Abstract
Infection with the human immunodeficiency virus (HIV) leads to a decrease in CD4+ T cells and disease progression within a decade of seroconversion. However, a small group of infected people, despite being infected by HIV for 10 or more years, remain clinically asymptomatic and have stable CD4+ cell counts without taking antiretroviral medication. To determine why these individuals, known as long-term survivors (LTS), remain healthy, the hematological profiles, viral load and properties, HIV coreceptor genotype, and anti-HIV immune responses of these people were compared with those of individuals who have progressed to disease (Progressors) over the same time period. Unlike Progressors, LTS have a low circulating viral load and a low number of HIV-infected cells. These differences in the levels of the viral load were not associated with a dominant biologic viral phenotype, varying growth kinetics of the virus, mutation in the cellular CCR5 gene, or the presence of neutralizing antibodies. Importantly, the difference in viral load could be explained by the enhanced ability of CD8+ cells from LTS to suppress HIV replication.
© 1998 by The American Society of Hematology.
STUDIES SUGGEST THAT 75% of human immunodeficiency virus (HIV) infections will lead to either a symptomatic clinical state or acquired immunodeficiency syndrome (AIDS) within 10 years.1-3 Typically, these individuals have a range of immune defects, high viral loads, and decreasing CD4+ cell counts.4 However, about 20% of infected people will remain asymptomatic for more than 10 years2 and a quarter of these will have stable CD4+ cell counts above 500 cells/μL without taking any antiretroviral medication.5 This latter group of asymptomatic individuals, who have been classified as long-term survivors (LTS)6 or long-term nonprogressors,7,8 represent an important group to study because of their potentially unique virological and immunological features. A number of parameters have been examined, but only a few characteristics have been found associated with a long-term healthy state after HIV infection. These include a correlation of a long-term asymptomatic clinical state with a low viral load,7,8 a 32-bp deletion in the CCR5 coreceptors gene,9 or differences in the biological features of the virus (eg, noncytopathic and cytopathic).10-12 Determination of attributes unique to LTS may provide insight into approaches to prevent the development of AIDS. The current studies were undertaken to evaluate several virological and immunological parameters in a well-defined group of LTS compared with an age-, race-, and sex-matched group of Progressors infected for the same time period (10 years).
MATERIALS AND METHODS
Study subjects.
All HIV seropositive individuals in this study were infected with HIV for 10 years or longer. During the initial visit a medical history was taken. Subjects were monitored every 2 to 3 months for changes in symptoms. A physical examination and the hematological profile, including CD4+ and CD8+ cell counts, were assessed at the time of each visit. HIV seronegative blood donors were provided by Irwin Memorial Blood Centers (San Francisco, CA) or randomly selected volunteers at the University of California at San Francisco (UCSF). All studies were approved by the Committee on Human Research, UCSF.
Blood samples.
Blood from LTS and Progressors was collected by venipuncture into Vacutainer tubes containing either EDTA or sodium heparin (Becton Dickinson, Franklin Park, NJ). EDTA-treated blood was used for complete blood and differential cell counts and flow cytometric studies. Blood containing heparin as an anticoagulant was used for the virological and immunological assays. All plasma samples were obtained and frozen away (−70°C) within 2 hours of drawing the blood from the subjects to prevent the loss of infectious virus.13
Flow cytometry.
Lymphocyte and CD8+ cell populations in the peripheral blood of LTS and Progressors were analyzed by flow cytometry using dual-color direct immunofluorescent staining of blood followed by red blood cell (RBC) lysis.14 A single laser flow cytometer (FACScan; Becton Dickinson), which discriminates forward and side light scatter, and two-color fluorescence was used with the Lysys II computer software program (Becton Dickinson) for analysis. Lymphocyte gates were confirmed by anti-CD45 and anti-CD14 fluorochrome antibody combination. Complete blood counts and differentials were performed by the Clinical Laboratories at UCSF using standard procedures.
Isolation of peripheral blood mononuclear cells, CD4+cells, and CD8+ cells.
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque (Sigma, St Louis, MO) gradient centrifugation of heparinized venous blood.15 The CD4+ and CD8+ cells were isolated from PBMC by positive selection using magnetic beads bearing anti-CD4 and anti-CD8 monoclonal antibodies (MoAbs) (Dynal, Lake Success, NY).16 Beads were removed from the cells by Detach-a-bead (Dynal) according to the manufacturer’s instructions. The purity of the cells obtained by the immunomagnetic bead isolation procedure was ≥95% CD4+, <1% CD8+, <1% CD19+, <1% CD56+, and <1% CD14+ for CD4+selected cells and ≥95% CD8+, <1% CD4+, <1% CD19+, <1% CD56+, and <1% CD14+ for CD8+ selected cells as determined by flow cytometry.14
Culture medium and reagents.
RPMI 1640 medium (BioWhittaker; Walkersville, MD) supplemented with 10% heat-inactivated (56°C, 30 minutes) fetal bovine serum (FBS) (Gemini Bioproducts, Calabasas, CA), 2 mmol/L glutamine (BioWhittaker), 100 U/mL penicillin, and 100 μg/mL streptomycin (BioWhittaker) was used as culture medium. PBMC and purified CD4+ cells (3 × 106/mL) were activated with 3 μg/mL of phytohemagglutinin (PHA; Sigma) in culture medium containing 10% natural IL-2 (T-stim [Collaborative Biomedical Products, Bedford, MA] with ≈20 U/mL of IL-2) or 10 U/mL of recombinant human IL-2 (Collaborative Biomedical Products). To help facilitate infection of PBMC and CD4+ cells, polybrene (Sigma; 2 μg/mL) in the culture medium was added to the cells (3 × 106/mL) 30 minutes before inoculation of virus.
Virus levels in the plasma of LTS and Progressors.
HIV RNA levels in the plasma of LTS and Progressors were determined using quantitative-competitive polymerase chain reaction (QC-PCR), described previously.17 The lowest level of detection of virus load in the plasma using this assay is 50 viral RNA copies/mL. The level of infectious virus in the plasma of LTS and Progressors was determined as reported.13 The p24 antigen levels in the plasma of the study subjects were measured by p24-specific enzyme-linked immunosorbent assay (ELISA) according to the methods outlined by the manufacturer (Coulter, Miami, FL).
Infectious center assay.
The frequency of infected cells in the peripheral blood of LTS and Progressors was determined using the infectious center assay.18,19 The results are presented as the frequency of infected cells in total PBMC which induced a positive reverse transcriptase (RT) activity20 (≥104 cpm/mL of culture fluid) in the cultured target human PBMC.
Isolation and determination of the biological phenotype of primary HIV isolates.
Two tissue culture systems (termed A-culture and B-culture) were used to measure the production of HIV from PBMC of LTS and Progressors.21 Briefly, in the A-culture, the PBMC of HIV-infected individuals were activated by PHA for 3 days followed 4 days later by the addition of PHA-stimulated PBMC from HIV-seronegative donors. In the B-culture, the PBMC from HIV-infected subjects (not activated in vitro) were cocultured with 3 × 106PHA-stimulated PBMC from uninfected donors. Primary virus isolates obtained from both these cultures were expanded in the PBMC from normal donors as described elsewhere.22 The 50% tissue culture infectious dose (TCID50) of a virus isolate was determined as described.23 The biological phenotype of the virus isolated from LTS and Progressors was determined by inoculation onto MT-2 cells.11,24 The virus was classified as syncytium-inducing (SI) phenotype if the inoculated MT-2 cells had a diameter of greater than 3 normal cells and the culture had RT activity20 of >104 cpm/mL of culture fluid.
Detection of the CCR5 Δ32 mutation.
Cells from each clinical specimen were resuspended in extraction buffer (10 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, 0.05% Tween 20, 0.05% NP40, 0.1 mg/mL Proteinase K) to a final cell density of 2 × 103 cells/μL and incubated at 100°C for 30 minutes. The cell lysate was then diluted 10-fold with specimen extraction buffer without Proteinase K and stored at −70°C. Each 100-μL polymerase chain reaction (PCR) consisted of a cell lysate from ≈104 cells or 50 ng genomic DNA in 50 mmol/L KCl, 10 mmol/L Tris-HCl, pH 8.3, 2 mmol/L MgCl2, 0.1 mmol/L dATP, dGTP, dCTP, 0.2 mmol/L dUTP, 0.4 μmol/L each primer, SYC658 and SYC659, 5 U AmpliTaq (Perkin-Elmer Corp, Norwalk, CT), and 2 U Amperase UNG (Perkin-Elmer). PCR conditions were 50°C for 2 minutes, 95°C for 1 minute, followed by 30 cycles of 95°C for 20 seconds and finally holding at 72°C, using the Perkin-Elmer Thermocycler 9600. A 177-bp and a 145-bp PCR product were amplified from wild-type (+) allele and Δ32 allele, respectively. The CCR5 Δ32 mutation was determined by analyzing 5 μL of each PCR reaction on a 3% NuSieve (FMC Bioproducts, Rockland, ME) and 1% agarose gel. The frequency of homozygous mutations in the CCR5 gene in the uninfected population has been reported to be 1%.25
Antibody neutralization assay.
The ability of plasma to neutralize primary HIV isolates was determined as described.26 The neutralization of HIV was assessed in duplicate wells using primary virus isolates (100 TCID50) mixed with serially diluted autologous or heterologous plasma. Plasma from some HIV-infected subjects was tested against more than one heterologous virus isolate. Plasma from HIV-seronegative donors was used as negative controls. Neutralization of virus by plasma was considered positive if a ≥66% reduction was observed in the RT activity, compared with the RT activity of control cultures containing plasma from HIV uninfected individuals.
CD8+ cell noncytotoxic anti-HIV response.
The extent to which the CD8+ cells from an infected subject can suppress HIV replication was determined by an acute infection assay27 using the cytopathic, syncytium-inducing, and β-chemokine-insensitive HIV-1SF33 strain.28This assay was chosen to measure CD8+ cell antiviral activity instead of an endogenous assay (ie, using naturally infected cells)16 because of the difficulties of obtaining CD4+ cells from Progressors. Briefly, PHA (3 μg/mL) stimulated CD4+ cells from HIV-seronegative donors were infected with 10,000 × TCID50 of HIV-1SF33. After 1 hour of incubation, the CD4+ cells were washed three times and mixed with CD8+ cells that were isolated from PBMC stimulated with PHA for 3 days before the acute assay was conducted. The acute assay was performed in the presence of 100 U/mL of recombinant human interleukin-2 (IL-2; Collaborative Biomedical Products). The ability of CD8+ cells to suppress HIV replication in 5 × 105 infected CD4+ cells was evaluated over a range of CD8+:CD4+ cell ratios from 0.25:1 to 4:1 (twofold dilutions) in 24-well tissue culture plates (Falcon, Lincoln Park, NJ). Culture fluid samples taken every 3 days were monitored for RT activity. The percent suppression was determined by comparing the RT activity in the culture fluids of the CD8+ and CD4+ cell cocultures with the RT activity of fluids from the infected CD4+ cells cultured alone at the time of peak virus production (6 days following initiation of the assay).16 27 The RT activity from the control-infected cultures was always ≥105 cpm/mL of culture fluid.
Statistical analyses.
The Mann-Whitney U-test was used to determine statistical significance of all studies except the evaluation of neutralizing antibodies and Δ32 mutations in CCR5 which used the Fisher Exact Test. Spearman’s coefficient of rank correlation was used to compare the CD8+ cell antiviral response with the viral load of LTS and Progressors. P values of ≤.05 were considered statistically significant.
RESULTS
Characteristics of the study subjects.
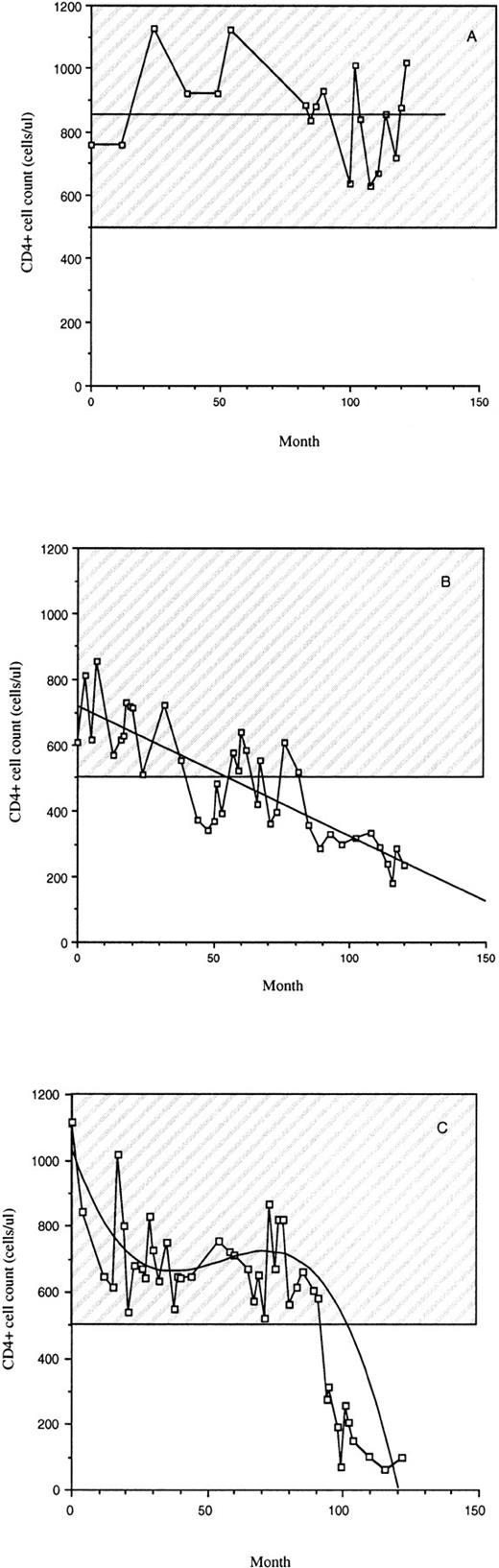
The subjects involved in this study were between 30 and 65 years old with a median age of 44. Most were white and nearly all were male. All of the subjects were infected for at least 10 years spanning the same period in time (±2 years) (Table 1). During their infection period, LTS remained clinically healthy with a stable CD4+ cell count above 500 cells/μL (Table 1 and Fig 1A). All Progressors at the time of the study had an AIDS diagnosis based on the 1993 Centers for Disease Control (USA) case criteria29 with <200 CD4+cells/μL (Fig 1). Progressors either had a slow decrease in CD4+ cell counts since infection (Fig 1B) or a high level of CD4+ cells for about 8 years followed by a decrease (Fig 1C). None of the LTS had received antiretroviral drugs (Table 1). Seventeen of the 21 Progressors were taking antiretroviral medication (nucleoside reverse transcriptase inhibitors), which were taken alone or in combination (Table 1). None of the subjects was taking HIV protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Subjects from these groups of LTS and Progressors were chosen at random for all the studies described.
Demographics of Study Subjects
| . | Long-Term Survivors . | Progressors . |
|---|---|---|
| No. of subjects | 21 | 21 |
| Years of age (mean ± SD) | 43 ± 6 | 44 ± 9 |
| Range of age | 36-58 | 30-65 |
| Race | 17 Whites | 20 Whites |
| 2 African Americans | 1 African American | |
| 1 Asian | ||
| 1 Hispanic | ||
| Gender (male/female) | 19/2 | 21/0 |
| Year of probable infection (mean year ± SD) | 1981 ± 2 yr | 1982 ± 2 yr |
| Year tested seropositive (mean year ± SD) | 1985 ± 1 yr | 1986 ± 2 yr |
| Symptoms | 21 Asymptomatic | 8 Kaposi’s Sarcoma |
| 5Pneumocystis carinii pneumonia | ||
| 2 Thrush | ||
| 2 Hairy leukoplakia | ||
| 2 Night sweats | ||
| 1 Mycobacterium aviumcomplex | ||
| 2 Toxoplasmosis | ||
| 1 Neuropathy | ||
| 2 Chronic diarrhea | ||
| 2 Low-grade fever | ||
| 21 Absolute CD4+ cell count <200 cells/μL | ||
| Antiretroviral medication | 21 None | 6 None 12 3′-azido-3-de oxythymidine |
| 4 2′,3′dideoxyino sine | ||
| 3 2′,3′ dideoxycyti dine | ||
| 6 2′,3′ didehydro- 3′-deoxythymi dine |
| . | Long-Term Survivors . | Progressors . |
|---|---|---|
| No. of subjects | 21 | 21 |
| Years of age (mean ± SD) | 43 ± 6 | 44 ± 9 |
| Range of age | 36-58 | 30-65 |
| Race | 17 Whites | 20 Whites |
| 2 African Americans | 1 African American | |
| 1 Asian | ||
| 1 Hispanic | ||
| Gender (male/female) | 19/2 | 21/0 |
| Year of probable infection (mean year ± SD) | 1981 ± 2 yr | 1982 ± 2 yr |
| Year tested seropositive (mean year ± SD) | 1985 ± 1 yr | 1986 ± 2 yr |
| Symptoms | 21 Asymptomatic | 8 Kaposi’s Sarcoma |
| 5Pneumocystis carinii pneumonia | ||
| 2 Thrush | ||
| 2 Hairy leukoplakia | ||
| 2 Night sweats | ||
| 1 Mycobacterium aviumcomplex | ||
| 2 Toxoplasmosis | ||
| 1 Neuropathy | ||
| 2 Chronic diarrhea | ||
| 2 Low-grade fever | ||
| 21 Absolute CD4+ cell count <200 cells/μL | ||
| Antiretroviral medication | 21 None | 6 None 12 3′-azido-3-de oxythymidine |
| 4 2′,3′dideoxyino sine | ||
| 3 2′,3′ dideoxycyti dine | ||
| 6 2′,3′ didehydro- 3′-deoxythymi dine |
CD4+ cell counts of LTS and individuals who progressed to AIDS (Progressors). Examples of CD4+ cell counts over a 10-year period in the peripheral blood of LTS (A) and Progressors (B and C) are provided. The hatched region represents the range of CD4+ cell counts of uninfected individuals.
CD4+ cell counts of LTS and individuals who progressed to AIDS (Progressors). Examples of CD4+ cell counts over a 10-year period in the peripheral blood of LTS (A) and Progressors (B and C) are provided. The hatched region represents the range of CD4+ cell counts of uninfected individuals.
Hematological and lymphocyte profiles of LTS and Progressors.
LTS had complete blood counts (CBC) within the range found in healthy HIV seronegative individuals (Table 2). In contrast, except for platelets (187 × 109/L), the CBC of Progressors were below normal and differed significantly from that of LTS (P < .02). Although the differential cell counts of these two clinical groups were still within the normal range, LTS had higher neutrophil, lymphocyte, monocyte, and basophil counts than the Progressors (Table 2). The eosinophil count was lower in LTS than Progressors but not significantly (P = .63).
Complete Blood and Differential Count of LTS and Progressors
| . | LTS . | Progressors . | Normal Range* . | PValue7-151 . |
|---|---|---|---|---|
| WBC (×109/L) | 6.9 ± 2.37-152 | 4.3 ± 2.0 | 4.5-13.2 | <.01 |
| RBC (×1012/L) | 4.83 ± 0.39 | 4.06 ± 0.74 | 4.4-5.9 | .02 |
| Hemaglobin (g/dL) | 14.9 ± 1.1 | 12.9 ± 1.8 | 13.6-17.5 | <.01 |
| Hematocrit (%) | 44.1 ± 2.8 | 37.6 ± 6.3 | 41-53 | <.01 |
| Platelets (×109/L) | 268 ± 52 | 187 ± 107 | 140-450 | <.01 |
| Neutrophils (×109/L) | 3.69 ± 1.77 | 2.00 ± 1.09 | 1.8-8 | <.01 |
| Lymphocytes (×109/L) | 2.42 ± 0.70 | 1.38 ± 0.86 | 1-6.1 | <.01 |
| Monocytes (×109/L) | 0.47 ± 1.5 | 0.35 ± 0.19 | 0-1.4 | .03 |
| Eosinophils (×109/L) | 0.15 ± 0.12 | 0.42 ± 1.20 | 0-0.8 | .63 |
| Basophils (×109/L) | 0.07 ± 0.04 | 0.03 ± 0.02 | 0-1.3 | <.01 |
| . | LTS . | Progressors . | Normal Range* . | PValue7-151 . |
|---|---|---|---|---|
| WBC (×109/L) | 6.9 ± 2.37-152 | 4.3 ± 2.0 | 4.5-13.2 | <.01 |
| RBC (×1012/L) | 4.83 ± 0.39 | 4.06 ± 0.74 | 4.4-5.9 | .02 |
| Hemaglobin (g/dL) | 14.9 ± 1.1 | 12.9 ± 1.8 | 13.6-17.5 | <.01 |
| Hematocrit (%) | 44.1 ± 2.8 | 37.6 ± 6.3 | 41-53 | <.01 |
| Platelets (×109/L) | 268 ± 52 | 187 ± 107 | 140-450 | <.01 |
| Neutrophils (×109/L) | 3.69 ± 1.77 | 2.00 ± 1.09 | 1.8-8 | <.01 |
| Lymphocytes (×109/L) | 2.42 ± 0.70 | 1.38 ± 0.86 | 1-6.1 | <.01 |
| Monocytes (×109/L) | 0.47 ± 1.5 | 0.35 ± 0.19 | 0-1.4 | .03 |
| Eosinophils (×109/L) | 0.15 ± 0.12 | 0.42 ± 1.20 | 0-0.8 | .63 |
| Basophils (×109/L) | 0.07 ± 0.04 | 0.03 ± 0.02 | 0-1.3 | <.01 |
*Values for the normal range were provided by the Clinical Laboratories of the Medical Center at the University of California, San Francisco.
P values obtained upon comparison of statistical significance between LTS and Progressors using the Mann-Whitney U test. Bold numbers indicate that the value is considered statistically significant.
Mean ± SD.
Within the lymphocyte population, the percent of CD19+cells (B lymphocytes) was similar among LTS, Progressors, and HIV-seronegative donors (Table 3). A lower percentage of CD56+/CD16+ lymphocytes (natural killer cells) was noted in the LTS compared with the Progressors, but this difference was not statistically different (P = .11). A higher percentage of T cells was found in LTS compared to Progressors and seronegative donors (mean percent CD3+ cells = 81%v 71% [P = .03]). The higher percentage of CD3+ cells in LTS compared with the HIV seronegative donors reflected the increased percentage of CD8+ cells (mean CD8+/CD3+ cells of 46% v 28%, respectively). The percent of CD4+ T cells in the lymphocyte population was significantly higher (P < .01) in LTS (mean percent CD4+/CD3+ cells = 35%) compared with Progressors (mean percent CD4+/CD3+ cells = 4%). In addition, the mean absolute CD4+ cell count of LTS (834 cells/μL) was significantly higher (P < .01) than that of Progressors (51 cells/μL) (Table 3). In contrast to the CD4+ cells, the percentage of CD8+ T cells in LTS (mean percent CD8+/CD3+ cells = 46%) was significantly lower (P = .03) than Progressors (mean percent CD8+/CD3+ cells = 63%), although the mean absolute CD8+ cell counts for both groups (1,122 v812 cells/μL) did not differ significantly (P = .15).
Lymphocyte Distribution of Peripheral Blood Cells From LTS and Progressors
| Lymphocyte Marker . | LTS . | Progressors . | HIVSeronegative Donors . | P Value* . |
|---|---|---|---|---|
| CD19 (%) | 9 ± 4† | 9 ± 6 | 11 ± 5 | .97 |
| CD56/CD16 (%) | 14 ± 7 | 21 ± 16 | 14 ± 7 | .11 |
| CD3 (%) | 81 ± 5 | 71 ± 18 | 71 ± 8 | .03 |
| CD4 (%) | 35 ± 7 | 4 ± 3 | 41 ± 6 | <.01 |
| CD4+cells/μL | 824 ± 208 | 51 ± 49 | 686 ± 217‡ | <.01 |
| CD8 (%) | 46 ± 10 | 63 ± 16 | 28 ± 9 | .03 |
| CD8+cells/μL | 1,122 ± 476 | 812 ± 708 | 443 ± 146 | .14 |
| CD4+:CD8+ cell ratio | 0.81 ± 0.31 | 0.07 ± 0.06 | 1.63 ± 63 | <.01 |
| Lymphocyte Marker . | LTS . | Progressors . | HIVSeronegative Donors . | P Value* . |
|---|---|---|---|---|
| CD19 (%) | 9 ± 4† | 9 ± 6 | 11 ± 5 | .97 |
| CD56/CD16 (%) | 14 ± 7 | 21 ± 16 | 14 ± 7 | .11 |
| CD3 (%) | 81 ± 5 | 71 ± 18 | 71 ± 8 | .03 |
| CD4 (%) | 35 ± 7 | 4 ± 3 | 41 ± 6 | <.01 |
| CD4+cells/μL | 824 ± 208 | 51 ± 49 | 686 ± 217‡ | <.01 |
| CD8 (%) | 46 ± 10 | 63 ± 16 | 28 ± 9 | .03 |
| CD8+cells/μL | 1,122 ± 476 | 812 ± 708 | 443 ± 146 | .14 |
| CD4+:CD8+ cell ratio | 0.81 ± 0.31 | 0.07 ± 0.06 | 1.63 ± 63 | <.01 |
*P values obtained upon comparison of statistical significance between LTS and Progressors using the Mann-Whitney U test. Bold numbers indicate that the value is considered statistically significant.
Mean ± SD.
Value for the normal range of CD4+ cells/μL provided by the Clinical Laboratories of the Medical Center at the University of California, San Francisco using standard protocols is 424 to 1,671 cells/μL.
Of the various CD8+ cell subpopulations analyzed (Table 4), only the percent of CD38+ cells differed significantly between LTS and Progressors. In LTS, the mean percent of CD8+ which were CD38+ was 62%, similar to the mean percent of CD8+ cells which were CD38+ in HIV uninfected donors (65%) (Table 4). Of the CD8+ cells from Progressors, 96% expressed the CD38 molecule which was statistically different from LTS (P < .01). Moreover, a lower percentage of CD8+ cells expressed HLA-DR in LTS compared with Progressors but this difference was not statistically significant (P = .06). In summary, aside from differences in the percentage of CD8+ cells, the LTS in contrast to the Progressors had a significantly lower number of CD8+ cells expressing the CD38 molecule.
CD8+ Cell Subsets of LTS and Progressors
| CD8+ Cell Marker (%) . | Long-Term Survivors . | Progressors . | HIVSeronegative Donors . | PValue* . |
|---|---|---|---|---|
| CD28 | 37 ± 9† | 35 ± 17 | 55 ± 16 | .34 |
| CD38 | 62 ± 14 | 96 ± 11 | 65 ± 10 | <.01 |
| HLA-DR | 54 ± 15 | 67 ± 17 | 19 ± 8 | .057 |
| CD45RO | 61 ± 10 | 66 ± 10 | 42 ± 15 | .29 |
| CD45RA | 56 ± 15 | 53 ± 9 | 74 ± 11 | .48 |
| CD57 | 52 ± 14 | 48 ± 15 | 20 ± 8 | .70 |
| CD62L | 32 ± 11 | 41 ± 13 | 56 ± 11 | .16 |
| CD26 | 15 ± 7 | 20 ± 13 | ND | .61 |
| CD25 | 3 ± 3 | 3 ± 2 | 3 ± 2 | .72 |
| CD122 | 34 ± 18 | 52 ± 27 | 52 ± 20 | .14 |
| CD8+ Cell Marker (%) . | Long-Term Survivors . | Progressors . | HIVSeronegative Donors . | PValue* . |
|---|---|---|---|---|
| CD28 | 37 ± 9† | 35 ± 17 | 55 ± 16 | .34 |
| CD38 | 62 ± 14 | 96 ± 11 | 65 ± 10 | <.01 |
| HLA-DR | 54 ± 15 | 67 ± 17 | 19 ± 8 | .057 |
| CD45RO | 61 ± 10 | 66 ± 10 | 42 ± 15 | .29 |
| CD45RA | 56 ± 15 | 53 ± 9 | 74 ± 11 | .48 |
| CD57 | 52 ± 14 | 48 ± 15 | 20 ± 8 | .70 |
| CD62L | 32 ± 11 | 41 ± 13 | 56 ± 11 | .16 |
| CD26 | 15 ± 7 | 20 ± 13 | ND | .61 |
| CD25 | 3 ± 3 | 3 ± 2 | 3 ± 2 | .72 |
| CD122 | 34 ± 18 | 52 ± 27 | 52 ± 20 | .14 |
*P values obtained upon comparison of statistical significance between LTS and Progressors using the Mann-Whitney U test. Bold numbers indicate that the value is considered statistically significant.
Mean percentage of CD8+ lymphocytes ± SD.
Quantification of virus in the plasma of LTS and Progressors.
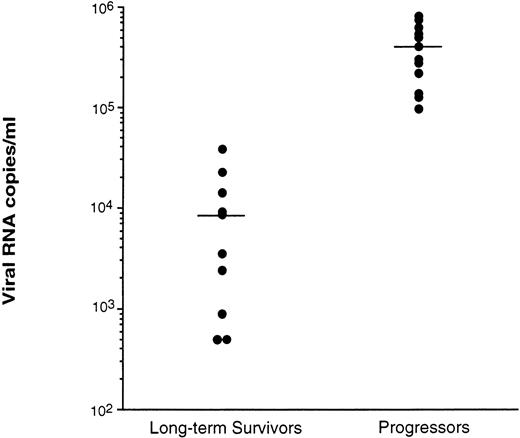
Virus levels in plasma were measured by two procedures: QC-PCR,17 which measures virus particles by the level of HIV RNA, and an infection assay,13 which measures the level of infectious virus particles in the plasma. Using the QC-PCR procedure, the LTS were shown to have significantly lower levels (P < .01) of HIV RNA in the plasma (mean of 9.5 × 103 copies/mL) compared with Progressors (mean of 3.6 × 105 RNA copies/mL) (Fig 2). Two of the 12 LTS tested had <5 × 102 RNA copies/mL. All Progressors had levels ≥9.8 × 104 RNA copies/mL.
Level of viral RNA detected in plasma of LTS and individuals who progressed to AIDS (Progressors). Plasmas from the peripheral blood of 10 LTS and 13 Progressors were recovered and stored at −70°C within 2 hours of venipuncture. Each plasma was then evaluated for the level of virus using quantitative-competitive PCR.17 Each point represents the level of HIV in the plasma from a different individual. Bars represent the mean value for the plasma of each group of individuals tested. The level of HIV in the plasma of LTS and Progressors was statistically different (P< .01) using the Mann-Whitney U test.
Level of viral RNA detected in plasma of LTS and individuals who progressed to AIDS (Progressors). Plasmas from the peripheral blood of 10 LTS and 13 Progressors were recovered and stored at −70°C within 2 hours of venipuncture. Each plasma was then evaluated for the level of virus using quantitative-competitive PCR.17 Each point represents the level of HIV in the plasma from a different individual. Bars represent the mean value for the plasma of each group of individuals tested. The level of HIV in the plasma of LTS and Progressors was statistically different (P< .01) using the Mann-Whitney U test.
As measured by the infection center assay, the LTS did not have detectable infectious virus in their plasma whereas HIV was detectable in the plasma of all Progressors (Table 5). The levels of infectious virus in the plasma of the Progressors varied (from titers of 2 to 32) (Table 5).
Infectious Virus and p24 Antigen Detected in the Plasma of LTS and Progressors
| Subject . | Infectious Virus Titer . | p24 Antigen . | ||||
|---|---|---|---|---|---|---|
| No. of Subjects . | Titer3-150 . | No. of Subjects . | Level (pg/mL) . | |||
| Negative . | Positive . | Negative . | Positive . | |||
| LTS | 12 | 0 | NA | 11 | 0 | NA |
| Progressors | 0 | 12 | undil. (2) | 7 | 4 | 186.93-151 |
| 2 (1) | 348.9 | |||||
| 4 (8) | 74.4 | |||||
| 32 (1) | 448.9 | |||||
| Subject . | Infectious Virus Titer . | p24 Antigen . | ||||
|---|---|---|---|---|---|---|
| No. of Subjects . | Titer3-150 . | No. of Subjects . | Level (pg/mL) . | |||
| Negative . | Positive . | Negative . | Positive . | |||
| LTS | 12 | 0 | NA | 11 | 0 | NA |
| Progressors | 0 | 12 | undil. (2) | 7 | 4 | 186.93-151 |
| 2 (1) | 348.9 | |||||
| 4 (8) | 74.4 | |||||
| 32 (1) | 448.9 | |||||
Abbreviations: NA, not applicable; undil., undiluted.
Number in parentheses indicates the number of individuals whose plasma was positive for infectious virus at the highest dilution shown.
Level of HIV p24 antigen in the plasma was detected by ELISA (see Materials and Methods). The four values listed represent the level of p24 antigen in the plasma of the four positive subjects.
Another means of determining the level of HIV in the plasma is by measuring the level of HIV p24 protein. In the plasma of 11 LTS tested the viral p24 antigen was below the limit of the assay used in our study (<5 pg of p24 HIV antigen/mL) and was found to be present in 4 of 7 Progressors (Table 5). The amount of p24 antigen found in the plasma from the 4 Progressors ranged from 74.4 to 448.9 pg/mL. Overall, our studies of virus particles in the plasma showed that in comparison to Progressors, LTS had a lower level of cell-free virus in their plasma and none was infectious in cell culture.
Ability to recovered virus from the PBMC and the number of infected cells in the peripheral blood of LTS and Progressors.
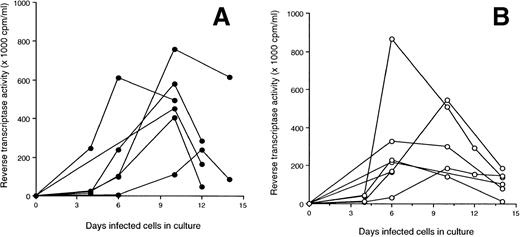
The A-culture and B-culture, two standard tissue culture techniques used to isolate HIV from cells in the peripheral blood,21were used to isolate virus, in vitro, from LTS and Progressors (see Materials and Methods). None of the PBMC from 10 LTS tested produced detectable virus in the A-culture (mean RT activity of 2.1 ± 1.3 × 103 cpm/mL) (Fig 3A). With the B-cultures, the PBMC from 7 of 10 LTS released HIV (mean RT activity of 110.3 ± 137.3 × 103 cpm/mL) (Fig 3B). All Progressors produced HIV in both the A- and B-culture (mean RT activity = 58.3 ± 35.4 and 285 ± 215.6 × 103cpm/mL, respectively) (Fig 3A and B). In comparison with the PBMC from Progressors, the PBMC of LTS produced significantly lower levels of virus in B-cultures (P = .02).
Production of HIV in culture fluids of PBMC from LTS and individuals who progressed to AIDS (Progressors). Two isolation methods [A-culture (A) and B-culture (B)] were used to detect HIV production from PBMC of LTS and Progressors. In the A-culture, the PBMC from each individual were stimulated with PHA for 3 days. Seven days later, PHA-activated PBMC from HIV-seronegative donors were added to these cultures. In the B-culture, PHA-activated PBMC from HIV-seronegative donors were added to unstimulated PBMC from LTS and Progressors. Culture fluids were monitored every 3 or 4 days for RT activity.20 Culture fluids were considered to contain HIV if the level of RT activity was ≥104 cpm/mL. The amount of HIV in the culture fluids of A- and B-cultures of LTS and Progressors was found to be statistically different (P < .01 and P = .02, respectively) using the Mann-Whitney U test.
Production of HIV in culture fluids of PBMC from LTS and individuals who progressed to AIDS (Progressors). Two isolation methods [A-culture (A) and B-culture (B)] were used to detect HIV production from PBMC of LTS and Progressors. In the A-culture, the PBMC from each individual were stimulated with PHA for 3 days. Seven days later, PHA-activated PBMC from HIV-seronegative donors were added to these cultures. In the B-culture, PHA-activated PBMC from HIV-seronegative donors were added to unstimulated PBMC from LTS and Progressors. Culture fluids were monitored every 3 or 4 days for RT activity.20 Culture fluids were considered to contain HIV if the level of RT activity was ≥104 cpm/mL. The amount of HIV in the culture fluids of A- and B-cultures of LTS and Progressors was found to be statistically different (P < .01 and P = .02, respectively) using the Mann-Whitney U test.
To determine the levels of infected PBMC in LTS and Progressors, infectious center assays were used.18 19 The LTS had almost a 2 log10 lower mean frequency of HIV-infected cells (1 in 9.5 × 105) in the PBMC than did Progressors (1 in 2 × 104 [P = .01]) (Fig 4). In 7 of 17 LTS examined, the frequency of infected cells was less than 1 in 106 PBMC. In contrast, the lowest frequency of infected cells found in Progressors was 1 in 105 cells (Fig 4). Thus, the number of infected cells in the peripheral blood was at least 50 times lower in LTS than Progressors. Overall, our studies indicate that like the levels in the plasma (Fig 2 and Table 5), a lower frequency of the infected peripheral blood cells are present in LTS relative to cells in the Progressors.
Frequency of infected cells in PBMC of LTS and individuals who progressed to AIDS (Progressors). PBMC from LTS and Progressors were serially diluted 10-fold and cultured with PHA-stimulated PBMC from HIV seronegative donors. The cocultures were evaluated for the lowest dilution of cells which produced positive particle-associated reverse transcriptase activity20(≥104 cpm/mL) in the culture fluids over a 30-day culture period. Each point represents the result from a different individual. Bars represent the mean value for the data obtained from the two groups of individuals tested. The number of infected cells in the peripheral blood of LTS and Progressors was statistically different (P = .01) using the Mann-Whitney U test.
Frequency of infected cells in PBMC of LTS and individuals who progressed to AIDS (Progressors). PBMC from LTS and Progressors were serially diluted 10-fold and cultured with PHA-stimulated PBMC from HIV seronegative donors. The cocultures were evaluated for the lowest dilution of cells which produced positive particle-associated reverse transcriptase activity20(≥104 cpm/mL) in the culture fluids over a 30-day culture period. Each point represents the result from a different individual. Bars represent the mean value for the data obtained from the two groups of individuals tested. The number of infected cells in the peripheral blood of LTS and Progressors was statistically different (P = .01) using the Mann-Whitney U test.
Biological properties of virus isolated from the peripheral blood of LTS and Progressors.
The majority of primary virus isolates from PBMC of the LTS (9 of 10) did not induce syncytium formation in MT-2 cells (Table 6), indicating an non-SI (NSI) phenotype. Infectious virus from the PBMC of 44% of the Progressors was of the NSI phenotype whereas 56% was of the SI phenotype.
Biologic Activity of Virus Isolated From PBMC and Plasma of LTS and Progressors
| Subject . | Source of Virus . | Phenotype of Primary Virus/Total Tested* . | |
|---|---|---|---|
| NSI . | SI . | ||
| LTS | PBMC | 9/10 (90%) | 1/10 (10%) |
| Progressors | PBMC | 7/16 (44%) | 9/16 (56%) |
| LTS | Plasma | Not tested4-151 | Not tested4-151 |
| Progressors | Plasma | 0/8 | 8/8 |
| Subject . | Source of Virus . | Phenotype of Primary Virus/Total Tested* . | |
|---|---|---|---|
| NSI . | SI . | ||
| LTS | PBMC | 9/10 (90%) | 1/10 (10%) |
| Progressors | PBMC | 7/16 (44%) | 9/16 (56%) |
| LTS | Plasma | Not tested4-151 | Not tested4-151 |
| Progressors | Plasma | 0/8 | 8/8 |
*Assayed with MT-2 cells as described in Materials and Methods.
Infectious virus could not be isolated from the plasma of the 12 individuals tested.
From the plasma of all eight Progressors evaluated, the infectious virus recovered was of the SI phenotype (Table 6). Notably, the biologic phenotype of HIV present in the plasma did not always correlate with the virus from the PBMC; HIV isolated from the plasma of two Progressors tested had virus from their PBMC which was of the NSI phenotype.
Despite the differences in the frequency of NSI and SI viruses obtained from PBMC of LTS and Progressors, the replication kinetics in normal PBMC of the primary isolates from LTS (Fig5A) and Progressors (Fig 5B) did not differ substantially. Thus, the biological properties of the virus isolated from LTS and Progressors may not explain the differences in the levels of virus observed between the two groups.
Kinetics of replication of primary HIV isolates from LTS and individuals who progressed to AIDS (Progressors). The ability of primary HIV isolates from 6 LTS (A) and 6 Progressors (B) to replicate in PHA-stimulated PBMC of HIV seronegative donors was evaluated over a 2-week period. The PBMC were infected with 100 TCID50 of virus, and replication of virus was indicated by the level of reverse transcriptase activity in the culture fluids.20
Kinetics of replication of primary HIV isolates from LTS and individuals who progressed to AIDS (Progressors). The ability of primary HIV isolates from 6 LTS (A) and 6 Progressors (B) to replicate in PHA-stimulated PBMC of HIV seronegative donors was evaluated over a 2-week period. The PBMC were infected with 100 TCID50 of virus, and replication of virus was indicated by the level of reverse transcriptase activity in the culture fluids.20
Prevalence of the CCR5 Δ32 mutation in LTS and Progressors.
Differences in the level of HIV found in LTS and Progressors (Figs 2and 3, Table 5) could be explained by the variability in the expression of HIV coreceptors. The presence of the Δ32 mutation in the CCR5 gene has been shown to be associated with a favorable clinical state in some infected individuals.30 In this study we evaluated whether the presence of the Δ32 mutation in the CCR5 gene of PBMC could explain the different levels of HIV in LTS and Progressors (Table 7).
In the LTS group, 8 of 21 individuals were heterozygous for the Δ32 deletion in the CCR5 gene. In contrast, only 3 of 20 Progressors tested had this mutation. This difference was not significantly different (P = .12). Moreover, no statistical difference was observed between the LTS and a group of HIV seronegative donors in which 5 of 24 subjects had a Δ32 deletion in one copy of the CCR5 gene (P = .12). None of the infected subjects tested had mutations in both copies of the gene encoding CCR5.
Neutralizing antibody in LTS and Progressors.
The level of neutralizing antibodies in the plasma could also explain the different levels of virus present in the plasma and peripheral blood cells of LTS and Progressors. The number of LTS (5 of 7) and Progressors (5 of 6) whose plasma was capable of preventing the replication of autologous virus was similar (Table 8). However, plasmas from 3 of the 7 LTS and none of the 6 Progressors tested was able to neutralize autologous virus at dilutions of 1:50 or more, although the difference observed was not significant (P = .1). The number of LTS whose plasma was capable of neutralizing heterologous virus at dilutions of 1:10 or less was slightly greater against virus from LTS compared with virus from Progressors (82% v 72%). Conversely, the number of Progressors whose plasma samples were able to neutralize heterologous virus was slightly greater if the virus was isolated from a Progressor compared with virus from an LTS (70% v 56%, respectively) (Table 8).
Heterozygosity for the ▵32 Mutation in CCR5 From LTS and Progressors
| CCR5 Genotype . | LTS . | Progressors . | HIV Seronegative Donor . |
|---|---|---|---|
| Wild type (+/+) | 13 | 17 | 19 |
| Δ32 Mutation (+/−) | 8 | 3* | 5 |
| CCR5 Genotype . | LTS . | Progressors . | HIV Seronegative Donor . |
|---|---|---|---|
| Wild type (+/+) | 13 | 17 | 19 |
| Δ32 Mutation (+/−) | 8 | 3* | 5 |
*Statistical significance between LTS and Progressors wasP = .12; Fisher’s Exact test.
Neutralization of Primary HIV Isolates by Plasma From LTS and Progressors
| Plasma . | Titer . | Virus Isolates . | ||
|---|---|---|---|---|
| Autologous . | Heterologous (LTS)* . | Heterologous (Progressors)* . | ||
| LTS | 0 | 2/7 (29)6-151 | 2/11 (18) | 7/25 (28) |
| 10 | 2/7 (29) | 7/11 (64) | 15/25 (60) | |
| 50 | 1/7 (14) | 1/11 (9) | 3/25 (12) | |
| 100 | 2/7 (29) | 1/11 (9) | 0/25 (0) | |
| Progressors | 0 | 1/6 (17) | 11/25 (44) | 7/23 (30) |
| 10 | 5/6 (83) | 12/25 (48) | 13/23 (57) | |
| 50 | 0/6 (0) | 5/25 (20) | 3/23 (13) | |
| 100 | 0/6 (0) | 0/25 (0) | 0/23 (0) | |
| Plasma . | Titer . | Virus Isolates . | ||
|---|---|---|---|---|
| Autologous . | Heterologous (LTS)* . | Heterologous (Progressors)* . | ||
| LTS | 0 | 2/7 (29)6-151 | 2/11 (18) | 7/25 (28) |
| 10 | 2/7 (29) | 7/11 (64) | 15/25 (60) | |
| 50 | 1/7 (14) | 1/11 (9) | 3/25 (12) | |
| 100 | 2/7 (29) | 1/11 (9) | 0/25 (0) | |
| Progressors | 0 | 1/6 (17) | 11/25 (44) | 7/23 (30) |
| 10 | 5/6 (83) | 12/25 (48) | 13/23 (57) | |
| 50 | 0/6 (0) | 5/25 (20) | 3/23 (13) | |
| 100 | 0/6 (0) | 0/25 (0) | 0/23 (0) | |
*Plasma from HIV-infected subjects was tested against one or more heterologous virus isolates.
The number of plasma samples which neutralized the HIV isolates was determined by comparison with the effect on virus replication by a plasma pool from control HIV seronegative donors. Number in parentheses indicates the percentage of plasma samples which neutralized the virus isolates by at least 66%.
CD8+ cell noncytotoxic anti-HIV activity in LTS and Progressors.
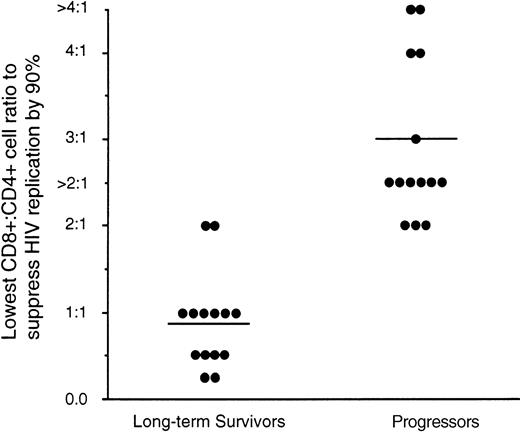
CD8+ cells from HIV-infected individuals have the ability to suppress HIV replication without killing the infected cell.31 This CD8+ cell noncytotoxic antiviral response has been shown to correlate with the clinical state of the infected individuals.16,32 Progression to disease correlates with a decrease in this CD8+ cell activity.33 Here we evaluated whether the variability in the level of HIV in LTS and Progressors can be associated with differences in the antiviral activity of their CD8+ cells. Using the acute assay system we evaluated the extent to which CD8+ cells can suppress HIV replication in CD4+cells infected with the β-chemokine–insensitive cytopathic HIV-1SF33 strain. An average CD8+:CD4+ cell ratio of 1:1 was needed for 90% suppression of HIV replication by CD8+ cells from LTS (Fig 6) whereas a mean ratio of 3:1 was needed for the CD8+ cells from Progressors to suppress HIV replication to a similar level (P < .01). The CD8+:CD4+ cell ratio needed to suppress HIV replication correlated directly with the viral load of the infected individual. (Spearman coefficient of rank correlation; P < .01).
Suppression of HIV replication by CD8+cells from LTS and individuals who progressed to AIDS (Progressors). The ability of CD8+ cells from 14 LTS and 14 Progressors to suppress HIV-1SF33 replication in CD4+cells was determined in an acute infection assay over a range of CD8+:CD4+ cell ratios of twofold dilutions ranging from 0.25:1 to 4:1. The level of antiviral activity of CD8+ cells shown represents the lowest CD8+:CD4+ cell ratio that achieved ≥90% reduction of RT activity relative to that observed in the fluid of CD4+ cells cultured alone. Each point represents the result from a different individual. A CD8+:CD4+ cell ratio greater than 2:1 was used in cases where the number of CD8+ cells from Progressors was limited such that the CD8+:CD4+ cell ratio of 4:1 could not be tested. For statistical analysis, the anti-HIV level was considered to be 3:1 (see Results). Bars represent the mean values of the data obtained for each group of individuals tested. The ratio of CD8+:CD4+ cells needed from LTS and Progressors to suppress HIV replication by 90% was statistically different (P < .01) using the Mann-Whitney U test.
Suppression of HIV replication by CD8+cells from LTS and individuals who progressed to AIDS (Progressors). The ability of CD8+ cells from 14 LTS and 14 Progressors to suppress HIV-1SF33 replication in CD4+cells was determined in an acute infection assay over a range of CD8+:CD4+ cell ratios of twofold dilutions ranging from 0.25:1 to 4:1. The level of antiviral activity of CD8+ cells shown represents the lowest CD8+:CD4+ cell ratio that achieved ≥90% reduction of RT activity relative to that observed in the fluid of CD4+ cells cultured alone. Each point represents the result from a different individual. A CD8+:CD4+ cell ratio greater than 2:1 was used in cases where the number of CD8+ cells from Progressors was limited such that the CD8+:CD4+ cell ratio of 4:1 could not be tested. For statistical analysis, the anti-HIV level was considered to be 3:1 (see Results). Bars represent the mean values of the data obtained for each group of individuals tested. The ratio of CD8+:CD4+ cells needed from LTS and Progressors to suppress HIV replication by 90% was statistically different (P < .01) using the Mann-Whitney U test.
Because of the limitation in the number of CD8+ cells recovered from the stimulated PBMC of 8 of the 14 Progressors, CD8:CD4+ cell ratios of ≤2:1 were used. Thus, the extent of CD8+ cell antiviral response could not be quantified further. For example, suppression of virus replication by ≥90% was not achieved in 6 of the 8 Progressors tested at a ratio of 2:1. Therefore, when scoring for statistical analysis we chose to designate the above as suppressing at a 3:1 CD8+:CD4+cell ratio. The CD8+ cells from 2 of the 14 Progressors did not suppress HIV replication by 90% at the highest CD8+:CD4+ cell ratio tested, 4:1.
DISCUSSION
LTS represent a small subset of infected individuals (≤5%) who, beyond the usual time to develop AIDS (10 years),1-3 remain healthy without receiving any antiviral medication.5Socioeconomic or behavioral characteristics apparently do not account for this long-term healthy state.2,34 Moreover, results suggesting that long-term survival is associated with certain major histocompatibility complex (MHC) alleles35,36 have not been consistently found.37
In an attempt to define unique features of LTS which enable them to remain clinically healthy, virological and immunological properties of these individuals were evaluated and compared with those of individuals, infected for the same length of time, but who have progressed to disease. In the present studies, two well-characterized cohorts were used to assess a large number of factors involved in HIV infection that may be uniquely associated with a long-term asymptomatic clinical condition. Attempts were made to rule out any possible influence of age, gender, ethnicity, or length of HIV infection on the analyses by closely matching the two clinical groups (Table 1). Emphasis was placed on hematological profiles and several virological and immunological parameters. Other reports7,8 38 have attempted to define some of the viral and immune components associated with long-term survival, but either a limited number of subjects was used, the subjects were infected for a shorter period of time (<10 years), or appropriate controls were not always included.
Aside from the amount of CD8+ cells and subset composition, we found a hematological profile in LTS (Tables 2-4) resembling that of uninfected individuals. The increase in CD8+ cells observed in infected individuals compared with HIV uninfected controls appears to reflect the expansion of memory (eg, increased CD45R0+ and CD62L−) and activated (eg, HLA-DR+) CD8+ cells (Table 4) associated with people infected with HIV.39
The only notable difference in the subsets of CD8+ cells between LTS and Progressors was a lower percentage of CD38+cells. A significantly (P < .01) lower percentage of CD38+CD8+ cells was found in LTS compared with the Progressors. The percentage of CD8+ cells expressing the CD38 molecule was similar between LTS and seronegative individuals. These findings are in agreement with those of other investigators who showed an increased percentage of CD38+CD8+cells associated with disease progression in HIV-infected individuals.40 The significance of a higher level of CD8+ cells expressing CD38 in Progressors relative to LTS is unclear at this time, but may reflect an increase in the number of cytotoxic T lymphocytes41 that could lead to the lysis of uninfected CD4+ cells.42
Examination of virological properties of LTS and Progressors indicated that the former group of infected individuals has low plasma viral loads (Fig 2, Table 5), low numbers of infected cells (Figs 3 and 4), and is infected with a less cytopathic virus strain (Table 6). Some of these observations have been reported by others in a limited number of subjects.7,8,43 In fact, we showed in this study that even when using optimal procedures to recover virus from the plasma, infectious virus could not be isolated from the plasma of all the 12 LTS evaluated (Table 5). This finding was in contrast to the ability to recover infectious virus from the plasma of all 12 Progressors that were tested. The inability to recover infectious virus (Table 5) in LTS despite the ability to detect HIV RNA (Fig 2) in the plasma most likely reflects a large number of defective particles found in plasma.44 The differences in the levels of virus in the infected individuals could not be explained by different growth kinetics of the virus (Fig 5), differential amounts of neutralizing antibodies (see below), or mutations in the CCR-5 receptor gene. Although the frequency of LTS heterozygous for the Δ32 deletion in the CCR5 gene was higher then those observed in the Progressors (Table7), it could not account for the healthy clinical state of the LTS. The majority of infected individuals were without this mutation (14 of 21 for LTS and 17 of 20 for Progressors) and the difference in the frequency of the heterozygous mutation in CCR5 of LTS and Progressors was not statistically significant (P = .12).
Although the results of our studies show a trend toward Progressors having more SI virus isolates and LTS having more NSI viruses (Table6), the characteristics of the dominant virus strain isolated from LTS and Progressors did not completely account for the difference in the levels of free virus and infected cells observed in our study (Figs 2and 4). Although some results have suggested that disease progression is associated with a change in virus phenotype from NSI type to an SI type,10,11 almost half the primary viruses isolated from PBMC of Progressors in our study were NSI (Table 6). Moreover, an SI virus was recovered from an LTS subject. These results are in agreement with the recent findings of other investigators who recovered NSI virus in 50% of Progressors and an SI virus from an LTS.43 Thus, the biologic phenotype of the virus does not always predict the clinical outcome of HIV infection. It is noteworthy that in two Progressors, the phenotype of the plasma and PBMC-derived viruses were different (Table 6). These findings suggest that different compartments may harbor different virus phenotypes.
Because anti-HIV antibody response has been implicated as being important in controlling the HIV infection,4 we examined the potential role of neutralizing antibodies in the plasma from LTS and Progressors to prevent HIV replication. In contrast to other studies,45 we used autologous virus strains to assess the neutralizing capacity of plasma. Neutralization measured by this method may have greater clinical relevance. An equal number of individuals from both groups had neutralizing antibodies directed against homologous and heterologous viral strains (Table 8). Thus, although loss of neutralizing antibodies and appearance of neutralization escape viruses have been associated with disease progression,46the presence or absence of neutralizing antibodies in the present studies did not account for the difference in the clinical outcome of long-term HIV infection.
The extent of the CD8+ cell noncytotoxic suppression of HIV replication (Fig 6) can provide an explanation for the low viral load (Fig 2), stable CD4+ cell counts (Fig 1 and Table 3), and favorable clinical outcome (Table 1) observed in LTS compared with Progressors. This conclusion was supported by two studies described here. In the first, A- and B-cultures of PBMC from LTS and Progressors (Fig 3A) give an indication of the relative ability of the CD8+ cells within the PBMC to suppress HIV replication.31 In the A-culture, PBMC from the infected individual are treated with the mitogen, PHA, which can activate the CD8+ cells and increase their ability to control virus replication.33 The absence of HIV recovery in the A-cultures from LTS, but the presence of virus in the A-cultures of all Progressors (Fig 3), reflects a diminished CD8+ cell response in Progressors.21 In the B-culture, because of the lack of PHA stimulation and the early addition of fresh permissive cells, the CD8+ cells are not usually as effective at suppressing virus replication. In these cultures, virus was recovered from LTS, as well as Progressors, but the level of virus produced by the PBMC of LTS was significantly lower than that of Progressors (P = .02). This finding could reflect the CD8+ cell antiviral activity as well as the low number of infected cells (Fig 4). In the second study, the acute infection assay, the purified CD8+ cells from LTS suppressed HIV replication more efficiently than CD8+ cells from the Progressors (Fig 6). Moreover, the low ratio of CD8+ to CD4+ cells needed to suppress HIV replication by ≥90% correlated directly with the low viral load in the plasma of LTS (P < .01). On average, at least three times more CD8+ cells from Progressors compared to LTS was needed to suppress 90% of HIV replication in CD4+ cells. In some cases, the CD8+ cells from Progressors were unable to control HIV replication (Fig 6).
These findings strongly support the conclusion that the ability of CD8+ cells to suppress HIV replication is important in controlling HIV infection, reducing the loss of CD4+ cells and preventing progression to disease in LTS. Progressors infected for the same length of time have a reduced capacity to control HIV replication in culture as shown by the need for many more CD8+ cells to suppress HIV replication (Fig 6). These results are in agreement with previous findings demonstrating a correlation between the ability of CD8+ cells to suppress HIV replication and a healthy clinical state.16 33
In summary, the LTS, despite their length of infection which generally leads to AIDS in a majority of infected individuals (>10 years), are clinically healthy without the need for antiretroviral medication. They usually have low levels of noncytopathic virus typically found in asymptomatic individuals who were recently infected. But, unlike most asymptomatic infected individuals who eventually progress to AIDS (Progressors), LTS appear to have the ability to maintain low viral levels, a high CD4+ cell count, and not develop disease. The CD8+ cell noncytotoxic antiviral response seems to play a major part in ensuring long-term survival. The results of this study suggest that sustaining this CD8+ cell antiviral function is important in preventing disease progression and has relevance in studies directed at developing anti-HIV immune-based therapies.
ACKNOWLEDGMENT
The authors thank Alan Landay and Janis Giorgi for their critical review of the manuscript, John Sninsky and Shirley Kwok for their advice and assistance on certain aspects of this study, Katharine Bossart and Roland Orque for technical assistance, and Chris Beglinger and Ann Murai for their assistance in the preparation of this manuscript.
Supported by a grant from the National Institutes of Health (RO1-AI30350). G.R.-T. was the recipient of a Fogarty International Fellowship.
Address reprint requests to Jay A. Levy, MD, Department of Medicine, Third Ave at Parnassus, University of California, San Francisco, CA 94143-1270.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. Production of HIV in culture fluids of PBMC from LTS and individuals who progressed to AIDS (Progressors). Two isolation methods [A-culture (A) and B-culture (B)] were used to detect HIV production from PBMC of LTS and Progressors. In the A-culture, the PBMC from each individual were stimulated with PHA for 3 days. Seven days later, PHA-activated PBMC from HIV-seronegative donors were added to these cultures. In the B-culture, PHA-activated PBMC from HIV-seronegative donors were added to unstimulated PBMC from LTS and Progressors. Culture fluids were monitored every 3 or 4 days for RT activity.20 Culture fluids were considered to contain HIV if the level of RT activity was ≥104 cpm/mL. The amount of HIV in the culture fluids of A- and B-cultures of LTS and Progressors was found to be statistically different (P < .01 and P = .02, respectively) using the Mann-Whitney U test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/9/10.1182_blood.v92.9.3105/5/m_blod42146003x.jpeg?Expires=1769135935&Signature=JEt~swC27ohR3d9J3Ufe3-NANvCiavic~dMqsmeIoft14XgwpeOSa1SSF61SBnK~Crh29G60P6OdK85grzATi791kxkZ2qvHe0-jseHA7k6EGgvckEqlW5CpkLGes9w1EoYoPSBekakEQWjhr2Ma3rlVZTcQpSMHz1g7BejyiwGb7ijHNS0BQ-ptw8VSWwlkNt2Qo~50Viq~vjvNkOZ6HeGgGExw8F1kKt6Ep-D4z1-owMAuWIrRURQ35AWKNfdbCjrkgcwR-q9svQURMCNwRSXjoxkI~uj7uAf2y2pnKyzQ7mq1V0zNPOEr7BRkl9QhLyGeDzWwzD4Nr0XvyFkkXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal