Abstract
Platelet membrane glycoprotein Ib (GPIb) is a major receptor for von Willebrand factor and thrombin, which plays a key role in the initial development of thrombi. Two polymorphisms (HPA-2 and VNTR) that affect phenotype have been described in GPIb. The relevance of these polymorphisms to thrombotic disease was investigated by genotypic identification in three case-control studies: 104 case patients with acute cerebrovascular disease (CVD), 101 case patients with acute coronary heart disease (CHD), 95 patients with deep venous thrombosis (DVT), and one control age-, sex-, and race-matched for each case patient. Results show that the C/B genotype of the VNTR and the HPA-2b polymorphisms of GPIb are strongly associated with increased risk of coronary heart disease and cerebral vascular disease but not with deep vein thrombosis. These two polymorphisms of GPIb may represent newly identified risk factors for arterial thrombotic disease, but not for venous thrombosis.
© 1998 by The American Society of Hematology.
ARTERIAL THROMBOSIS is a principal human affliction causing approximately 50% of the nonaccident deaths in developed nations. Over the past 2 decades, numerous studies have reported platelet hyperaggregability in patients with coronary heart disease (CHD), thrombotic cerebrovascular disease (CVD), and peripheral vascular disease.1-4 Shear-induced binding of von Willebrand factor (vWF) to platelet glycoprotein (GP) Ibα, which occurs at high levels in diseased arteries and arterioles partially obstructed by atherosclerosis or vasospasm, induces the activation and aggregation of platelets without the addition of any agonist.5-9
GPIbα is a transmembranous platelet glycoprotein having a molecular weight (Mr) of 143,000 that forms a noncovalent complex with GPIbβ, GPIX, and GPV. There are approximately 25,000 GPIbα molecules per platelet.10 Two polymorphisms of GPIbα have been described. Size polymorphism was first described by Moroi et al,11 who noted four variants of GPIbα, which were designated A, B, C, and D in order of decreasing molecular mass, from 168 kD for variant A to 153 kD for variant D. The polymorphism was shown to result from a variable number of tandem repeats (VNTR) of 39 bp in the macroglycopeptide region of GPIbα: either one (D allele), two (C allele), three (B allele), or four (A allele). Each repeat leads at the protein level to additions of 13-amino acids and, thus, moves the vWF-binding domain farther out of the platelet membrane.12 13 A potential consequence of this structural change is to expose the molecule to greater shear forces, which may lower the threshold for shear-induced interaction with vWF and subsequent platelet activation.
Another polymorphism within the GPIbα coding region, a Thr in HPA-2a (Kob; Sib[b] negative) → Met in HPA-2b (Koa; Sib[a]) at position 145,14 is related to the antigenicity of HPA-2,15 which suggests that the aminoacidic change should cause a variation in the structure of the GPIbα molecule. The HPA-2 polymorphism is located close to the vWF and the high-affinity thrombin binding sites16,17 and might therefore influence the receptor function of these variants. DNA typing has recently showed that the HPA-2 polymorphism is in linkage disequilibrium with the VNTR polymorphism described above.18 HPA-2a is associated with the D or C variants, whereas HPA-2b is linked with the longest B and A alleles.
Different frequencies of these GPIbα variants have been found among distinct populations.10 In Caucasians, the C (HPA-2a) allele is the most prevalent, with a frequency of 80%, followed by the D (HPA-2a) and B (HPA-2b) alleles with a similar 10% frequency, whereas the largest variant A (HPA-2b) is uncommon.10 19-22
The aim of the present study was to perform an analysis of the two polymorphisms of GPIbα, one affecting the distance by which the active sites of platelet GPIbα extend from the surface and the other altering the vicinity of the GPIbα active sites, in relation with the development of arterial thrombotic disorders. Thus, we conducted two retrospective case-control studies both in survivors from acute cerebrovascular events and in patients diagnosed of acute coronary syndromes and examined the relevance of those polymorphisms in a venous thromboembolism setting.
MATERIALS AND METHODS
Selection of case patients and control subjects.
Studies on case patients and control subjects were approved by the local ethics committee, and all participants gave their informed consent. Genotypic analyses were performed on 104 consecutive CVD patients referred to our institution and diagnosed with transient ischemic attack (TIA; n = 31), minor stroke (n = 44), or cerebral infarction (n = 29). Diagnosis was attained according to the classification of cerebrovascular diseases of the National Institute of Neurological Disorders and Stroke ad Hoc Committee.23 One hundred one consecutive patients who survived a primary acute coronary event and were admitted to the Coronary Unit with an established diagnosis of myocardial infarction (n = 69) or unstable angina (n = 32), according to the World Health Organization criteria,24were also enrolled in the CHD study. Moreover, 95 consecutive patients with a confirmed diagnosis (by compression ultrasonography or contrast venography) of deep venous thrombosis (DVT) were evaluated. All included cases of CHD, CVD, or DVT were age- and sex-matched to a different control who had no documented history of vascular disease. All case patients and control subjects were Mediterranean Caucasians. CVD and CHD controls were additionally chosen approximating criteria to match selected risk factors for arterial thrombotic disease (smoking history, blood pressure, total serum cholesterol level, and diabetes status) with their respective case patient. These 300 distinct controls were selected by reviewing patient charts from a population of patients admitted to the hospital who had no documented history of vascular disease. Prespecified subgroup analyses were performed with stratification by age (≤60 or >60 years of age), sex, type of coronary acute event (myocardial infarction or unstable angina), and type of cerebrovascular acute event (TIA, minor stroke, or cerebral infarction). For the DVT case/control study, the intake of oral contraceptives, the presence of lupus anticoagulant, and the prevalence of two genetic risk factors for this disease (factor V Leiden and prothrombin 20210 A allele) were investigated. The identification of such genetic markers was performed as previously described.25 26
Blood collection and DNA isolation.
Blood samples were obtained by atraumatic venipuncture collection into 1:10 volume of EDTA (Vacutainer; Becton Dickinson, Meylon, France). Total genomic DNA was obtained from peripheral blood after lysis with sodium dodecyl sulfate (SDS) and proteinase K treatment of buffy coat. DNA was purified using phenol/chloroform and ethanol precipitation.
DNA amplification of the macroglycopeptide region (VNTR) and genotyping of HPA-2.
Identification of the VNTR polymorphism of the GPIbα gene was performed by genomic polymerase chain reaction (PCR) of the macroglycopeptide region using two oligonucleotide primers: VNTR-F3 and VNTR-B4 (corresponding to nucleotides 4202-4223 and 4378-4400, respectively; nucleotide number according to Wenger et al27), with modifications from Ishida et al.13To confirm these results, we performed a second genomic PCR using a different set of primers: VNTR-F2 (nucleotides 3915-3938) and VNTR-B5 (4372-4393). PCR products were electrophoresed through acrylamide gels and stained with AgNO3, as previously described.25
Two oligonucleotide primers, HPA-2F and HPA-2B (nucleotides 3435-3455 and 4001-4021, respectively), were used in a PCR to generate a 587-bp fragment of the GPIbα gene involving the HPA-2 polymorphism (modified from Ishida et al13). Amplified products were digested withBsaHI (New England BioLabs, Beverly, MA), electrophoresed through 7% acrylamide gels, and stained with AgNO3. Confirmation of the HPA-2 genotype was achieved in all cases using single-strand conformation polymorphism analysis (SSCP).28
Genotyping of both VNTR and HPA-2 was always performed blinded as to whether the DNA sample was from a case patient or a control.
Statistical analysis.
The Student’s t-test was used to compare age. All other variables were analyzed by the χ2 test. A P value ≤.05 was considered to indicate statistical significance. The strength of the association of the polymorphisms with the occurrence of thrombotic events was estimated by calculation of the odds ratio (OR) with the EpiInfo software (Division of Surveillance and Epidemiology, CDC, Atlanta, GA) and the Cornfield method for the calculation of 95% confidence intervals (CI).
RESULTS
Characteristics of the study population.
Table 1 shows the age and sex of the study subjects. No significant differences were found in the prevalence of selected risk factors for arterial thrombosis (hypercholesterolemia, diabetes, hypertension, or smoking) among patients and controls in the CVD and CHD case/control studies. However, significant differences were observed for the family history of CVD and/or CHD between these patients and controls.
Age and Sex of Subjects of Study and Prevalence of Selected Risk Factors for Arterial and Venous Thrombosis Among Case Patients and Controls
| . | CVD . | CHD . | . | DVT . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n = 104) . | Controls (n = 104) . | P-150 . | Patients (n = 101) . | Controls (n = 101) . | P-150 . | Patients (n = 95) . | Controls (n = 95) . | P-150 . | ||
| Age (yr) | Age (yr) | |||||||||
| Range | 24-88 | 24-89 | .996 | 34-85 | 32-86 | .595 | Range | 19-87 | 19-87 | .984 |
| Mean ± SD | 65.8 ± 13.8 | 65.8 ± 14.5 | 62.9 ± 11.1 | 63.7 ± 10.9 | Mean ± SD | 61.0 ± 14.7 | 61.0 ± 14.7 | |||
| Male sex (%) | 51.9 | 51.9 | 1 | 73.3 | 73.3 | 1 | Male sex (%) | 56.8 | 56.8 | 1 |
| Risk factors (%) | Risk factors (%) | |||||||||
| Hypertension-151 | 46.2 | 38.5 | .262 | 49.5 | 38.6 | .119 | FV Leiden +/− | 11.6 | 4.2 | .059 |
| Current/former smoker | 29.8 | 32.7 | .654 | 51.5 | 51.5 | 1 | FII 20210 A/G | 9.5 | 1.1 | .009 |
| Hypercholesterol emia-152 | 31.7 | 26.9 | .446 | 42.6 | 36.6 | .388 | OCs | 1.0 | 0 | .316 |
| Type I or II diabetes | 35.6 | 28.6 | .299 | 37.6 | 31.7 | .375 | LA | 0 | 0 | 1 |
| Family history-153 | 52.9 | 36.6 | .018 | 61.4 | 31.7 | .001 | ||||
| . | CVD . | CHD . | . | DVT . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (n = 104) . | Controls (n = 104) . | P-150 . | Patients (n = 101) . | Controls (n = 101) . | P-150 . | Patients (n = 95) . | Controls (n = 95) . | P-150 . | ||
| Age (yr) | Age (yr) | |||||||||
| Range | 24-88 | 24-89 | .996 | 34-85 | 32-86 | .595 | Range | 19-87 | 19-87 | .984 |
| Mean ± SD | 65.8 ± 13.8 | 65.8 ± 14.5 | 62.9 ± 11.1 | 63.7 ± 10.9 | Mean ± SD | 61.0 ± 14.7 | 61.0 ± 14.7 | |||
| Male sex (%) | 51.9 | 51.9 | 1 | 73.3 | 73.3 | 1 | Male sex (%) | 56.8 | 56.8 | 1 |
| Risk factors (%) | Risk factors (%) | |||||||||
| Hypertension-151 | 46.2 | 38.5 | .262 | 49.5 | 38.6 | .119 | FV Leiden +/− | 11.6 | 4.2 | .059 |
| Current/former smoker | 29.8 | 32.7 | .654 | 51.5 | 51.5 | 1 | FII 20210 A/G | 9.5 | 1.1 | .009 |
| Hypercholesterol emia-152 | 31.7 | 26.9 | .446 | 42.6 | 36.6 | .388 | OCs | 1.0 | 0 | .316 |
| Type I or II diabetes | 35.6 | 28.6 | .299 | 37.6 | 31.7 | .375 | LA | 0 | 0 | 1 |
| Family history-153 | 52.9 | 36.6 | .018 | 61.4 | 31.7 | .001 | ||||
Abbreviations: CVD, cerebrovascular disease; CHD, coronary heart disease; DVT, deep venus thrombosis; FV, factor V; FII, factor II; OCs, oral contraceptives; LA, lupus anticoagulant.
Student’s t-test (for age) and the χ2 test (for all other variables) were used to compare the values for case patients and controls.
Hypertension was defined as a systolic blood pressure ≥140 mm Hg at the time of admission to the hospital.
Hypercholesterolemia was defined as a total serum cholesterol level ≥5.72 mmol/L (220 mg/dL) at the time of admission to the hospital.
Family history was defined as at least one first-degree relative who had suffered from CVD and/or CHD thromboembolic episodes.
Genotyping of VNTR polymorphism and frequency of genotypes.
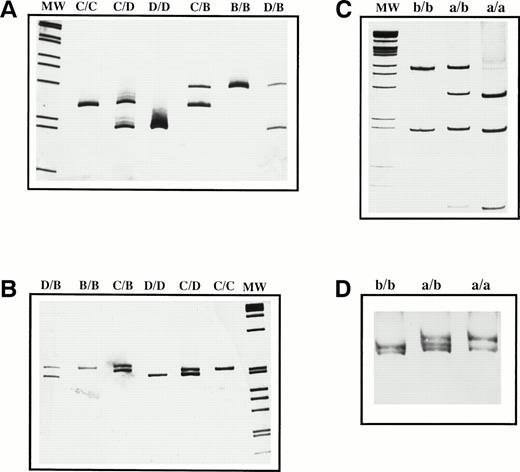
The amplification by PCR of the region of GPIbα encoding the VNTR polymorphism with the VNTR-F3 and VNTR-B4 oligonucleotide primers resulted in the identification of three visible fragments of 276, 237, and 198 bp on acrylamide gels, corresponding to the alleles B, C, and D, respectively (Fig 1A). To confirm the VNTR genotype, we performed a second PCR of the region using oligonucleotide primers VNTR-F2 and VNTR-B5 and amplified three different fragments of 557 bp (B allele), 518 bp (C allele), and 479 bp (D allele) (Fig 1B). The genotype obtained by these two PCR coincided in all samples tested. No allele A carriers were found.
Genetic determination of the VNTR and HPA-2 regions of GPIb. PCR analysis of the VNTR polymorphism was performed with two different sets of primers: VNTR F3/B4 (A) and VNTR F2/B5 (B). HPA-2 identification was performed by PCR-ASRA–BsaHI digestion (C) and confirmed by SSCP (D). Amplified products were resolved by acrylamide gel electrophoresis and stained with AgNO3. The genotype of the VNTR and HPA-2 polymorphisms is indicated at the top of each lane. MW represents the 1-kb marker ladder (GIBCO-BRL, Life Technologies, Barcelona, Spain).
Genetic determination of the VNTR and HPA-2 regions of GPIb. PCR analysis of the VNTR polymorphism was performed with two different sets of primers: VNTR F3/B4 (A) and VNTR F2/B5 (B). HPA-2 identification was performed by PCR-ASRA–BsaHI digestion (C) and confirmed by SSCP (D). Amplified products were resolved by acrylamide gel electrophoresis and stained with AgNO3. The genotype of the VNTR and HPA-2 polymorphisms is indicated at the top of each lane. MW represents the 1-kb marker ladder (GIBCO-BRL, Life Technologies, Barcelona, Spain).
The genotypes and allelic frequencies of the VNTR polymorphism in the case/control studies are summarized in Table 2. Genotype analyses showed an association of the C/B genotype and CVD (P = .0114; OR, 2.83; 95% CI, 1.16 to 7.07). A similar association was observed between the C/B genotype and CHD (P = .0047; OR, 2.84; 95% CI, 1.28 to 6.41). The prevalence of the B allele was significantly higher in the group of CVD patients than in the respective control group (12.02%v 5.77%; P = .0256; OR, 2.23; 95% CI, 1.04 to 4.86). The B allele was also more frequently identified among CHD case patients than in controls, although this difference did not reach significant levels (14.36% v 8.91%; P = .0878; OR, 1.71; 95% CI, 0.88 to 3.35). No statistically significant difference related to age, sex, and type of coronary or cerebrovascular acute event was detected in relationship to the VNTR genotype (data not shown).
Frequency of Genotypes and Haplotypes of the VNTR in Case Patients and Controls
| . | CVD . | CHD . | DVT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients (%) . | No. of Controls (%) . | P* (OR) . | No. of Patients (%) . | No. of Controls (%) . | P* (OR) . | No. of Patients (%) . | No. of Controls (%) . | P* (OR) . | |
| Genotype | P = .1761† | P = .0768† | P = .3153† | ||||||
| C/C | 70 (67.3) | 76 (73.1) | .363 | 55 (54.5) | 67 (66.3) | .084 | 65 (68.4) | 58 (61.1) | .288 |
| C/B | 22 (21.2) | 9 (8.7) | .011 (2.83) | 28 (27.7) | 12 (11.9) | .005 (2.84) | 10 (10.5) | 9 (9.5) | .809 |
| C/D | 9 (8.7) | 16 (15.4) | .136 | 16 (15.8) | 17 (16.8) | .849 | 16 (16.8) | 23 (24.2) | .209 |
| B/B | 1 (1.0) | 1 (1.0) | — | 0 | 2 (2.0) | — | 0 | 2 (2.1) | — |
| D/B | 1 (1.0) | 1 (1.0) | — | 1 (1.0) | 2 (2.0) | — | 2 (2.1) | 3 (3.2) | — |
| D/D | 1 (1.0) | 1 (1.0) | — | 1 (1.0) | 1 (1.0) | — | 2 (2.1) | 0 | — |
| Haplotype (%) | P = .0439† | P = .2311† | P = .5726† | ||||||
| C | 82.2 | 85.1 | .426 | 76.2 | 80.7 | .276 | 82.1 | 77.9 | .305 |
| B | 12.0 | 5.8 | .026 (2.23) | 14.4 | 8.9 | .088 (1.71) | 6.3 | 8.4 | .432 |
| D | 5.8 | 9.1 | .191 | 9.4 | 10.4 | .739 | 11.6 | 13.7 | .537 |
| . | CVD . | CHD . | DVT . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients (%) . | No. of Controls (%) . | P* (OR) . | No. of Patients (%) . | No. of Controls (%) . | P* (OR) . | No. of Patients (%) . | No. of Controls (%) . | P* (OR) . | |
| Genotype | P = .1761† | P = .0768† | P = .3153† | ||||||
| C/C | 70 (67.3) | 76 (73.1) | .363 | 55 (54.5) | 67 (66.3) | .084 | 65 (68.4) | 58 (61.1) | .288 |
| C/B | 22 (21.2) | 9 (8.7) | .011 (2.83) | 28 (27.7) | 12 (11.9) | .005 (2.84) | 10 (10.5) | 9 (9.5) | .809 |
| C/D | 9 (8.7) | 16 (15.4) | .136 | 16 (15.8) | 17 (16.8) | .849 | 16 (16.8) | 23 (24.2) | .209 |
| B/B | 1 (1.0) | 1 (1.0) | — | 0 | 2 (2.0) | — | 0 | 2 (2.1) | — |
| D/B | 1 (1.0) | 1 (1.0) | — | 1 (1.0) | 2 (2.0) | — | 2 (2.1) | 3 (3.2) | — |
| D/D | 1 (1.0) | 1 (1.0) | — | 1 (1.0) | 1 (1.0) | — | 2 (2.1) | 0 | — |
| Haplotype (%) | P = .0439† | P = .2311† | P = .5726† | ||||||
| C | 82.2 | 85.1 | .426 | 76.2 | 80.7 | .276 | 82.1 | 77.9 | .305 |
| B | 12.0 | 5.8 | .026 (2.23) | 14.4 | 8.9 | .088 (1.71) | 6.3 | 8.4 | .432 |
| D | 5.8 | 9.1 | .191 | 9.4 | 10.4 | .739 | 11.6 | 13.7 | .537 |
Abbreviations: CVD, cerebrovascular disease; CHD, coronary heart disease; DVT, deep venous thrombosis; OR, odds ratio.
χ2 test was used to compare the value of a particular VNTR genotype/haplotype among case patients and controls.
χ2 test was used to compare the values of the distribution of VNTR genotypes/haplotypes among case patients and controls.
By contrast, the DVT group of patients did not differ from the control group either in the C/B genotype frequency (10.53% v 9.47%;P = .8089) or in the B allele frequency (6.32% v8.42%; P = .4322).
Genotyping of HPA-2 polymorphism and frequency of genotypes.
The HPA-2a allele displayed a restriction pattern with BsaHI of 270, 201, and 116 bp, whereas the presence of two bands (386 and 201 bp) was distinctive of the HPA-2b allele (Fig 1C). HPA-2 genotyping, as determined by the SSCP method, was in complete concordance with results obtained with BsaHI digestion (Fig 1D).
Table 3 summarizes the genotyping data for all subjects of study. We found a significant association between HPA-2b and acute cerebrovascular events. Thus, of the 104 such patients, 22.11% carried at least one b allele, as compared with 10.58% of the controls (OR, 2.40; 95% CI, 1.04 to 5.63; P = .0244). Similarly, the prevalence of HPA-2b (percentage of subjects that were either heterozygous [a/b] or homozygous [b/b]) was significantly higher among CHD subjects than among controls (OR, 2.09; 95% CI, 0.98 to 4.49; P = .0375). No statistically significant difference related to age, sex, and type of coronary or cerebrovascular acute event was detected in relationship to the HPA-2 genotype (data not shown).
Genotypes of the HPA-2 Polymorphism in Case Patients and Controls
| . | CVD . | CHD . | DVT . | |||
|---|---|---|---|---|---|---|
| No. of Patients (%) . | No. of Controls (%) . | No. of Patients (%) . | No. of Controls (%) . | No. of Patients (%) . | No. of Controls (%) . | |
| Genotype | P = .0244 | OR = 2.40 | P = .0375 | OR = 2.09 | P = .6729 | |
| a/a | 81 (77.9) | 93 (89.4) | 74 (73.3) | 86 (85.2) | 83 (87.4) | 81 (85.3) |
| a/b + b/b | 22 + 1 (22.1) | 10 + 1 (10.6) | 27 + 0 (26.7) | 13 + 2 (14.9) | 12 + 0 (12.6) | 12 + 2 (14.7) |
| . | CVD . | CHD . | DVT . | |||
|---|---|---|---|---|---|---|
| No. of Patients (%) . | No. of Controls (%) . | No. of Patients (%) . | No. of Controls (%) . | No. of Patients (%) . | No. of Controls (%) . | |
| Genotype | P = .0244 | OR = 2.40 | P = .0375 | OR = 2.09 | P = .6729 | |
| a/a | 81 (77.9) | 93 (89.4) | 74 (73.3) | 86 (85.2) | 83 (87.4) | 81 (85.3) |
| a/b + b/b | 22 + 1 (22.1) | 10 + 1 (10.6) | 27 + 0 (26.7) | 13 + 2 (14.9) | 12 + 0 (12.6) | 12 + 2 (14.7) |
χ2 test was used to compare the values of the distribution of HPA-2 genotypes among case patients and controls.
Abbreviations: CVD, cerebrovascular disease; CHD, coronary heart disease; DVT, deep venous thrombosis; OR, odds ratio.
The prevalence of the HPA-2b allele was 12.63% in DVT patients and 14.74% in controls, a value that was not significantly different (P = .6729).
DISCUSSION
The study of genetic variations determining arterial thrombosis has already shown polymorphisms of plasma proteins29-37 or endothelial cell surface glycoproteins38-40 that can give rise to independent risk factors for thrombosis and/or atherosclerosis. Considering the interest to know whether polymorphisms of platelet glycoprotein receptors also represent risk factors in arterial thrombosis, to date only the association between the HPA-1b (PlA2) polymorphism of the GPIIIa has been reported in acute coronary thrombosis.41 However, we,42like others,43-48 found that association controversial.
To assess the association between the VNTR and HPA-2 polymorphisms of the GPIbα gene and arterial thrombosis, we compared the risk of CVD or CHD among case and control subjects matched for sex, race, and age. Considering that genetic and environmental factors act additively or synergistically to determine that individual’s risk of arterial thrombosis, our strategy for identification of polymorphisms predisposing to the disease was to avoid overrepresentation of classic vascular risk factors in the CVD and CHD groups compared with controls. Thus, we tried to approximate risk factors between every case patient and a single control who was age- and sex-matched. The association between the presence of the C/B genotype and arterial thrombosis was remarkably significant: for CHD and CVD, the presence of the C/B genotype was associated with almost a threefold increase in risk. The HPA-2b polymorphism of GPIbα, which is in linkage disequilibrium with A and B variants of the VNTR polymorphism,18 was also significantly associated with the occurrence of acute coronary events and with the development of CVD. By contrast, no statistically significant differences in the prevalence of the VNTR or HPA-2 polymorphisms were found between the case patients with DVT and the control subjects. Interestingly, the C/B (HPA-2a/b) genotype was found in 60% of subjects with a family history of CVD and/or CHD, whereas all other genotypes were present in 40% of those individuals. Thus, the findings of the present study suggest that the VNTR C/B and the HPA-2a/b genotypes of GPIα are associated with arterial thrombotic events. In agreement with our results, during the preparation of this manuscript, data from the Japanese population showed that the largest VNTR A and the HPA-2b alleles increased, in a way similar to that in our study, the risk of coronary heart disease,49 confirming that these GPIbα polymorphisms represent newly identified genetic risk factors for arterial thrombosis in Caucasian and Japanese populations.
Considering the present case/control studies, several points should be borne in mind when interpreting results. First, the study was performed in arterial thrombotic events survivors. Therefore, a survival bias cannot be avoided in the disease-association study, and it is likely that early mortality from CHD or CVD in patients could lead to an underestimation of the prothrombotic polymorphisms. Second, our study refers to the association between the VNTR and HPA-2 polymorphisms of GPIbα and arterial thrombosis, specifically in the Mediterranean Caucasian population. The relevance of these polymorphisms should be investigated in other populations and with prospective and family studies.
We cannot exclude the possibility that the VNTR and HPA-2 polymorphisms of the GPIbα gene could be simply genetically linked to the causative gene. Nevertheless, these two changes in the GPIbα could influence the susceptibility to arterial thrombosis. The single amino acid substitution (Thr145/Met145) in the neighbouring of the active sites of GPIbα16,17 and the alloantibody formation,15 determined by the HPA-2 polymorphism, suggest that the aminoacidic change might cause a conformational variation in the structure of the GPIbα molecule that may therefore be able to affect its function. On the other hand, the addition of repeats to the macroglycopeptide region of GPIbα, determined by the VNTR polymorphism, increases the distance between the ligand-binding domains of GPIbα and the platelet plasma membrane. The key question, as suggested by others,10,50 is whether variations in the distance by which the active sites of GPIbα extend from the surface influence platelet adhesion and the predisposition for pathologic thrombi to form by altering the susceptibility of platelets to shear-induced activation. Our study shows a significant association of C/B genotype carriers with the development of acute arterial thrombotic events. These findings support the previous hypothesis suggesting that the higher or lower number of repeats, and thus the greater or smaller overall length of the macroglycopeptide region of GPIbα, would have a prothrombotic or antihemostatic effect, respectively.12 Being that the HPA-2 polymorphism is genetically linked to the VNTR polymorphism, whether the HPA-2b is per se associated with arterial events or by means of its linkage disequilibrium with the size polymorphism of GPIbα remains to be elucidated. The finding of a stronger association of the C/B genotype with either CVD (OR, 2.83) or CHD (OR, 2.84), than between HPA-2 (a/b + b/b) and CVD (OR 2.40) or CHD (OR, 2.09; 95% CI, 0.98 to 4.49), could suggest a more relevant role of the VNTR polymorphism.
To date, very few studies have attempted to assess the influence of the HPA-2 and VNTR polymorphisms in platelet function. Mazzucato et al22 have reported no functional abnormalities in the binding of vWF to the platelet GPIbα as a function of the HPA-2 genotype, and we have found comparable basal activation, aggregability, and ristocetin-induced binding of vWF in platelets from three subjects VNTR B/B (HPA-2 b/b) and in platelets from three individuals VNTR C/C (HPA-2 a/a) (data not shown). By contrast, a chronic, but not transient, enhanced platelet aggregation response to shear stress in stroke patients has been recently reported,4which suggests a genetic predisposition of platelets in the development of arterial vascular disease. Further studies under conditions that resemble those found in arterial circulation and diseased arteries, such as the in vitro shear-induced platelet activation, could be helpful to appropriately explore GPIbα function and the role of VNTR and HPA-2 polymorphisms.
The proposed involvement of a structural change that exposes the GPIbα to greater shear forces opens up the possibility of identifying individuals who would most benefit from drug therapy with agents that interfere with shear-stress–induced platelet responses that have been shown to beneficially affect arterial thrombosis51-55 and, thus, to improve clinical outcome.
Supported by FIS 97/1150. J.C. is Contratado de Reincorporación en la Universidad de Murcia. R.G.-C. is a FIS Fellow (95/1401).
Address reprint requests to Vicente Vicente, MD, Centro Regional de Hemodonación. C/. Ronda de Garay s/n, 30003 Murcia, Spain; e-mail: vvg@fcu.um.es.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal