Abstract
Although all blood cells are derived from hematopoietic stem cells, the regulation of this production system is only partially understood. Negative feedback control mediated by erythropoietin and thrombopoietin regulates erythrocyte and platelet production, respectively, but the regulation of leukocyte levels is less well understood. The local regulatory mechanisms within the hematopoietic stem cells are also not well characterized at this point. Because of their dynamic character, cyclical neutropenia and other periodic hematological disorders offer a rare opportunity to more fully understand the nature of these regulatory processes. We review the salient clinical and laboratory features of cyclical neutropenia (and the less common disorders periodic chronic myelogenous leukemia, periodic auto-immune hemolytic anemia, polycythemia vera, aplastic anemia, and cyclical thrombocytopenia) and the insight into these diseases afforded by mathematical modeling. We argue that the available evidence indicates that the locus of the defect in most of these dynamic diseases is at the stem cell level (auto-immune hemolytic anemia and cyclical thrombocytopenia seem to be the exceptions). Abnormal responses to growth factors or accelerated cell loss through apoptosis may play an important role in the genesis of these disorders.
© 1998 by The American Society of Hematology.
REGULATION OF HEMATOPOIESIS
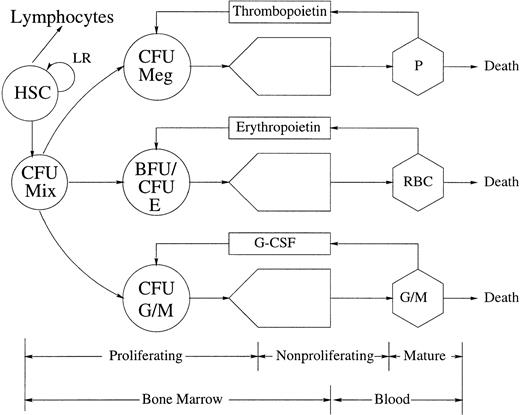
MATURE BLOOD CELLS and recognizable precursors in the bone marrow ultimately derive from a small population of morphologically undifferentiated cells, the hemopoietic stem cells (HSC), which have a high proliferative potential and sustain hematopoiesis throughout life (Fig 1). The earliest HSC are totipotent and have a high self-renewal capacity.1-3These qualities are progressively lost as the stem cells differentiate. Their progeny, the progenitor cells, or colony-forming units (CFUs), are committed to one cell lineage. They proliferate and mature to form large colonies of erythrocytes, granulocytes, monocytes, or megakaryocytes. The growth of CFUs in vitro depends on lineage-specific growth factors, such as erythropoietin (EPO), thrombopoietin (TPO), granulocyte colony-stimulating factor (G-CSF), monocyte colony-stimulating factor (M-CSF), and granulocyte-monocyte colony-stimulating factor (GM-CSF).
The architecture and control of hematopoiesis. This figure gives a schematic representation of the architecture and control of platelet (P), red blood cell (RBC), and monocyte (M) and granulocyte (G) (including neutrophil, basophil, and eosinophil) production. Various presumptive control loops mediated by TPO, EPO, and G-CSF are indicated, as well as a local regulatory (LR) loop within the totipotent HSC population. CFU (BFU) refers to the various colony (burst) forming units (Meg, megakaryocyte; Mix, mixed; E, erythroid; G/M, granulocyte/monocyte) that are the in vitro analogs of the in vivo committed stem cells (CSC).
The architecture and control of hematopoiesis. This figure gives a schematic representation of the architecture and control of platelet (P), red blood cell (RBC), and monocyte (M) and granulocyte (G) (including neutrophil, basophil, and eosinophil) production. Various presumptive control loops mediated by TPO, EPO, and G-CSF are indicated, as well as a local regulatory (LR) loop within the totipotent HSC population. CFU (BFU) refers to the various colony (burst) forming units (Meg, megakaryocyte; Mix, mixed; E, erythroid; G/M, granulocyte/monocyte) that are the in vitro analogs of the in vivo committed stem cells (CSC).
EPO ajusts erythropoiesis to the demand for O2 in the body. A decrease in tissue pO2 levels (in response to any one of a number of factors) leads to an increase in the renal production of EPO. This, in turn, leads to an increased cellular production by the primitive erythroid precursors (colony-forming units-erythroid [CFU-E]) and, ultimately, to an increase in the erythrocyte mass and hence the tissue pO2 levels. This increased cellular production triggered by EPO is due, at least in part, to an inhibition of preprogrammed cell death (apoptosis)4 5 in the CFU-E and their immediate progeny. Thus, EPO mediates a negative feedback such that an increase (decrease) in the erythrocyte mass leads to a decrease (increase) in erythrocyte production.
The mechanisms regulating granulopoiesis are not as well understood. G-CSF, the primary controlling agent of granulopoiesis, is a completely sequenced high molecular weight molecule6 produced by a number of tissues (fibroblasts, endothelial, and epithelial) and circulating cells (monocytes). G-CSF is absolutely essential for the growth of the granulocytic progenitor cells colony-forming units-granulocyte (CFU-G) in vitro.7 CFU-G colony growth is a sigmoidally increasing function of increasing G-CSF concentration.8,9 One of the modes of action of G-CSF, along with several other cytokines, is to decrease apoptosis.7,10-12 Additionally, there is a clear shorting of the neutrophil maturation time under the action of G-CSF.13
The important role of G-CSF for the in vivo control of granulopoiesis was demonstrated by Lieschke et al.14 They showed that mice lacking G-CSF (due to an ablation of the G-CSF gene in embryonal stem cells) have pronounced neutropenia and reduction of the marrow granulocyte precursor cells by a factor of 50%. The administration of exogenous G-CSF corrects the neutropenia in 1 day and restores the marrow composition to that typical of a normal wild-type mouse within 4 days. G-CSF also rapidly corrects neutropenia of diverse causes in humans15-20 and other mammals.21-23
Several studies have shown an inverse relation between circulating neutrophil density and serum levels of G-CSF.24-27 Coupled with the in vivo dependency of granulopoiesis on G-CSF, this inverse relationship suggests that the neutrophils would regulate their own production through a negative feedback, as is the case with erythrocytes, in which an increase (decrease) in the number of circulating neutrophils would induce a decrease (increase) in the production of neutrophils through the adjustement of G-CSF levels. Although mature neutrophils bear receptors for G-CSF and for GM-CSF, the role of these receptors in governing neutrophil production is not yet known.
The regulation of thrombopoiesis presumably involves similar negative feedback loops. Megakaryopoiesis can be separated in two processes: the proliferation and differentiation of megakaryocytic progenitor cells, and the complex process of maturation of precursor cells, which includes a variable number of endomitotic nuclear divisions, cytoplasmic growth and maturation, and the development of platelet-specific structures.
Until recently, the regulation of megakaryopoiesis was thought to include two separated control mechanisms28,29: first, a regulatory loop mediated by a megakaryocte colony stimulating antigen (Meg-CSA) responding to megakaryocyte demand and acting on the proliferation of colony-forming unit-megakaryocte (CFU-Meg); second, a thrombocytopenia-activated control of the maturation of megakaryocytes, mediated by TPO. Several growth factors, such as interleukin-11 (IL-11), IL-6, IL-3, and GM-CSF possess either one (but not both) of these activities and promote platelet production in vivo. However, none was found to be specific to the megakaryocytic lineage.28
A lineage-specific factor that is the ligand for Mpl receptor has been cloned recently that has both Meg-CSA and TPO activity.30Plasma TPO levels are increased in thrombocytopenic patients.31 Administration of TPO to nonhuman primates induces up to sixfold to sevenfold increases in the platelet counts.31,32 It is now thought that the Mpl ligand mediates a negative feedback loop regulating platelet production.33
There are more than 15 other cytokines acting on hematopoiesis,6 with broad, redundant actions.6,34 In vitro studies have shown that IL-3 and stem cell factor (SCF; Kit ligand) are involved with the survival of HSC, whereas IL-6, IL-11, IL-12, and G-CSF have synergestic effects on the entry into cycling of dormant HSC.17,35 In contrast to the committed progenitors, the growth of HSC in vitro thus depends on the interaction of several cytokines. The action of G-CSF on the cycling of HSC in vitro is supported by in vivo effects. Whereas suppression of G-CSF only affects granulopoiesis,14 G-CSF administration can result in multilineage recovery36 and modify the kinetics of colony-forming units-spleen (CFU-S).37 Little is known about how the self-maintenance of the HSC population is achieved. HSC are usually in a dormant state but are triggered to proliferate after transplantation into irradiated hosts.38 The specific mechanisms regulating the differentiation commitment of HSC are unknown.39Self-maintainance of HSC depends on the balance between self-renewal and differentiation. Mechanisms that could support auto-regulatory feedback control loops controlling HSC kinetics are starting to be investigated.40
PERIODIC HEMATOLOGICAL DISORDERS
Cyclical Neutropenia (CN)
General features.
CN has been the most extensively studied periodic hematological disorder. Its hallmark is a periodic decrease in the circulating neutrophil numbers from normal values to very low values. In humans, it occurs sporadically or as an autosomal dominantly inherited disorder, and the period is typically reported to fall in the range of 19 to 21 days,41 although recent data indicate that longer periods occur in some patients.42 Our understanding of CN has been greatly aided by the discovery that the grey collie suffers from a very similar disease. The canine disorder closely resembles human CN with the exception of the period that ranges from 11 to 15 days43 and the maximum neutrophil counts, which are higher than for humans. For reviews see.41 44-50
It is now clear that in both human CN42,51-53 and the grey collie,43 54 there is not only a periodic decrease in the circulating neutrophil levels, but also a corresponding oscillation of platelets, often the monocytes and eosinophils and occasionally the reticulocytes and lymphocytes (Fig 2A). The monocyte, eosinophil, platelet, and reticulocyte oscillations are generally from normal to high levels, in contrast to the neutrophils, which oscillate from near normal to extremely low levels. Often (but not always), the period of the oscillation in these other cell lines is the same as the period in the neutrophils.
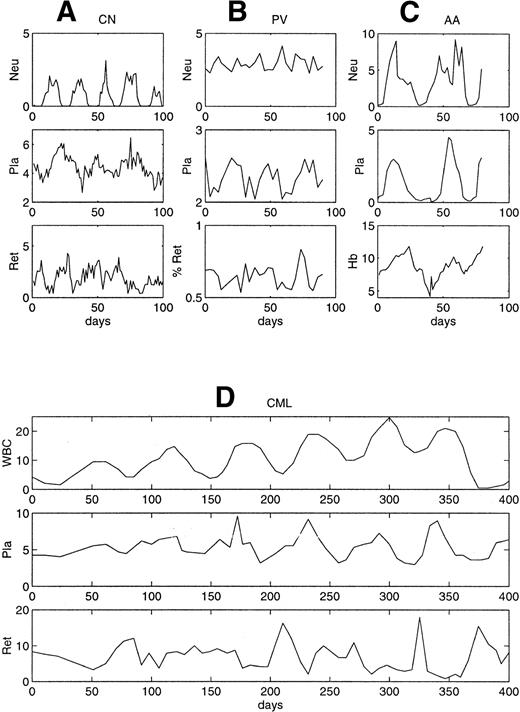
Representative patterns of circulating cell levels in four periodic hematological disorders considered in this review. (A) CN,54 (B) PV,55 (C) AA,56 and (D) periodic CML.57 The density scales are neutrophils, 103 cells/μL; white blood cells, 104cells/μL; platelets, 105 cells/μL; reticulocytes, 104 cells/μL; and Hb, g/dL.
Representative patterns of circulating cell levels in four periodic hematological disorders considered in this review. (A) CN,54 (B) PV,55 (C) AA,56 and (D) periodic CML.57 The density scales are neutrophils, 103 cells/μL; white blood cells, 104cells/μL; platelets, 105 cells/μL; reticulocytes, 104 cells/μL; and Hb, g/dL.
The clinical criteria for a diagnosis of CN have varied widely, although the criteria used by Dale et al58 are widely accepted. These criteria are that a patient must display an absolute neutrophil count (ANC) less than 0.5 × 109/L at least 3 to 5 consecutive days per cycle for each of three regularly spaced cycles. Usually, this requires blood cell counts three times per week for 6 to 8 weeks. Most patients will also have significant symptoms (malaise and anorexia) and signs (eg, fever, mouth ulcers, and lymphoadenopathy) during their neutropenic periods. Sometimes it is difficult to make the diagnosis with certainty. Using periodogram analysis, some patients classified as having CN do not in fact display any significant periodicity, whereas other patients classified with either congenital or idiopathic neutropenia do display significant cycling.42
Origin.
Transplantation studies show that the origin of the defect in CN is resident in one of the stem cell populations of the bone marrow.59-64 Studies of bone marrow cellularity throughout a complete cycle in humans with CN show that there is an orderly cell density wave that proceeds successively through the myeloblasts, promyelocytes, and myelocytes and then enters the maturation compartment before being manifested in the circulation.54,65 Further studies have shown that this wave extends back into the CFU-G,66 CFU-E,67-70 as well as in the burst-forming unit-erythroid (BFU-E) and colony-forming unit–granulocyte-macrophage (CFU-GM),69 71suggesting that it may originate in the totipotent HSC populations.
Studies in the grey collie9,72 and in humans8,73 show that the responsiveness of granulocyte committed progenitor cells to G-CSF is greatly attenuated in CN compared with normal. Patients also differ from normal in their requirements for GM-CSF but not for IL-3.73
In CN, the levels of colony-stimulating activity (CSA-related to G-CSF) fluctuate inversely with the circulating neutrophil levels and in phase with the peak in monocyte numbers.74-76 EPO levels oscillate approximately in phase with the reticulocyte oscillation.75 Dunn et al77 have also shown a periodic stimulation/repression of CFU-S (the HSC) by conditioned media derived from cyclic neutropenic marrow. It is unclear if these correlations and inverse correlations between levels of circulating cells and putative humoral regulators are related to the cause of CN or are simply a secondary manifestation of some other defect.
Effect of phlebotomy and hypertransfusion.
The effect of bleeding and/or hypertransfusion on the hematological status of grey collies gives interesting results.78 In the untreated grey collie, EPO levels cycle out of phase with the reticulocytes and virtually in phase with the neutrophil counts. After phlebotomy (bleeding of between 10% and 20% of the blood volume), the cycles in the neutrophils and reticulocytes continue as they had before the procedure, and there is no change in the relative phase between the cycles of the two cell types. Hypertransfusion (with homologous red blood cells) completely eliminates the reticulocyte cycling (as long as the hematocrit level remained elevated), but has no discernible effect on the neutrophil cycle. Most significantly, when the hematocrit level decreases back to normal levels and the reticulocyte cycle returns, the phase relation between the neutrophils and the reticulocytes is the same as before the hypertransfusion. These observations suggest that the source of the oscillations in CN is relatively insensitive to any feedback regulators involved in peripheral neutrophil and erythrocyte control, whose levels would be modified with the alteration of the density of circulating cells, and is consistent with a relatively autonomous oscillation in the HSC (see “Models of the Autoregulatory Control of HSC” below).
Effect of cytokine and lithium therapy.
In both the grey collie72,79 and in humans with CN,80-82 administration of G-CSF leads to an increase in the mean value of the peripheral neutrophil counts by a factor of as much as 10 to 20 and is associated with a clear improvement of the clinical symptoms. However, G-CSF does not obliterate the cycling in humans, but rather induces an increase in the amplitude of the oscillations and a decrease in the period of the oscillations in all the cell lineages, from 21 to 14 days.80 In human and canine CN, GM-CSF leads to an increase in neutrophil count by a factor of between 1.5 and 3.5, which is much less than achieved by G-CSF. In one report, CM-CSF obliterated cycling.82 Although recombinant canine stem cell factor (rc-SCF) does not cause neutrophillia in grey collies, it does obliterate the oscillations of CN. Lithium therapy in grey collies69,83 has uniformly yielded an elimination of the severe neutropenic phases and a diminution in the amplitude of the oscillations without any apparent change in the period of the oscillation. In humans, there are variable results with lithium,84,85 and the largest study showed lack of efficacy and toxicity problems.86
Other Periodic Hematological Disorders Associated With Bone Marrow Defects
Periodic chronic myelogenous leukemia (CML).
CML is a hematopoietic stem cell disease characterized by granulocytosis and splenomegaly.87 In 90% of the cases, the hematopoietic cells contain a translocation between chromosomes 9 and 22 that leads to the shortening of chromosome 22, refered to as the Philadelphia (Ph) chromosome. The disease is acquired and results from the malignant transformation of a single pluripotential stem cell, as shown by the presence of a single G-6PD isoenzyme in the red blood cells, neutrophils, eosinophils, basophils, monocytes, and platelets in women with CML who are heterozygotes for isoenzymes A and B.88 The leukocyte count is greater than 100 × 109/L in 50% of the cases and it increases progressively in untreated patients. The platelet and reticulocyte counts can also be mildly elevated. In most cases, the disease eventually develops into acute leukemia.
Morley et al89 was the first to describe oscillations in the leukocyte count of CML patients in 1967. Several other cases of cyclic leukocytosis in CML have now been reported.57,90-104The leukocyte count usually cycles with an amplitude of 30 to 200 × 109 cells/L and with periods ranging from approximately 30 to 100 days. Oscillations of other blood elements in association with CML have been observed. The platelets and sometimes the reticulocytes then oscillate with the same period as the leukocytes, around normal or elevated numbers. There have been no specific studies of hematopoiesis in patients with periodic CML. There is also a report of one patient with periodic acute myelogenous leukemia.97
Polycythemia vera (PV) and aplastic anemia (AA).
PV is characterized by an increased and uncontrolled proliferation of all the hematopoietic progenitors and it involves, like CML, the transformation of a single pluripotential stem cell. Two patients with PV were reported with cycling of the reticulocyte, platelet, and neutrophil counts in one case (Fig 2B) and cycling only of the reticulocyte counts in the other. The period of the oscillations was 27 days in the platelets, 15 days in the neutrophils, and 17 days in the reticulocytes.55
Cytokine-induced cycling.
G-CSF is routinely used in a variety of clinical settings, eg, to treat chronic neutropenia or to accelerate recovery from bone marrow transplant and/or chemotherapy.58 G-CSF may induce oscillations in the level of circulating neutrophils of neutropenic individuals.42,106-108 When these oscillations arise, they always seem to be of relatively low period (on the order of 7 to 15 days), and their origin is unclear. There has also been one report of GM-CSF–induced 40-day cycling in a patient with CML after bone marrow transplant.109
Induction of cycling by chemotherapy or radiation.
Several reports describe induction of a CN-like condition by the chemotherapeutic agent cyclophosphamide. In mongrel dogs on cyclophosphamide, the observed period was on the order of 11 to 17 days, depending on the dose of cyclophosphamide.110,111 In a human undergoing cyclophosphamide treatment, cycling with a period of 5.7 days was reported.112 Gidáli et al113observed oscillations in the granulocyte count and the reticulocyte counts with 3 weeks periodicity in mice after mild irradiation. They observed an overshooting regeneration in the reticulocytes and the thrombocytes but not in the granulocytes. Whereas the CFU-S returned to normal levels rapidly, the proliferation rate of CFU-S stayed abnormally elevated.
Five CML patients receiving hydroxyurea showed oscillations in their neutrophils, monocytes, platelets, and reticulocytes with periods in the range of 30 to 50 days.95 In one patient, an increase of the hydroxurea dose led to a cessation of the oscillations. Chikkappa et al114 report a CN-like condition (period between 15 and 25 days) in a patient with multiple myeloma after 3 years of chemotherapy.
A 89Sr-induced cyclic erythropoiesis has been described in two congenitally anemic strains of mice, W/Wv and S1/S1d.115-117W/Wv mice suffer from a defect in the HSC, and in S1/S1d mice the hematopoietic micro-environment is defective.
The induction of cycling by 89S can be understood as a response to elevated cell death (see “Models of the Autoregulatory Control of HSC” below), as can the dynamic effects of chemotherapy.
Periodic Hematological Disorders of Peripheral Origin: Auto-Immune Hemolytic Anemia (AIHA) and Cyclical Thrombocytopoiesis
Periodic AIHA is a rare form of hemolytic anemia in humans.118 Periodic AIHA, with a period of 16 to 17 days in hemoglobin and reticulocyte counts, has been induced in rabbits by using red blood cell auto-antibodies.119
Cyclic thrombocytopenia, in which platelet counts oscillate from normal to very low values, has been observed with periods between 20 and 40 days120-136 (reviewed by Cohen and Cooney126). Although it has been claimed that oscillations could be detected in the platelet counts of normal individuals with the same range of periods,137 138 this conclusion may not be statistically justified.
HYPOTHESES FOR THE ORIGIN OF PERIODIC HEMATOPOIESIS
In clinical reports of periodic diseases affecting hematopoiesis, oscillations have usually been observed only in the blood counts without examinations of bone marrow precursors and progenitor cells. However, even in the case of CN in which the kinetics of hematopoiesis have been extensively studied, the mechanisms responsible for the onset of periodic oscillations are still unknown. A number of mathematical models have been put forward that suggest possible mechanisms for the origin of oscillations in hematopoiesis. These models fall into two major categories. The first group identifies the origin of the oscillations with the loss of stability in peripheral control loops adjusting the production rate of blood precursors to the number of mature cells in the blood and mediated by TPO, EPO, and G-CSF (Fig 1). The second group is based on the assumption that oscillations arise in stem cell populations as a consequence of the loss of stability of auto-regulatory (local and LR) control loops (Fig 1). A few investigators have also modeled interactions between these two types of control loops (see Dunn139 and Fisher140 for reviews).
Models of the Peripheral Control of Hematopoiesis
It is well known that simple negative feedback systems, such as the erythroid control system, have a tendency to oscillate. The relative detail known about erythrocyte production control has not escaped the attention of modelers who have mathematically explored ways to explain the results of laboratory manipulations in rodents,141-147and rabbits119,148 and the nature of the human erythropoietic regulatory system in health and disease.149-152
The control of erythropoiesis by EPO can be modeled by a single delayed differential equation describing the rate of change of RBC production as a function of the death rate of circulating erythrocytes, the rate of change of cell production after a given perturbation of the circulating RBC number, and the maturation time of erythrocyte progenitors. The transition from damped to stable oscillations, which characterizes the onset of periodic hematopoiesis, depends on the modification of one or several of these controlling parameters. In AIHA, the death rate of circulating RBC is increased, whereas the other parameters lie within normal ranges. The mathematical modeling of the control of RBC production by EPO indicates that such an increase in the destruction rate of circulating erythrocytes will induce periodic fluctuations of erythropoiesis around a low average, with periods similar to the ones observed in AIHA.153-155 From a modeling perspective, the laboratory version of rabbit AIHA is thus one of the best understood periodic hematological diseases.
Similar mathematical treatments have been applied to the control of granulopoiesis and megakaryopoiesis, even though the existence of functional peripheral feedback in these systems is still hypothetical.
A few investigators have formulated models for the regulation of thrombopoiesis138,156-159 assuming the existence of a negative feedback loop mediated by TPO. Bélair and Mackey160 specifically considered cyclical thrombocytopenia. They speculated that elevations in the random destruction rate of platelets could give rise to the characteristic patterns observed in cyclical thrombocytopenia. Although modeling results based on this assumption yielded results qualitatively consistent with the clinical data, there is still much room for further study of this problem.
Several models of granulopoiesis incorporate a peripheral negative feedback loop.161-172 In the grey collie and in CN patients, the survival of circulating neutrophils is normal.51 This finding implies that there is not a periodic elevation of the peripheral death rate of neutrophils in CN, but rather a periodic modulation of marrow cell production. An alteration of the peripheral control of granulopoiesis has been proposed as the mechanism of CN by several investigators.56,110,111 173-185
There is experimental evidence of alterations in the kinetics of granulopoiesis in CN, ie, the modification of the distribution of maturation time and the subnormal responsiveness of granulocytic progenitors to CSF. However, recent modeling indicates that these are insufficient to account for a destabilization of the putative peripheral feedback control of granulocytopoiesis.186Moreover, the lack of effect of hypertransfusion or phlebotomy on either cycle (neutrophil or reticulocyte) strongly implies that there is not a direct role of peripheral feedback loops in the origin of the cycling in CN.
A few attempts have also been made to model periodic CML based on the peripheral control of granulopoiesis171,187,188 but are also unsatisfactory in the sense that the models have assumptions on the kinetic of granulopoiesis in CML that are now known to be biologically unrealistic.189 190
The occurrence of oscillations in several blood elements in CN and CML strongly suggests that the oscillations are not a consequence of a lineage-specific regulatory loop but rather of regulatory mechanisms affecting all hematopoiesis. The existence of a peripheral feedback loop controlling granulopoiesis can thus not be supported by the occurence of oscillations in CN and CML.
Models of the Autoregulatory Control of HSC
Except for AIHA and cyclical thrombocytopenia, the periodic hematological disorders that have been described are characterized by the occurence of oscillations in many or all of the peripheral cellular elements (neutrophils, platelets, lymphocytes, and reticulocytes). Many have speculated that the origin of these oscillations is in the common HSC population feeding progeny into the differentiated cell lines.
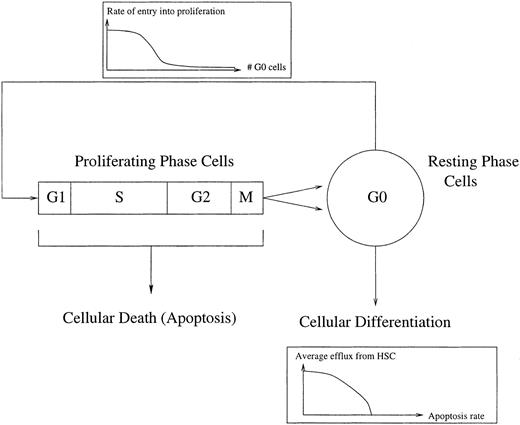
Mackey191 193 proposed that there could be a loss of stability in the stem cell population independent of feedback from peripheral circulating cell types. He analyzed a mathematical model for a stem cell population in which the proportion of cells entering proliferation depends on the size of the population in G0(Fig 3). The efflux into the different commited cell lineages depends on the size of the population, which varies with the rate of proliferation and the cell death rate.
A schematic representation of the control of HSC regeneration. Proliferating phase cells include those cells in G1, S (DNA synthesis), G2, and M (mitosis), whereas the resting phase cells are in the G0 phase. Local regulatory influences are exerted via a cell number dependent variation in the rate of entry into proliferation. Differentiation into all of the comitted stem cell populations occurs from the G0population, whereas there is a loss of proliferating phase cells due to apoptosis. See Mackey191 and Milton and Mackey192 for further details.
A schematic representation of the control of HSC regeneration. Proliferating phase cells include those cells in G1, S (DNA synthesis), G2, and M (mitosis), whereas the resting phase cells are in the G0 phase. Local regulatory influences are exerted via a cell number dependent variation in the rate of entry into proliferation. Differentiation into all of the comitted stem cell populations occurs from the G0population, whereas there is a loss of proliferating phase cells due to apoptosis. See Mackey191 and Milton and Mackey192 for further details.
Other investigators194-202 have considered the dynamics of auto-regulatory stem cell populations from a modeling perspective applied to various experimental and clinical situations. Nečas et al40 203-205 have developed such a modeling study based on experimental evidence that the number of stem cells entering proliferation is controlled by the number of DNA-synthesizing cells. Cell to cell interactions or cytokines that are essential for the growth of HSC in vitro, such as IL-3 and SCF, could be involved in the autoregulation of HSC proliferation.
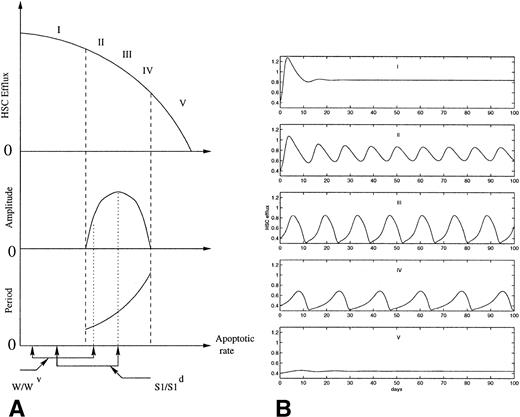
Because of the delay in the autoregulation loop, due to the time necessary for replicating cells to go through the S phase and the mitotic phase, this system has a tendency to oscillate. In normal conditions it is assumed that the population does not oscillate. Mackey191 showed that an abnormally large irreversible cell loss within the proliferating compartment, which can represent either apoptosis or other cell death, would induce oscillations of the number of stem cells. Figure 4 shows the variations in the amplitude and the period of the oscillations predicted by the model as the apoptotic rate is increased. An increase in the apoptotic rate of HSC also induces a decrease in the average efflux from the HSC to all the cell lineages. This is consistent with the occurence of oscillations in all the blood elements in some patients with AA, in which all the blood cell counts are low, as well as the oscillations observed in several cell lineages after chemotherapy or radiation. The range of periods obtained by Mackey depends largely on the rate of irreversible cell loss and on the cell cycle time delay. Depending on the value of these parameters, the period can vary from 16 to 43 days in humans and from 9 to 26 days in dogs. In most periodic hematological disorders, large differences consistent with these values have been observed in the period of the oscillations between different individuals.
(A) Schematic representation of the effects of increasing apoptotic rate on the HSC dynamics, as predicted from the model proposed previously.191,192 The diagram shows the effect of administering the same dose of 89Sr to W/Wv and S1/S1d mice on the amplitude and the period of the oscillations. The dashed lines show the onset and the end of oscillations as the apoptotic rate increases. (B) Computer simulations of the normalized HSC efflux predicted191 for increasing apoptotic rates (from I to V). As the apoptotic rate is increased, the period of the oscillations increases and the amplitude increases and decreases consecutively.
(A) Schematic representation of the effects of increasing apoptotic rate on the HSC dynamics, as predicted from the model proposed previously.191,192 The diagram shows the effect of administering the same dose of 89Sr to W/Wv and S1/S1d mice on the amplitude and the period of the oscillations. The dashed lines show the onset and the end of oscillations as the apoptotic rate increases. (B) Computer simulations of the normalized HSC efflux predicted191 for increasing apoptotic rates (from I to V). As the apoptotic rate is increased, the period of the oscillations increases and the amplitude increases and decreases consecutively.
The model also gives a plausible explanation for the 89Sr induction of cyclic erythropoiesis in the two congenitally anemic strains of mice, W/Wv and S1/S1d (Fig 4). Assume that the difference between W/Wv and S1/S1dmice is solely related to differences in the rate of apoptosis.206. (The observation that S1/S1d mice are more refractory to erythropoietin than W/Wv mice suggests that the apoptotic rate is higher in S1/S1d.) The results of Mackey191 predict that a higher rate of apoptosis would increase the likelihood that an oscillation in erythrocyte number occurs. Indeed, in contrast to W/Wv mice, approximately 40% of S1/S1d mice have spontaneous oscillations in their hematocrit.115,116 In both strains of mice, a single dose of 89Sr is sufficient to increase apoptosis into a range associated with oscillations in erythrocyte number. Because the apoptotic rate for the S1/S1d mice is greater than that for W/Wv mice before 89Sr, it is reasonable to expect that it will also be higher after administration of equal doses of 89Sr to both strains of mice. As predicted, the period of the oscillation is longer, the amplitude is larger, and the mean hematocrit is lower for S1/S1d mice than they are for W/Wv mice.192
The Particular Cases of CN and CML
Destabilization of an early HSC population resulting in oscillations with a large range of periods in all the blood elements after radiation or chemotherapy and in AA may also be at the origin of the oscillations observed in CN and CML. Modification of any of the parameters in the model described in Mackey191 (Fig 3) can potentially induce the onset of oscillations.
Even though all the blood elements are often oscillating in periodic CML and CN, the defect in these two disorders primarily affects granulopoiesis. The abnormal responsiveness of CFU-G explains the low ANC levels in CN, which may induce an alteration in the cytokine levels. The fact that G-CSF is the only cytokine shown to alter the period of the oscillations in all the blood elements shows that it plays a crucial role in the mechanism at the origin of the oscillations in CN. The finding that G-CSF not only regulates granulopoiesis, but also affects the kinetics of early HSC suggests that its effect on the oscillations in CN may be mediated through the modification of HSC dynamics. In vitro studies of the effect of G-CSF suggest that it increases the rate of entry into cycling, which is likely to destabilize the steady state while increasing the average size of the population. This is consistent with the mildly elevated platelet, monocyte, and lymphocyte counts often observed in CN. Similar mechanisms may also induce the oscillations in CML.
The mechanisms underlying the complex dynamical features of CN and periodic CML will more likely be understood with the use of models that include both the local autoregulation of early stem cells and its relation with the more mature hemopoietic compartments. The multilevel effect of cytokines such as G-CSF suggests indeed that strong relationships exist between the regulation of early and late hematopoietic compartments.
CONCLUSION
This review focuses on the clinical and laboratory findings in periodic hematopoietic diseases, including CN, periodic CML, AA, PV, AIHA, and cyclical thrombocytopenia. With the exception of the latter two, the available evidence indicates a broad involvement of the entire hematopoietic system, because cycling is typically observed in more than one of the mature hematopoietic cell types.
Cycling in one or several hematopoietic cell lineages is probably much more frequent than reported and would be detected if serial blood counts were systematically performed. These observations suggest a major derangement of the dynamics of one or more of the stem cell populations such that they become unstable and generate sustained oscillations that are manifested in more than one of their progenitor lines. Mathematical modeling studies suggest that there are several ways in which the HSC dynamics can be destabilized and give rise to oscillations if they are controlled by an autoregulatory loop. The finding that lineage-specific cytokines also have an effect on early HSC regulation implies that oscillations could arise as a result of the alteration of only one compartment, such as observed in CN and CML. Analysis of the effect of the cytokines on the dynamical features of these disorders, through modeling studies, may be a key to the understanding of the nature of early hematopoiesis regulation.
Supported by the Natural Sciences and Engineering Research Council (NSERC Grant No. OGP-0036920, Canada), the National Institutes of Health (NIH Grant No. 18951, USA), Le Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (FCAR Grant No. 98ER1057, Québec), and the École Normale Supérieure de Paris.
Address reprint requests to Michael C. Mackey, PhD, Departments of Physiology, Physics, and Mathematics, Center for Nonlinear Dynamics in Physiology and Medicine, McGill University, McIntyre Drummond Building, 3655 Drummond St, Montreal H3G 1Y6, Quebec, Canada.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal