogen subunit cDNAs6 12 in combination as indicated below the lanes. When all four subunit cDNAs were cotransfected, E cDNA was included at a twofold excess. Fibrinogen was immunoprecipitated from cell lysates (A) and culture medium (B) with either antifibrinogen (f) or anti-VI (VI) as indicated above the lanes, and subunits were separated under reducing conditions. Migration of the γ chain as a doublet in cell extracts (see also Figs 5A and 6A) may reflect the presence of the nonglycosylated precursor.
Synthesis and secretion of E- and -fibrinogen by transfected COS cells. Cells were transfected with stoichiometric proportions of pBC-vectors containing fibrinogen subunit cDNAs6 12 in combination as indicated below the lanes. When all four subunit cDNAs were cotransfected, E cDNA was included at a twofold excess. Fibrinogen was immunoprecipitated from cell lysates (A) and culture medium (B) with either antifibrinogen (f) or anti-VI (VI) as indicated above the lanes, and subunits were separated under reducing conditions. Migration of the γ chain as a doublet in cell extracts (see also Figs 5A and 6A) may reflect the presence of the nonglycosylated precursor.
Transfected COS cells secrete mostly fully assembled fibrinogen hexamers.12 To identify αE-containing fibrinogen from among the other fibrinogen species, immunoprecipitation of culture medium with two antibodies was used. Anti-VI immunoprecipitated only those consisting of αE, β, and γ, whereas antifibrinogen collected every fibrinogen species, yielding a profile on reduced SDS-PAGE that included, when present, all four fibrinogen subunits αE, α, β, and γ (Fig 1B). In immunoprecipitates of the medium of cells transfected with αE/β/γ cDNAs alone, the three fibrinogen subunits were detected at roughly similar molar ratios, irrespective of the antibody used (anti-VI or antifibrinogen). Because anti-VI is highly specific for the αE chain (see Fig 1A, lanes 1 and 3; Fig 2, lane 3), immunoprecipitating β or γ only when bound to αE, it follows that the bulk of the αE secreted by these cells is packaged in fully assembled fibrinogen molecules. Cells transfected additionally with the cDNA for common α (the αE/α/β/γ-transfectants) secrete both α-fibrinogen and αE-fibrinogen as indicated by the presence of αE, β, and γ chains in the anti-VI immunoprecipitates and all four chains in the antifibrinogen immunoprecipitates. Although αE/α/β/γ-transfectants expressed significant amounts of the common α chain, none was found in their anti-VI–immunoprecipitated culture medium, suggesting that no mixed molecules of the structure ααE(βγ)2were exported.
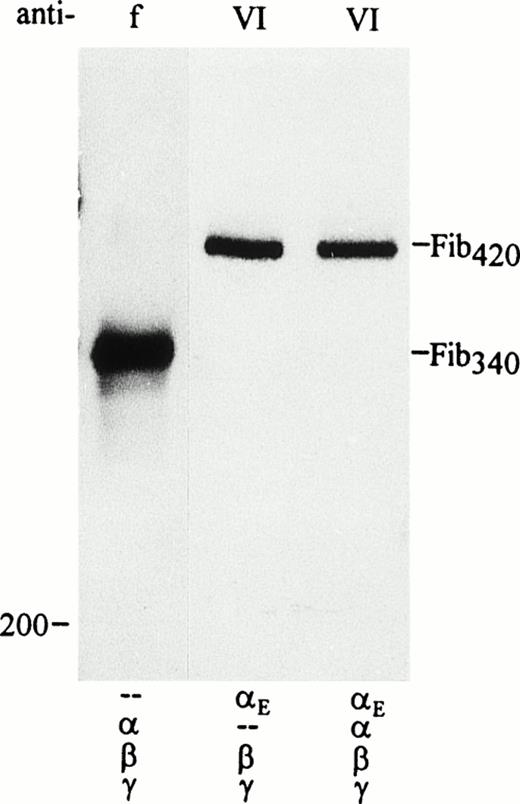
Secretion of Fib420 by transfected COS cells in the presence and absence of the common chain. Cells were transfected with pED4–Neo-vectors containing fibrinogen subunit cDNAs in combination as indicated below each lane; E cDNA was included at a fivefold excess. Fibrinogen was immunoprecipitated from culture medium with either antifibrinogen (f) or anti-VI (VI) as indicated and run under nonreducing conditions. Traces of incompletely assembled or single subunits could be seen in the lower part of the gel only after overexposure of the film, confirming that most of the chains detected in the medium upon reduction of the immunoprecipitates in Fig1B were indeed components of hexameric fibrinogen molecules.
Secretion of Fib420 by transfected COS cells in the presence and absence of the common chain. Cells were transfected with pED4–Neo-vectors containing fibrinogen subunit cDNAs in combination as indicated below each lane; E cDNA was included at a fivefold excess. Fibrinogen was immunoprecipitated from culture medium with either antifibrinogen (f) or anti-VI (VI) as indicated and run under nonreducing conditions. Traces of incompletely assembled or single subunits could be seen in the lower part of the gel only after overexposure of the film, confirming that most of the chains detected in the medium upon reduction of the immunoprecipitates in Fig1B were indeed components of hexameric fibrinogen molecules.
The issue of mixed versus symmetrical molecules was explored further by separating the secreted fibrinogen species under nonreducing conditions. As seen in Fig 2, αE/β/γ-transfectants secreted a high molecular weight species (Fib420) that is significantly larger than that of the α/β/γ-transfectants (Fib340). Most striking, however, is that only one αE-containing species is secreted when all four chains are cotransfected (αE/α/β/γ-transfectants); this product comigrates with Fib420. No mixed molecules, containing αE as well as α and therefore migrating at a position intermediate between Fib420 and Fib340, were detected.
These findings with heterologous host cells support our earlier hypothesis that formation of symmetrical fibrinogen molecules, (αEβγ)2, is energetically favored over that of the mixed molecules ααE(βγ)2,1 driven perhaps by alternative disulfide bond configurations either between the two αE chains’ VI-domains to form a fourth nodule and/or connecting the VI-domains to N-terminal cysteines that could belong, in principle, to any subunit chain, even αEitself. The latter would serve to tether the VI-domains to the central nodule, consistent with the tetranodular images observed in many published electron micrographs of fibrinogen.14-16
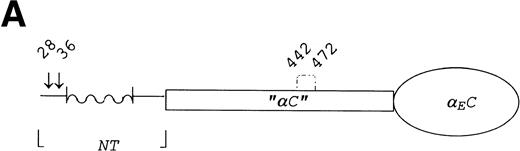
To test this alternative disulfide bond hypothesis, we looked for qualitative changes in the fibrinogen species secreted by COS cells that had been transfected with subunit cDNAs coding for either wild-type chains or chains in which specific cysteines were converted to serines (or alanines) by site-directed mutagenesis. All four cysteines in the VI-domain of human αE were substituted, individually and in combination; changes in the N-terminus and in the “αC” region of the αE chain were also introduced (illustrated in Fig 3). In addition, N-terminal substitution mutants at sites in the β and γ chains (β65S and γ8S/9S) were evaluated for their potential contribution to Fib420 formation.
Schematic representation of targeted amino acid positions in the E subunit for site-directed mutagenesis. Residues are numbered as done previously.6 Arrows or simple vertical lines indicate positions of those cysteines converted, either singly or in combination, to serine or alanine by site-directed mutagenesis. (A) The three regions of the E chain: the N-terminus (NT) containing a large -helical segment, the so-called “C” region, and the C-terminal globular VI-domain (EC domain); (B) the VI-domain, potential glycosylation sites in the wild-type sequence at Asn667 and Asn812 are marked, respectively, with closed and open diamonds. The glycosylation site at Asn791 introduced by the Cys793 → Ser change is marked with a striped diamond. Putative loops connecting cysteines E613/644 as well as E780/793 are drawn by analogy with the intrachain loops formed by homologous cysteines in the βC and γC domains.
Schematic representation of targeted amino acid positions in the E subunit for site-directed mutagenesis. Residues are numbered as done previously.6 Arrows or simple vertical lines indicate positions of those cysteines converted, either singly or in combination, to serine or alanine by site-directed mutagenesis. (A) The three regions of the E chain: the N-terminus (NT) containing a large -helical segment, the so-called “C” region, and the C-terminal globular VI-domain (EC domain); (B) the VI-domain, potential glycosylation sites in the wild-type sequence at Asn667 and Asn812 are marked, respectively, with closed and open diamonds. The glycosylation site at Asn791 introduced by the Cys793 → Ser change is marked with a striped diamond. Putative loops connecting cysteines E613/644 as well as E780/793 are drawn by analogy with the intrachain loops formed by homologous cysteines in the βC and γC domains.
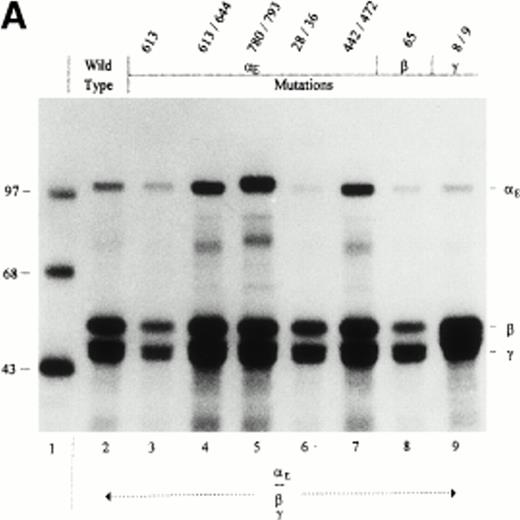
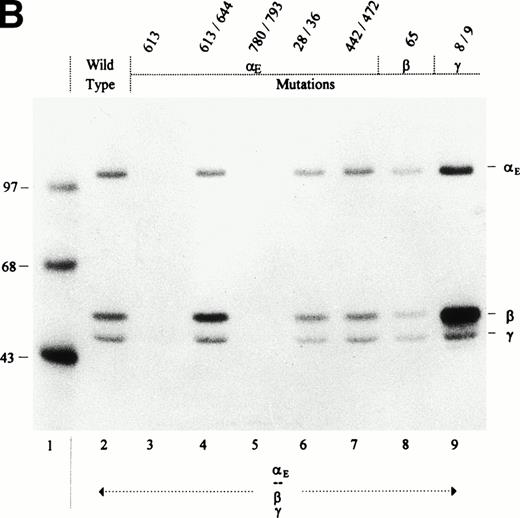
To establish whether any of the above cysteine mutations per se affected Fib420 secretion, they were initially examined in αE/β/γ-transfectants, ie, in the absence of the major α chain (Fig 4). All mutant fibrinogen subunits were expressed, as shown by analysis of cell lysates (Fig 4A). Despite some variation in the amount of fibrinogen secreted by the different mutants, it is clear that αE613S and αE780S/793S substitutions abolished secretion of Fib420 (Fig 4B). Although the double mutation αE613S/644S, which knocks out the putative first loop in the VI-domain, has no discernable effect on Fib420secretion, the single αE613S mutant and its mutated disulfide bond partner (αE644S; Table 1) are inhibitory. In either of these single mutations, a reactive thiol group introduced by an unpaired cysteine appears to interfere nonspecifically with the assembly and/or secretion process. Of note, the αE780S/793S mutation creates a neo–N-glycosylation site (NNS) at Asn791. That carbohydrate is attached to this site can be seen from the upward mobility shift of αE780S/793S in Fig 4A, lane 5. To test whether the extra carbohydrate chain is responsible for blocking secretion, we changed Cys793 to alanine instead of serine in mutant αE780S/793A. This particular mutation also blocked secretion (Table 1), indicating that the missing (second) intrachain loop and not the carbohydrate attachment is responsible for lack of Fib420 export in αE780S/793S mutants.
Cys → Ser changes in the E, β, and γ subunits: Negative and neutral effects on the secretion of Fib420. Cys → Ser changes were made in the fibrinogen subunits at the positions indicated above the lanes. COS cells were transfected with pED4-vectors containing E, β, and γ (either wild-type or mutant) cDNAs as shown; Econstructs were included at a fivefold excess. Fibrinogen was immunoprecipitated, from cell lysates (A) and culture medium (B), with antifibrinogen and run under reducing conditions. The size of protein markers (lane 1) is displayed in kD in the left margin.
Cys → Ser changes in the E, β, and γ subunits: Negative and neutral effects on the secretion of Fib420. Cys → Ser changes were made in the fibrinogen subunits at the positions indicated above the lanes. COS cells were transfected with pED4-vectors containing E, β, and γ (either wild-type or mutant) cDNAs as shown; Econstructs were included at a fivefold excess. Fibrinogen was immunoprecipitated, from cell lysates (A) and culture medium (B), with antifibrinogen and run under reducing conditions. The size of protein markers (lane 1) is displayed in kD in the left margin.
Effect of E Subunit Mutations on Secretion of Fib420 in COS Cells Transfected With E, β, and γ cDNAs
| Mutation . | Fib420 Secretion . |
|---|---|
| Wild-type | + |
| 28-S and 36-S | + |
| 442-S and 472-S | + |
| 613-S | − |
| 644-S | − |
| 780-S | − |
| 793-S | − |
| 613-S and 644-S | + |
| 780-S and 793-S | − |
| 780-S and 793-A | − |
| 613-S and 780-S | − |
| 644-S and 793-S | − |
| 613-S, 644-S, 780-S, and 793-S | − |
| 667-Q | + |
| 812-Q | + |
| Mutation . | Fib420 Secretion . |
|---|---|
| Wild-type | + |
| 28-S and 36-S | + |
| 442-S and 472-S | + |
| 613-S | − |
| 644-S | − |
| 780-S | − |
| 793-S | − |
| 613-S and 644-S | + |
| 780-S and 793-S | − |
| 780-S and 793-A | − |
| 613-S and 780-S | − |
| 644-S and 793-S | − |
| 613-S, 644-S, 780-S, and 793-S | − |
| 667-Q | + |
| 812-Q | + |
Cys → Ser (S); Cys → Ala (A); Asn → Gln (Q).
In addition to the VI-domain cysteines, we evaluated substitutions of cysteines in other parts of the Fib420 molecule that might be available for alternative disulfide bonding with the VI-domain, reasoning that those cysteines were not found to be critical for export of Fib340. The first pair of these was further upstream in the αE chain, Cys442 and Cys472, which correspond to positions in the αC region of the common α chain known to form an intrachain loop10; mutant αE442S/472S had no effect on Fib420 secretion in αE/β/γ-transfectants (Fig 4). Other candidate cysteines that we evaluated, located in the N-termini of the constituent fibrinogen chains (αE Cys28 and Cys36, β Cys65, and γ Cys8 and Cys9), are involved in forming the symmetrical disulfide bridges that hold the αβγ trimers together; only one out of the four symmetrical bonds is absolutely required for Fib340 hexamer assembly and secretion.7,8 10None of these mutations, αE28S/36S, β65S, or γ8S/9S, when cotransfected with complementary wild-type chains, interfered with proper secretion of Fib420molecules in the αE/β/γ-transfectants (Fig 4).
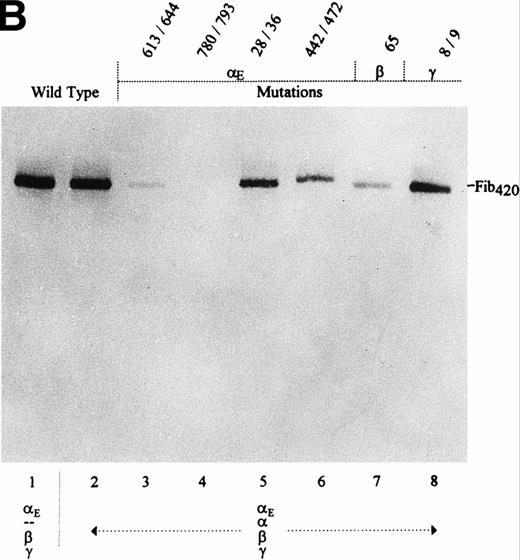
Having established that Fib420 production was possible despite substitution of particular residues, the same mutations were examined in αE/α/β/γ-transfectants. This test of the alternative disulfide bond configuration hypothesis could be expected to yield secretion of mixed molecules (containing both αE and α) whenever cysteines involved in bonds favoring homodimer formation were replaced. For each set of mutations, it was first shown that simultaneous transfection with all four cDNAs leads to expression of all four subunits (Fig 5A) and that for the subunits comprising Fib420, ie, the αE, β, and γ chains, the levels of expression were comparable to those of the αE/β/γ-transfectants (Fig4A). The medium of the transfected cells was then immunoprecipitated with anti-VI for detection of secreted αE-containing fibrinogen (Fig 5B). Although secretion with mutated subunits was less efficient, in no case were mixed molecules detectable as species migrating faster than that of the αE/β/γ-transfectant. It follows that selective incorporation of two αE subunits in the presence of the abundant α chain is not dependent on disulfide bridges involving cysteines in the VI-domain of αE or on available cysteine partners further upstream in the αE chain itself or in the other two subunits present in the central core of the fibrinogen molecule. These results with recombinant molecules are consistent with trypsin digests of α′-fibrinogen,4 the lamprey equivalent of Fib420, which appears to be a similarly symmetrical molecule but which has αE-like chains derived from a separate α′ gene17 instead of being alternatively spliced α gene products as in higher vertebrates.3 6
Effect of E, β, and γ subunit mutations on Fib420 secretion in presence of the wild-type subunit: No secretion of mixed molecules. Cys → Ser changes were made in the fibrinogen subunits at the positions indicated above the lanes. COS cells were transfected with either wild-type or mutant E, β, and γ cDNAs together with wild-type cDNA in the combinations shown; E constructs were included at a fivefold excess. Fibrinogen was immunoprecipitated from cell lysates (A) with antifibrinogen and run under reducing conditions; from the culture medium (B), fibrinogen was immunoprecipitated with anti-VI and run under nonreducing conditions. As described previously,1due to the differential proteolytic susceptibility of and E subunits in cell lysates, a proteolytic fragment derived only from the chain, not from E, appears as a band migrating below the γ chain doublet in panel A (compare also Fig 1A).
Effect of E, β, and γ subunit mutations on Fib420 secretion in presence of the wild-type subunit: No secretion of mixed molecules. Cys → Ser changes were made in the fibrinogen subunits at the positions indicated above the lanes. COS cells were transfected with either wild-type or mutant E, β, and γ cDNAs together with wild-type cDNA in the combinations shown; E constructs were included at a fivefold excess. Fibrinogen was immunoprecipitated from cell lysates (A) with antifibrinogen and run under reducing conditions; from the culture medium (B), fibrinogen was immunoprecipitated with anti-VI and run under nonreducing conditions. As described previously,1due to the differential proteolytic susceptibility of and E subunits in cell lysates, a proteolytic fragment derived only from the chain, not from E, appears as a band migrating below the γ chain doublet in panel A (compare also Fig 1A).
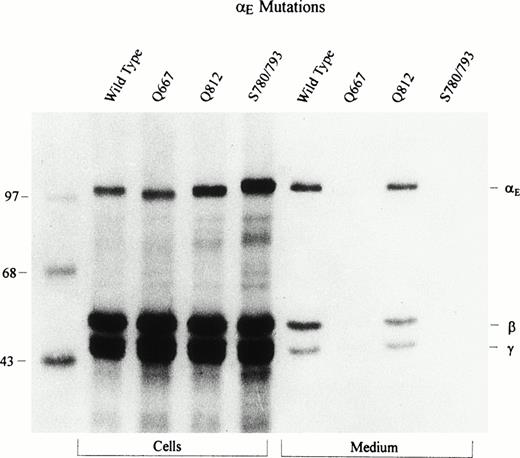
In contrast to the predominant human α chain, which has no carbohydrate, human αE is N-glycosylated.1 We knocked out each of the two potential glycosylation sites, αE667 and αE812, by changing asparagines to glutamines. These mutated αE chains showed a mobility shift comparable in magnitude to that fortuitously generated in mutant αE780S/793S with its second active glycosylation site. Examination of lysates of αE/β/γ-transfectants in Fig 6 revealed that the mutant αE812Q comigrates with wild-type αE, whereas αE667Q migrates faster, suggesting that normally carbohydrate is attached to Asn667 and not to Asn812. αE780S/793S, as noted above, is larger than wild-type αE because of an additional carbohydrate moiety at Asn791. Thus, the stepwise increase in molecular weight of αE667Q, αE812Q, and αE780S/793S reflects the attachment of zero, one, and two carbohydrate chains, respectively.
Mutation of potential N-glycosylation sites in the E VI-domain: Determination of carbohydrate attachment site. The VI-domain’s two potential glycosylation sites, at Asn667 and Asn812, were changed to Gln; a new site at Asn791 had been introduced by the Cys793 → Ser change. COS cells were transfected with either wild-type or these mutant E cDNAs together with the β and γ subunit cDNAs. Fibrinogen was immunoprecipitated from cell lysates and culture medium with antifibrinogen and run under reducing conditions. Upon overexposure of the film, Fib420 subunits are clearly detectable in the culture medium of the Q667 mutant.
Mutation of potential N-glycosylation sites in the E VI-domain: Determination of carbohydrate attachment site. The VI-domain’s two potential glycosylation sites, at Asn667 and Asn812, were changed to Gln; a new site at Asn791 had been introduced by the Cys793 → Ser change. COS cells were transfected with either wild-type or these mutant E cDNAs together with the β and γ subunit cDNAs. Fibrinogen was immunoprecipitated from cell lysates and culture medium with antifibrinogen and run under reducing conditions. Upon overexposure of the film, Fib420 subunits are clearly detectable in the culture medium of the Q667 mutant.
In mutant αE667Q, secretion of Fib420 is reduced to less than 20% of wild-type levels (Fig 6; upon overexposure of the film the subunits are clearly detectable), suggesting possible involvement of carbohydrate attachment at this site in secretion. However, from an earlier finding that Fib420 secretion was not appreciably inhibited when tunicamycin blocked N-glycosylation in HepG2 cells,1 it is apparent that reduced secretion of αE667Q mutant Fib420 results not from the absence of sugar but rather from a conformational change introduced by replacing Asn with Gln.
DISCUSSION
This study shows, in transfected COS cells, that formation of secreted αE-containing fibrinogen hexamers is possible with the αE, β, and γ subunit building blocks alone. Importantly, the predominant secreted species of αE-containing fibrinogen is the homodimeric (αEβγ)2, even in the presence of the common α chain (ie, in αE/α/β/γ-transfectants); no mixed molecules of the composition ααE(βγ)2 are observed (Figs 1 and 2). In other words, the heterologous COS cell system mimics the hepatic Fib420 assembly process as evaluated in the cell line HepG2, where αE-homodimeric fibrinogen molecules are formed preferentially in the presence of an abundant supply of α chains, the αE:α chain ratio being roughly 1:20.1
This investigation of human αE-containing fibrinogen contradicts prior notions regarding the obligatory presence of common α chains for assembly and secretion.6 Inability of cells used in previous COS experiments to match the performance of hepatoma cells (HepG2), which assemble αE and α chains into fibrinogen with equal efficiency, may account for the anomalous earlier findings. Exploiting the current COS cells’ fidelity with regard to Fib420 synthesis and secretion, studies were extended to analyze the molecule’s glycosylation sites as well as the requirement for specific cysteines in connection with its secretion.
Of the two potential glycosylation sites in the Fib420’s unique αEC domain, the tripeptide at Asn667 is conserved in all vertebrates, including lamprey, whereas the one at Asn812 is conserved only in mammals.3 17 By substituting Gln for Asn we have now shown that it is the highly conserved Asn667 site that has carbohydrate attached in the αE chain of human Fib420 (Fig 6). Thus, Fib420 has a total of six carbohydrate moieties among its six component chains. All of the sites have the consensus tripeptide sequence NXT, with X being a positively charged residue: arginine in α and β, lysine in γ. Whereas the γ chain bears carbohydrate in a more upstream region of the chain, both αE and β chains use sites in the C-terminal region. Although the αEC and βC attachment sites do not align with each other, they are both located between the homologous first and second disulfide loops.
With its four extra cysteines per αEC domain, Fib420 has the potential to form 33 disulfide bonds. The positions of the four cysteines in αEC are invariant among αE homologs throughout vertebrate evolution and align precisely with cysteines in the βC and γC domains,3,6 17 each of which has two intrachain disulfide bridges. The results from substituting serine or alanine residues for the VI-domain cysteines (Figs 4 and 5, Table 1) are consistent with the existence of similar bonds between the cysteine pairs at αE613/αE644 and at αE780/αE793. The finding that the individual αE613S and αE644S mutations block secretion but not the double mutation αE613S/644S strongly suggests that there is an intrachain loop formed by Cys613 and Cys644. Fib420 secretion is prevented by double mutation of the cysteines presumably forming the domain’s second disulfide loop (αE780/793), indicating a vital role for them, and not those of the potential first loop, in Fib420 export from the cell.
A number of protein sequences have been identified that bear C-terminal domains homologous to αEC, βC, and γC. In this family of fibrinogen-related domains (FREDs),18 which contains at least 14 unique members at latest count, the cysteines of the first and second disulfide loops are preserved. With only one exception,19 the 12 residues of the second loop are highly conserved; those of the first loop, varying in number from 24 to 30, constitute the sequence with the lowest conservation in the entire family of FREDs. In the context of our findings regarding Fib420 secretion, the second loop’s higher degree of conservation may reflect a more critical role in secretion of the parent molecule.
It should be mentioned that the C-terminal region of the β chain actually has three intrachain disulfide bonds. In the large loop formed by cyteines β201/286, which is essential for secretion,10only β Cys286 is part of the βC domain proper.6Although the loop is conserved among all vertebrate fibrinogen β chains, there is no cysteine corresponding to β Cys286 in any other member of the FRED family, implying a highly specific function.
Structurally, the αE chain differs significantly from the β and γ chains by virtue of the large “αC” region (identical to the truncated C-terminus of the common α chain) that tethers the subunit’s globular C-terminus (αEC) to its α-helical N-terminus. Overall, the “αC” tether, encoded by exon V of the α gene, is poorly conserved among the fibrinogens of higher vertebrates.20 However, the disulfide loop it contains, homologous to the human cysteines at positions 442/472, is highly conserved, although it does not play a role in either Fib340 or Fib420 secretion10 (Figs4 and 5). An apparent conformational change in Fib420occurs in the αE442S/472S mutant as a result of eliminating a disulfide-bridged loop, and it is reflected, even in this high-molecular-weight range, in distinctly slower migration on SDS-PAGE relative to all the other species that were generated (Fig 5). This is consistent with the slower migration noted previously of the α442S/472S mutant relative to wild-type α chains under nonreducing SDS-PAGE conditions10 and gives credence to the hypothesis that the loops in the “αC”-region of αE make the Fib420 molecule more compact. It remains to be seen whether the α442S/472S Fib420 mutant displays neoepitopes and/or is more susceptible to proteolytic attack.
Based on sequence comparison,6 the globular fold of the αEC domain is expected to be very similar to the folds of the βC and γC domains, which were recently determined at atomic resolution and which were found to be virtually superimposable.21-23 However, the dependence of secretion on the integrity of the two homologous intradomain disulfide bridges is distinctly different for each subunit. In the βC region, only the first loop is required; in the αEC region, it is the second loop; and in the γC region both loops play a critical part. These observations support different structural roles for the subunits in the process of fibrinogen assembly, a notion put forward more than a decade ago.24 25
The mechanism that favors formation of the symmetrical Fib420 molecule over that of a mixed αE/α-containing molecule in the presence of excess α chains is not known at present. We previously speculated that alternative disulfide bridges, either between the VI-domains and/or the VI-domain and the center of the molecule, might provide the impetus for homodimer formation. Without directly determining disulfide bridges between particular cysteines, the experiments using αE/α/β/γ-transfectants (Fig 5) do not support that hypothesis; specific mutations in αE’s VI-domain and the N-termini of all the αE, β, and γ chains, designed to remove cysteines potentially available for alternative disulfide bridges, failed to reduce production of (αEβγ)2 in favor of mixed ααE(βγ)2 molecules. Thus, a different mechanism based on noncovalent interactions must be advanced to explain symmetrical incorporation of αE chains into Fib420 against all stoichiometric odds. Given the high negative charge borne by each αEC domain,6 it is conceivable that chaperone proteins associated with nascent fibrinogen26 27 may play a critical role in balancing the spatial charge distribution.
NOTE ADDED IN PROOF
As this report was being processed for publication, the assignments of bound cysteine pairs and carbohydrate attachment site in the αEC domain were confirmed by x-ray crystallographic analysis of a recombinant version of the domain.28
ACKNOWLEDGMENT
We thank our colleague K.M. Hertzberg for many valuable contributions to the manuscript.
Supported in part by the National Institutes of Health through grants to G.G. (HL 51050) and C.M.R. (HL 37457) and by the American Heart Association through a grant to G.G.
Address reprint requests to Gerd Grieninger, PhD, New York Blood Center, 310 E 67th St, New York, NY 10021; e-mail:ggrien@server.nybc.org.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal