Abstract
Extracorporeal exposure of peripheral blood mononuclear cells to the photosensitizing compound 8-methoxypsoralen and ultraviolet A radiation has been shown to be effective in the treatment of several T-cell–mediated diseases, including cutaneous T-cell lymphoma and rejection after organ transplantation. We present 21 patients (10 men and 11 women) with hematological malignancies with a median age of 36 years (range, 25 to 55 years) who had received marrow grafts from sibling (n = 12) or unrelated (n = 9) donors. Six patients had acute graft-versus-host disease (GVHD) grade II to III not responding to cyclosporine A (CSA) and prednisolone when referred to extracorporeal photochemotherapy (ECP). In 15 patients, 2 to 24 months after bone marrow transplantation (BMT), extensive chronic GVHD with involvement of skin (n = 15), liver (n = 10), oral mucosa (n = 11), ocular glands (n = 6), and thrombocytopenia (n = 3) developed and was unresponsive to conventional therapy, including steroids. All patients were treated with ECP on 2 consecutive days every 2 weeks for the first 3 months and thereafter every 4 weeks until resolution of GVHD. ECP was tolerated excellently without any significant side effects. After a median of 14 cycles of ECP, acute GVHD resolved completely in 4 of 6 patients (67%) and partially in another 2 patients. Cutaneous chronic GVHD completely resolved in 12 of 15 (80%) patients. Contractures of knees and elbows due to scleroderma resolved partially. Oral mucosal ulcerations resolved in all patients. Seven of 10 patients (70%) with liver involvement had complete responses after ECP. After discontinuation of ECP, no severe infections were observed. Our findings suggest that ECP is a safe and effective adjunct therapy for both acute and extensive chronic GVHD with skin and visceral involvement and resistance to conventional therapy.
© 1998 by The American Society of Hematology.
DESPITE IMPROVEMENTS in posttransplant immunosuppression, up to 30% of HLA-identical marrow graft recipients and up to 90% of patients receiving marrow from unrelated donors still develop significant acute graft-versus-host disease (GVHD).1-3 Prednisone has been shown to be effective in the treatment of established acute GVHD.4 However, patients not responding to corticosteroids are at high risk of death due to infections. Chronic GVHD affects 50% of long-term marrow transplant survivors and is lethal in 20% to 40% of affected patients, despite aggressive treatment.1,5 Primary therapy for extensive chronic GVHD includes corticosteroids and cyclosporine A (CSA).6 Other therapeutic options are thalidomide, azathioprine, psoralen and ultraviolet A (PUVA), and monoclonal antibodies.1,7 8 However, these therapies are often unsuccessful in patients with extensive multiorgan involvement and are associated with significant therapy-related complications. Considering the toxicity and incomplete response rates of conventional treatment for chronic GVHD, alternative approaches are needed for patients who do not respond to first-line therapy.
Extracorporeal photochemotherapy (ECP) is currently being used for the treatment of cutaneous T-cell lymphoma, selected autoimmune diseases, and rejection after organ transplantation.9-12 ECP consists of infusion of UVA irradiated autologous peripheral blood mononuclear cells collected by apheresis and incubated with 8-methoxypsoralen (8-MOP). Recently, ECP has been used in treatment of severe GVHD.13-15
We present here our experience in patients with extensive chronic and steroid-refractory severe acute GVHD treated with ECP.
MATERIALS AND METHODS
Patients.
Twenty-one patients (10 men and 11 women; median age, 36 years; range, 25 to 55 years) underwent allogeneic marrow transplantation for acute myeloid leukemia (n = 5), acute lymphoblastic leukemia (n = 4), chronic myeloid leukemia (n = 8), non-Hodgkin’s lymphoma (n = 3), or severe aplastic anemia (n = 1). Their pretransplant characteristics are shown in Table 1. All were conditioned for transplantation with cyclophosphamide (CY) and fractionated total body irradiation (TBI); etoposide was also administered in 3 patients.
Overall Patient Characteristics
| No. of patients | 21 |
| Median age (yr) | 36 |
| Sex (male/female) | 10/11 |
| Diagnosis | |
| AML | 5 |
| ALL | 4 |
| CML | 8 |
| NHL | 3 |
| SAA | 1 |
| Conditioning regimen | |
| CY/fTBI | 18 |
| CY/VP16/fTBI | 3 |
| GVHD prophylaxis | |
| CSA/MTX | 17 |
| CSA | 3 |
| CSA/Pred | 1 |
| BM donors | |
| Serologic and LBT match | 18 |
| Serologic and LBT mismatch | 3 |
| Related/unrelated | 12/9 |
| Sex mismatch | 13 |
| Female donor to male recipient | 7 |
| Median donor age (yr) | 38 |
| Range | 20-62 |
| No. of patients | 21 |
| Median age (yr) | 36 |
| Sex (male/female) | 10/11 |
| Diagnosis | |
| AML | 5 |
| ALL | 4 |
| CML | 8 |
| NHL | 3 |
| SAA | 1 |
| Conditioning regimen | |
| CY/fTBI | 18 |
| CY/VP16/fTBI | 3 |
| GVHD prophylaxis | |
| CSA/MTX | 17 |
| CSA | 3 |
| CSA/Pred | 1 |
| BM donors | |
| Serologic and LBT match | 18 |
| Serologic and LBT mismatch | 3 |
| Related/unrelated | 12/9 |
| Sex mismatch | 13 |
| Female donor to male recipient | 7 |
| Median donor age (yr) | 38 |
| Range | 20-62 |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; NHL, non-Hodgkin’s lymphoma; SAA, severe aplastic anemia; CY, cyclophosphamide; fTBI, fractionated total body irradiation; VP16, etoposide; CSA, cyclosporine A; MTX, methotrexate; Pred, methylprednisone; BM, bone marrow; LBT, ligation based typing of class II.
Marrow donors were HLA-identical siblings in 12 patients, HLA-identical unrelated donors in 6 patients, and 1-antigen mismatched unrelated donors in 3 patients. Bone marrow (BM) was harvested and infused as previously described.16 For GVHD prophylaxis, 17 patients received CSA and methotrexate (MTX) according to the Seattle protocol,2 3 patients received CSA only,2 and 1 patient received CSA and prednisolone.17 Patients were hospitalized in isolation rooms with laminar air-flow or reverse isolation. They received antimicrobial prophylaxis with nonabsorbable antibiotics and Pneumocystis carinii prophylaxis with cotrimoxazole. For cytomegalovirus (CMV)-prophylaxis, patients received acyclovir as described.18 Packed red blood cells (RBCs) were administered to maintain hemoglobin concentrations greater than 8.0 g/dL and platelet transfusions were administered to keep the platelet count greater than 20 × 109/L. All patients received CMV-negative blood products. Five patients received granulocyte colony-stimulating factor (G-CSF) starting on day 1 after marrow infusion until neutrophil recovery.
All patients had central venous catheters implanted on the day of admission. Catheters were removed between days 100 and 150 after BM transplantation (BMT). Informed consent was obtained from all patients or their guardians.
Patients referred for ECP received baseline evaluations, including a history and physical examination, complete blood count with differential, and complete chemistry panel. When indicated, patients were evaluated by an opthalmologist. Skin, liver, or mucous membrane biopsies were performed as clinically indicated. Physical assessment and laboratory studies were repeated every 2 weeks during the study.
Patients with extensive chronic GVHD received Pneumocystis carinii and pneumococcal prophylaxis, as well as intravenous Ig replacement if infections occurred in the setting of Ig deficiency. Supportive care with artificial tear replacements, sun-blocking creams, and oral caloric supplements was administered as required.
At study entry, all patients were in hematologic remission with donor marrow engraftment.
One patient has been previously reported.13
Data were analyzed as of January 31, 1998. Survival rates were estimated by the Kaplan-Meier method.
Evaluation criteria.
From 1993 on, all consecutive patients with chronic extensive GVHD were treated with ECP; from 1996 on, patients with clinicopathologic diagnosis of acute GVHD resistant to steroid treatment were included. Because of the limited capacity at our institution, only a small number of patients with acute GVHD could receive ECP.
The clinical diagnosis of GVHD was confirmed by histopathology of the skin (see below) and, if indicated, liver biopsies and clinically graded as 0 through IV for acute GVHD by the criteria reported19 and as none, limited, or extensive for chronic GVHD.20 Progressive onset chronic GVHD developed as a direct extension of acute GVHD, quiescent chronic GVHD occurred after resolution of acute GVHD, and de novo chronic GVHD was not preceeded by acute GVHD. Diagnosis was established upon review of clinical, laboratory, and histologic data by previously published criteria.19-22 Complete organ responses of chronic GVHD were defined as resolution of skin, joint, oral mucosa, liver, or ocular manifestations. Partial responses were defined as a greater than 50% response in organ involvement, but less than a complete response. No change was defined as stable organ involvement, despite the tapering of other immunosuppressive agents by at least 50% of the dosage. No response referred to progressive worsening of chronic GVHD and the inability to taper other medications.
ECP.
ECP was performed using the UVAR photopheresis system (Therakos, West Chester, PA), as described.9,13 Briefly, 240 mL of buffy coat and 300 mL of plasma were collected during each treatment by a standard apheresis procedure and diluted with 200 mL of saline solution. 8-MOP (0.2 mg; Gerot, Vienna, Austria) was added to the final enriched lymphocyte solution containing 6 × 109cells, as described.21 The solution was passed as a film, 1-mm thick, through a disposable plastic device, exposed to a UVA light source (2 J/cm2/cell) for 90 minutes, and then returned to the patient. The mean treatment time for the photopheresis procedure was 3.5 hours. Only peripheral vein catheters were used.
Treatment protocol.
ECP was initiated when the white blood cell count was greater than 1 × 109/L. Patients were treated on 2 consecutive days at 2-week intervals for the first 3 months and thereafter every 4 weeks until resolution of GVHD. All adverse effects observed during the treatments were recorded. Informed consent was obtained by the patients, and the use of ECP was approved by the local medical ethics committee.
Skin biopsy.
Four-millimeter punch biopsies were performed at onset of cutaneous GVHD, before ECP, and after 6 months on ECP. Skin biopsies were fixed in formalin, embedded in paraffin, and stained with haematoxylin and eosin. Sections were evaluated under the light microscope. The histopathologic diagnosis of acute cutaneous GVHD grade II to IV was based on the criteria and grading as suggested by Lerner et al.22 Thus, basal cell vacuolization with or without further epidermal changes was graded as cutaneous GVHD I, whereas the additional presence of single necrotic/apoptotic keratinocytes (mummified cells) and the presence of satellite lymphocytes was diagnosed as cutaneous GVHD II. Basal cleft formation together with the above-described histological changes was used for establishing grade III, and frank necrosis of the epidermis was regarded as a hallmark for grade IV cutaneous GVHD. In addition to the epidermal changes, mononuclear infiltrates were described as perivascular in acute cutaneous GVHD.23 More pronounced epidermal changes in addition to basal cell vacuolization, necrotic/apoptotic keratinocytes, satellite lymphocytes, hyperkeratosis, and acanthosis together with a sparse bandlike infiltrate at the dermo-epidermal junction composed by mononuclear cells were regarded as a typical lichen planus-like pattern according to Shulman et al.24 Whenever atrophy of the epidermis with coarse collagen bundles and fibrosis/sclerosis of the dermis with loss of skin appendages was observed, the histologic diagnosis of scleroderma-like cutaneous GVHD was made.
RESULTS
Response of patients with acute GVHD.
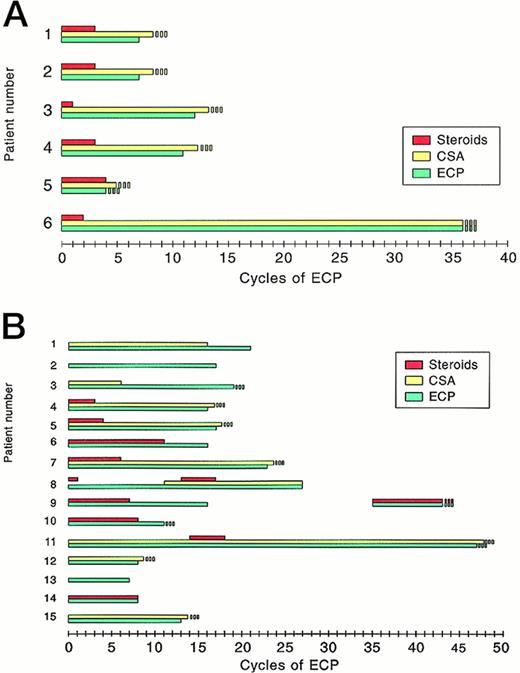
Six patients experienced acute GVHD II to III at a median of 16 days (range, 12 to 21 days) after BMT and did not respond to CSA and corticosteroids (2 mg to 10 mg/kg body weight) administered for a median of 28 days, as shown in Table2. Therefore, ECP was started after a median of 46 days after BMT, allowing a rapid reduction of corticosteroids and discontinuation after a median of 38 days without a further increase in GVHD activity. In 4 of 6 patients, ECP was terminated after 9 to 13 cycles (Fig 1A) when complete resolution of GVHD was observed (Table 2). Clinical results were confirmed by histologic evaluation of skin biopsies with absence of basal cell vacuolization and apoptotic cells as well as of satellite lymphocytes. Perivascular mononuclear infiltrates within the upper dermis appeared reduced or were absent. Immunosuppression with CSA was continued, but the dosage was subsequently reduced (Fig 1A). In one of these patients (patient no. 4), chronic limited GVHD of skin and ocular mucosa evolved 28 months after BMT and 17 months after discontinuation of ECP. Another patient (patient no. 1) developed chronic GVHD with chronic wasting syndrome and keratoconjunctivitis 150 days after BMT. Two patients who achieved partial resolution are still under ECP.
Results of ECP in Patients With Acute GVHD
| Patient No. . | Acute GVHD . | Steroids Before ECP . | Therapy at Start of ECP . | Interval BMT-ECP (d) . | No. of ECP Cycles . | Duration of ECP (mo) . | Response to ECP . | Current Therapy . | Time Off ECP (mo) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade . | Onset (d) . | Max. Dose (mg) . | Duration (d) . | Skin . | Liver . | |||||||
| 1 | II | 12 | 4 | 14 | CSA + MP | 30 | 9 | 5 | CR | NA | CSA | 1 |
| 2 | II | 14 | 4 | 23 | CSA + MP | 37 | 9 | 5 | CR | CR | CSA | 1 |
| 3 | III | 18 | 3 | 29 | CSA + MP | 56 | 13 | 7 | CR | NA | CSA | 1 |
| 4 | II | 21 | 2 | 49 | CSA + MP | 70 | 12 | 9 | CR | NA | NONE | 17 |
| 5 | III | 15 | 10 | 22 | CSA + MP | 37 | 5 | 3 | PR | NA | CSA | NA* |
| 6 | III | 13 | 2 | 32 | CSA + MP | 45 | 36 | 24 | PR | CR | CSA | NA* |
| Patient No. . | Acute GVHD . | Steroids Before ECP . | Therapy at Start of ECP . | Interval BMT-ECP (d) . | No. of ECP Cycles . | Duration of ECP (mo) . | Response to ECP . | Current Therapy . | Time Off ECP (mo) . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade . | Onset (d) . | Max. Dose (mg) . | Duration (d) . | Skin . | Liver . | |||||||
| 1 | II | 12 | 4 | 14 | CSA + MP | 30 | 9 | 5 | CR | NA | CSA | 1 |
| 2 | II | 14 | 4 | 23 | CSA + MP | 37 | 9 | 5 | CR | CR | CSA | 1 |
| 3 | III | 18 | 3 | 29 | CSA + MP | 56 | 13 | 7 | CR | NA | CSA | 1 |
| 4 | II | 21 | 2 | 49 | CSA + MP | 70 | 12 | 9 | CR | NA | NONE | 17 |
| 5 | III | 15 | 10 | 22 | CSA + MP | 37 | 5 | 3 | PR | NA | CSA | NA* |
| 6 | III | 13 | 2 | 32 | CSA + MP | 45 | 36 | 24 | PR | CR | CSA | NA* |
Abbreviations: Max, maximum; CSA, cyclosporine A; MP, methylprednisolone; CR, complete remission; PR, partial remission; NA, not applicable; NA*, still under ECP.
Therapy of (A) acute and (B) chronic GVHD. On the x-axis, the cycles of extracorporeal photochemotherapy (1 cycle = 1 therapy on 2 consecutive days) are shown; on the y-axis, the number of patients treated is shown.
Therapy of (A) acute and (B) chronic GVHD. On the x-axis, the cycles of extracorporeal photochemotherapy (1 cycle = 1 therapy on 2 consecutive days) are shown; on the y-axis, the number of patients treated is shown.
Response of patients with chronic GVHD.
Fifteen patients had extensive chronic GVHD after a median of 178 days after BMT. Eight had a progressive presentation of chronic GVHD, 6 had a quiescent onset, and 1 patient presented with de novo chronic GVHD, as shown in Table 3. Histologically, 7 patients had sclerodermoid, 5 had combined lichenoid and sclerodermatous, and 3 had lichen planus-like GVHD, as described.23 Before ECP, immunosuppressive therapy consisted of CSA (n = 11), steroids up to 2 mg/kg body weight (n = 13), azathioprine (n = 1), thalidomide (n = 2), and PUVA (n = 2). Because patients experienced increasing GVHD activity, ECP was started after a median of 12 months (range, 3 to 44 months) after BMT (Table3). At the initiation of ECP, 5 patients (33%) were receiving CSA alone, 3 (20%) were receiving CSA and steroids, 4 (27%) were receiving steroids alone, 1 (7%) were receiving steroids and thalidomide, and 2 patients (13%) were receiving no systemic immunosuppressive therapy. Patients received 7 to 47 cycles (median, 18 cycles) of ECP within 4 to 31 months, as shown in Table 3 and Fig 1B. During ECP, steroid therapy could be discontinuated after a median of 80 days. As of January 31, 1998, 11 patients are off ECP, including 1 (patient no. 8) with abrupt discontinuation of ECP after relapse of high- grade non-Hodgkin’s lymphoma. Four patients are currently under ECP because of partial resolution of GVHD manifestations (n = 3) or recurrence of GVHD (n = 1) 3 months after first discontinuation of ECP. Five patients remain under immunosuppressive therapy.
Results of ECP in Patients With Chronic GVHD
| Patient No. . | Acute GVHD . | Chronic GVHD . | Therapy Before ECP . | BMT-ECP (mo) . | No. of ECP Cycles . | Duration of ECP (mo) . | Response to ECP . | Current Therapy . | Time Off ECP (mo) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade . | Onset (d) . | Type . | Histo . | Onset (d) . | Skin . | Joints . | Mouth . | Liver . | Ocular . | Thrombopenia . | |||||||
| 1 | III | 10 | P | S | 110 | CSA | 6 | 21 | 15 | CR | PR | CR | CR | NA | CR | A | 25 |
| 2 | II | 14 | P | S | 100 | CSA, MP | 17 | 17 | 14 | CR | NA | CR | NA | NA | NA | NONE | 9 |
| 3 | I | 19 | P | L + S | 110 | CSA, MP | 17 | 19 | 11 | PR | NA | CR | CR | NA | NA | NONE | NA |
| 4 | II | 11 | P | L | 63 | CSA, MP | 3 | 16 | 10 | CR | NA | NA | NA | NA | NA | NONE | 27 |
| 5 | II | 15 | Q | S | 229 | CSA, MP | 13 | 17 | 10 | CR | PR | CR | NA | CR | NA | A | 16 |
| 6 | I | 19 | P | L + S | 154 | MP | 8 | 16 | 8 | CR | NA | CR | CR | PR | NA | NONE | 1 |
| 7 | II | 16 | Q | S | 397 | CSA, MP, T | 23 | 23 | 22 | CR | PR | CR | CR | NA | NA | CSA | 13 |
| 8 | II | 23 | Q | S | 161 | MP, A | 11 | 27 | 18 | PR | PR | CR | PR | PR | NA | NONE | NA* |
| 9 | II | 20 | P | S | 74 | CSA, MP | 3 | 16 | NA | CR | NA | CR | CR | PR | NA | MP, A | NA* |
| 10 | I | 16 | P | L + S | 100 | CSA, MP, T, PUVA | 14 | 11 | 6 | CR | NA | CR | PR | PR | NC | NONE | NA* |
| 11 | II | 20 | P | L + S | 83 | CSA, MP | 5 | 47 | 31 | PR | NA | CR | NC | NC | CR | CSA | NA* |
| 12 | II | 20 | Q | L + S | 120 | CSA | 4 | 8 | 4 | CR | NA | NA | CR | NA | NA | NONE | 22 |
| 13 | 0 | NA | N | S | 730 | MP, PUVA | 44 | 7 | 6 | CR | NA | NA | NA | NA | NA | NONE | 16 |
| 14 | III | 11 | Q | L | 140 | MP | 11 | 8 | 5 | CR | NA | CR | CR | NA | NA | NONE | 21 |
| 15 | II | 22 | Q | L | 104 | CSA, MP | 4 | 12 | 11 | CR | NA | NA | NA | NA | NA | NONE | 24 |
| Patient No. . | Acute GVHD . | Chronic GVHD . | Therapy Before ECP . | BMT-ECP (mo) . | No. of ECP Cycles . | Duration of ECP (mo) . | Response to ECP . | Current Therapy . | Time Off ECP (mo) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade . | Onset (d) . | Type . | Histo . | Onset (d) . | Skin . | Joints . | Mouth . | Liver . | Ocular . | Thrombopenia . | |||||||
| 1 | III | 10 | P | S | 110 | CSA | 6 | 21 | 15 | CR | PR | CR | CR | NA | CR | A | 25 |
| 2 | II | 14 | P | S | 100 | CSA, MP | 17 | 17 | 14 | CR | NA | CR | NA | NA | NA | NONE | 9 |
| 3 | I | 19 | P | L + S | 110 | CSA, MP | 17 | 19 | 11 | PR | NA | CR | CR | NA | NA | NONE | NA |
| 4 | II | 11 | P | L | 63 | CSA, MP | 3 | 16 | 10 | CR | NA | NA | NA | NA | NA | NONE | 27 |
| 5 | II | 15 | Q | S | 229 | CSA, MP | 13 | 17 | 10 | CR | PR | CR | NA | CR | NA | A | 16 |
| 6 | I | 19 | P | L + S | 154 | MP | 8 | 16 | 8 | CR | NA | CR | CR | PR | NA | NONE | 1 |
| 7 | II | 16 | Q | S | 397 | CSA, MP, T | 23 | 23 | 22 | CR | PR | CR | CR | NA | NA | CSA | 13 |
| 8 | II | 23 | Q | S | 161 | MP, A | 11 | 27 | 18 | PR | PR | CR | PR | PR | NA | NONE | NA* |
| 9 | II | 20 | P | S | 74 | CSA, MP | 3 | 16 | NA | CR | NA | CR | CR | PR | NA | MP, A | NA* |
| 10 | I | 16 | P | L + S | 100 | CSA, MP, T, PUVA | 14 | 11 | 6 | CR | NA | CR | PR | PR | NC | NONE | NA* |
| 11 | II | 20 | P | L + S | 83 | CSA, MP | 5 | 47 | 31 | PR | NA | CR | NC | NC | CR | CSA | NA* |
| 12 | II | 20 | Q | L + S | 120 | CSA | 4 | 8 | 4 | CR | NA | NA | CR | NA | NA | NONE | 22 |
| 13 | 0 | NA | N | S | 730 | MP, PUVA | 44 | 7 | 6 | CR | NA | NA | NA | NA | NA | NONE | 16 |
| 14 | III | 11 | Q | L | 140 | MP | 11 | 8 | 5 | CR | NA | CR | CR | NA | NA | NONE | 21 |
| 15 | II | 22 | Q | L | 104 | CSA, MP | 4 | 12 | 11 | CR | NA | NA | NA | NA | NA | NONE | 24 |
Abbreviations: P, progressive; Q, quiescent; N, de novo; S, sclerodermoid; L, lichen planus-like; L + S, lichenoid and sclerodermatous; T, thalidomide; A, azathioprine; NC, no change; NA*, still under ECP.
Response rates for different organ involvement are shown in Table 3. Twelve of 15 patients (80%) treated for chronic cutaneous GVHD achieved a complete response with clinically normal appearing skin and resolution of postinflammatory hyperpigmentation and fibrosis/sclerosis (Fig 2). No difference in response rates in sclerodermatous or lichenoid involvement was observed. All patients with oral involvement showed a complete response. Seven of 10 patients with liver involvement (70%) experienced complete resolution, as seen in normalization of serum bilirubin and alkaline phosphatase levels. Both patients with chronic wasting syndrome markedly gained weight. Two of 3 patients (67%) with thrombocytopenia had an increase in peripheral blood platelet counts during ECP. All patients with contractures experienced partial improvement in joint mobility. In 1 patient (17%), ocular symptoms resolved completely; ocular symptoms resolved partially in 4 patients (66%).
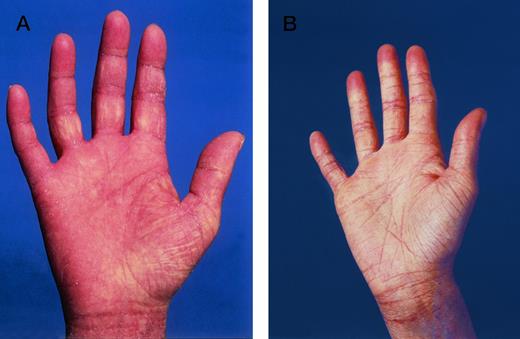
Lichenoid GVHD involvement of the palms in patient no. 6 (Fig 1A) (A) before and (B) in complete remission after ECP.
Lichenoid GVHD involvement of the palms in patient no. 6 (Fig 1A) (A) before and (B) in complete remission after ECP.
Infectious and toxic complications.
Nine of 15 patients (60%) experienced infections after the onset of chronic GVHD before ECP. The most common infections were bacteremia in patients with persistent central venous catheters (2 Staphylococcus, 1 Pseudomonas, 1 Hemophilus influenzae, and 1 Pneumococcus), pneumonia (2 Pneumococcus and 2 idiopathic), and CMV reactivation, as shown in Table 4. During ECP, the predominant infectious complications were pansinusitis and pneumonia (1 Streptococcus, 1 Hemophilus influenzae, 1 Pneumococcus, and 1 idiopathic). No lethal infections occurred. After termination of ECP, no further infections requiring antimicrobial or antiviral therapy were seen.
Infections in Patients With Chronic GVHD
| . | Before ECP (no. of patients/episodes) . | During ECP (no. of patients/episodes) . | ||
|---|---|---|---|---|
| Bacterial | ||||
| Bacteremia | 3 | 5 | 3 | 3 |
| Pneumonia | 3 | 4 | 2 | 4 |
| Pansinusitis | 2 | 2 | 4 | 7 |
| Bronchitis | 2 | 2 | 1 | 3 |
| Otitis/Parotitis | 0 | 0 | 2 | 2 |
| Viral | ||||
| Herpes stomatitis | 1 | 1 | 2 | 2 |
| Herpes zoster | 2 | 2 | 2 | 2 |
| CMV reactivation | 6 | 7 | 2 | 3 |
| Fungal | ||||
| Stomatitis | 2 | 2 | 2 | 2 |
| Fever of unknown origin | 1 | 1 | 1 | 1 |
| . | Before ECP (no. of patients/episodes) . | During ECP (no. of patients/episodes) . | ||
|---|---|---|---|---|
| Bacterial | ||||
| Bacteremia | 3 | 5 | 3 | 3 |
| Pneumonia | 3 | 4 | 2 | 4 |
| Pansinusitis | 2 | 2 | 4 | 7 |
| Bronchitis | 2 | 2 | 1 | 3 |
| Otitis/Parotitis | 0 | 0 | 2 | 2 |
| Viral | ||||
| Herpes stomatitis | 1 | 1 | 2 | 2 |
| Herpes zoster | 2 | 2 | 2 | 2 |
| CMV reactivation | 6 | 7 | 2 | 3 |
| Fungal | ||||
| Stomatitis | 2 | 2 | 2 | 2 |
| Fever of unknown origin | 1 | 1 | 1 | 1 |
Three of 6 patients with severe acute GVHD had a decrease in their ANC after the first cycles of ECP without infectious complications. Two to 5 days later, their ANC increased again. One patient experienced heparin-induced thrombocytopenia and another patient experienced hemolytic uremic syndrome that resolved after CSA discontinuation and plasmapheresis.
Survival.
All 6 patients treated for acute GVHD and 14 of 15 patients (93%) with chronic GVHD are alive and well, with a median follow-up time after termination of ECP of 5 months for acute GVHD and 15 months for chronic GVHD. One patient died of a relapse of high-grade non-Hodgkin’s lymphoma 3 years after BMT. In the surviving patients, Karnofsky performance scores improved from 50% before ECP to at least 90% after ECP, respectively.
DISCUSSION
ECP has been shown to be effective in the treatment of cutaneous T-cell lymphoma, autoimmune diseases such as pemphigus vulgaris, systemic lupus erythematosus, progressive systemic sclerosis, and rejection after organ transplantation.9-12 Because of the pathogenetic and clinical similarities of chronic GVHD to diseases responsive to ECP, this treatment modality has been already used in selected patients with severe resistant chronic GVHD.13-15 25 There, complete responses have been reported in patients with skin (6/8), oral mucosal (3/6), liver (1/3), lung (1/5), and gut (1/2) involvement.
Our report is the first to provide detailed information of the so-far largest series of patients receiving ECP for chronic GVHD. Considering the fact that only patients with histologically proven extensive chronic GVHD were included in the study and that 87% had received prior systemic immunosuppression without response, our results with ECP appear to be very promising. Skin and oral mucosal affection due to chronic GVHD completely resolved in 80% and 100% of patients, respectively. Even liver involvement resolved completely in 70% of patients. Partial responses were seen in patients with joint involvement or keratoconjunctivitis.
Conventional treatment of GVHD, which usually includes prolonged and high-dose corticosteroids, may be responsible for severe side effects, including Cushing’s syndrome, hypertension, renal failure, or life-threatening infections. In our study at the initiation of ECP, all patients with acute and 8 of 15 patients (53%) with chronic GVHD were receiving steroids. Once ECP was started, gradual improvements allowed a timely reduction and, finally, discontinuation of steroids without an increase in GVHD activity. No deaths due to infections or metabolic complications were observed in our patients. Respiratory infections and particularly sinusitis are frequently seen in patients with chronic GVHD resulting from a combination of the sicca syndrome in the sinuses and a predisposition to bacterial infections.5 Reactivation of latent CMV infection occurs in approximately 70% of CMV-seropositive marrow graft recipients and 30% of CMV-seronegative patients whose donors are seropositive.26 The use of ECP in our group of patients did not increase the rate of infections compared with results previously published.5,6 In fact, our findings confirm reports on patients treated with ECP without increased sensitivity to bacterial or viral infections.25
Because the risk of central venous catheter-related infectious complications has to be considered in immunosuppressed patients, all ECP treatments were performed by additional access through suitable veins in the forearms. In view of the fact that no septicemia occurred in our patients, we recommend this approach for future patients.
So far, only a few patients with acute GVHD treated with ECP without achieving response have been reported.14,25 In our study, complete resolution of both skin and liver involvement was seen in 4 of 6 patients with steroid-resistant acute GVHD, and 2 additional patients are responding but currently still under ECP. Aside from a reversible decrease in peripheral blood neutrophil counts during the first cycles of ECP, when hematologic regeneration after BMT was still incomplete, no major side effects were observed in these patients. The fact that in our study ECP was administered over a substantially longer time period as compared with previous reports14 25 may explain the response rates achieved in our patients.
Our treatment schedule is based on the experience gained in patients treated with ECP where ECP administered on 2 consecutive days appeared to be more effective than single-day treatment courses.9,12,25 Because our patients with extensive chronic GVHD differed with regard to prior immunosuppressive therapies and duration of GVHD on the one hand and the aim of this study was to assess the efficacy and feasibility of ECP in these patients on the other hand, treatment modalities applied in addition to ECP and therapy duration varied. So far, after a median observation time of 12 months after discontinuation of ECP, only 3 patients (14%) had recurrence of GVHD. ECP was tolerated excellently, without any significant side effects. The hemolytic uremic syndrome that had resolved after CSA discontinuation was probably not related to ECP, because similar observations have been reported on patients treated with CSA therapy alone.27
The exact mechanisms by which ECP leads to the described responses in GVHD as well as other T-cell–mediated diseases have not been elucidated. The hypothesis that ECP may be involved in augmenting the apoptotic process leading to deletion of graft reactive cells after tolerance induction by donor bone marrow is supported by recent observations and points to further directions of investigations.28-31
In summary, our results demonstrate that ECP is a safe and efficacious adjunct therapy for patients with both acute and chronic GVHD. However, randomized studies are mandatory to evaluate the impact of extracorporeal photochemotherapy on the course of GVHD and overall survival.
ACKNOWLEDGMENT
The authors thank the dedicated nurses of our stem cell transplant program and the ECP unit, our fellows and house staff, the medical technicians, and the physicians who referred patients to our unit.
Address reprint requests to Hildegard T. Greinix, MD, AKH Wien, Klinik fuer Innere I, Knochenmarktransplantation, Waehringer Guertel 18-20, A-1090 Vienna, Austria.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal