To the Editor:
Although familial thrombocytopenias have been described in the literature since the early 1960s,1 they are a rare and heterogeneous group of disorders characterized by varying modes of inheritance. A number of autosomal dominant thrombocytopenias have been described, but their rarity has limited their detailed clinical and genetic analyses. In three kindreds to date,2-4 a unique autosomal dominant thrombocytopenia has been described with a predisposition to acute myelocytic leukemia termed familial platelet disorder-acute myelocytic leukemia (FPD-AML). A potential FPD-AML gene locus was recently mapped to the long arm of chromosome (21q22.1-.2) by linkage analysis in one family.5 We now extend these observations by reporting a new kindred with a similar phenotype, which maps to an overlapping chromosomal region, suggesting that a defect in a single gene may underlie the FPD-AML phenotype.
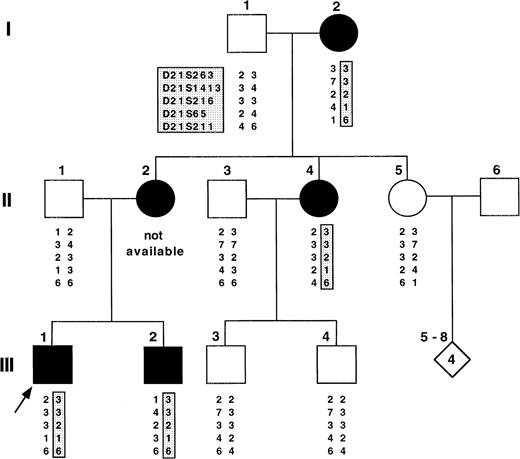
The FPD-AML pedigree involves three generations of family with five affected individuals of mixed Czechoslovakian and Hungarian background (see Fig 1). Clinical history and blood specimens were available from 11 available family members, each of whom consented to participate in this study. There was no history of consanguinity in this family.
Pedigree of the family under study with FPD-AML. The arrow identifies the proband (III-2). The FPD phenotype is shown in black. The STRP studied are shown here and cosegregation of linked haplotypes in family members with FPD is shown in the shaded box. II-2 developed AML and was posttransplantation when DNA samples were collected so that her analysis was inferred and shown in parenthesis. Five other flanking markers were genotyped and had negative lod scores. For clarity, these data are not shown.
Pedigree of the family under study with FPD-AML. The arrow identifies the proband (III-2). The FPD phenotype is shown in black. The STRP studied are shown here and cosegregation of linked haplotypes in family members with FPD is shown in the shaded box. II-2 developed AML and was posttransplantation when DNA samples were collected so that her analysis was inferred and shown in parenthesis. Five other flanking markers were genotyped and had negative lod scores. For clarity, these data are not shown.
The proband (III-1) presented at the age of 4 years for hypospadias repair. He had easy bruising since infancy and was found preoperatively to have a platelet count of 106,000/μL. His family history was notable for his mother (II-2), maternal aunt (II-4), and maternal grandmother (I-2) having low platelet counts and a bleeding diathesis. In addition to thrombocytopenia, the index patient and his sibling (III-2) both had hypospadias and umbilical hernia.
The proband's mother (II-2), at 41 years of age, developed progressive anemia and neutropenia and was shown to have a myelodysplastic syndrome (refractory anemia). Within 6 months of diagnosis of myelodysplasia, increased bone marrow blast counts above 30% were noted. Cytogenetics were normal, as were fluorescent in situ hybridization (FISH) studies for monosomy 5 and 7. Immunohistochemical typing of her blasts confirmed acute myelogenous leukemia, FAB-M1.
Because of the previously reported linkage of FPD-AML to chromosome 21q22 in one family,5 candidate locus linkage analyses were performed. Genotyping and two-point lod scores at 10 precisely mapped chromosome 21 polymorphic marker loci showed evidence for linkage at marker loci overlapping the previously defined FPD-AML region (eg, maximum two-point lod score at D21S65 = 1.682). These findings were extended by multipoint analyses, where the putative disease gene locus was placed at each map interval betweenD21S263 and D21S211. Maximum lod scores greater than 3 were obtained at each map interval (Table1). Lastly, there was no evidence for allele sharing between this kindred and the French-Canadian pedigree previously reported, confirming distinct ancestry.
Multipoint Linkage Analysis
| Gene Order . | Recombination Fractions . | Maximum Lod Score . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| D21S263-FPD-AML-D21S1413-D21S216-D21S65-D21S211 | 0 | 0 | 0 | 0 | 0.125 | 3.440 |
| D21S263-D21S1413-FPD-AML-D21S216-D21S65-D21S211 | 0 | 0 | 0 | 0 | 0.125 | 4.439 |
| D21S263-D21S1413-D21S216-FPD-AML-D21S65-D21S211 | 0 | 0 | 0 | 0 | 0.125 | 3.809 |
| D21S263-D21S1413-D21S216-D21S65-FPD-AML-D21S211 | 0 | 0 | 0 | 0.046 | 0.062 | 3.771 |
| Gene Order . | Recombination Fractions . | Maximum Lod Score . | ||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ||
| D21S263-FPD-AML-D21S1413-D21S216-D21S65-D21S211 | 0 | 0 | 0 | 0 | 0.125 | 3.440 |
| D21S263-D21S1413-FPD-AML-D21S216-D21S65-D21S211 | 0 | 0 | 0 | 0 | 0.125 | 4.439 |
| D21S263-D21S1413-D21S216-FPD-AML-D21S65-D21S211 | 0 | 0 | 0 | 0 | 0.125 | 3.809 |
| D21S263-D21S1413-D21S216-D21S65-FPD-AML-D21S211 | 0 | 0 | 0 | 0.046 | 0.062 | 3.771 |
All linkage analyses were undertaken assuming that FPD-AML is caused by a mutation in a single diallelic autosomal dominant gene with a population frequency of 0.0001, and that the phenotype is incompletely penetrant (90% lifetime probability of manifesting disease in carriers, and no chance of manifesting disease in noncarriers). The map-specific multipoint lod scores above were calculated at each marker interval between D21S263 andD21S211 using the MLINK and LINK-MAP programs as implemented in FASTLINK v 2.3P.8 These analyses assumed 1 cM distance between markers (except 2 cM between D21S65 andD21S211) and that there was no interference or sex differences in the recombination fractions. The five recombination fractions were estimated from the genotype data and indicate that there was only one recombinant haplotype (in one unaffected individual).
These results support the hypothesis that an inherited mutation in a gene mapping to the previously defined FPD-AML locus caused inherited thrombocytopenia and predisposition to AML in this kindred. Therefore, these data provide additional evidence for a singular genetic defect underlying the clinical phenotype of FPD-AML. Significantly, our results corroborate clinical and genetic data for the existence of a gene on chromosome 21q that is critical to normal hematopoiesis, and when dysregulated, may result in acquired hematologic disorders, such as myelodysplasia or AML. Candidate genes in the FPD-AML locus include: acute myelogenous leukemia (AML1), the interferon-α/β receptor (IFNAR), the Down syndrome critical region 1 (DSCR1), phosphoribosylglycinamide formyltransferase gene (GART), SON, and the cytokine receptor 2-4 (CRF2-4) (http://www.ncbi.nih.gov/Science96). TheAML-1 gene and DSCR1 region are of particular interest because of well-established associations with translocations (chromosome 8;21) in acute myelogenous leukemia, FAB-M2, involving theAML-1 gene, and childhood megakaryocytic leukemia, FAB-M7, in Down syndrome.6 7
Presently, the concurrent risk factors for developing AML in affected individuals is unknown. The risk of developing leukemia from this disorder appears to increase with age, and carries an overall life-time risk of ∼30%.5 It may well be that a single defective FPD-AML allele results in thrombocytopenia, while accumulation of a defect in the second allele results in the development of the myelogenous leukemic state. Because our findings support a unique genetic locus linked to a predisposition for AML in a subset of families with inherited thrombocytopenia, we believe that genetic screening evaluating linkage patterns and counseling should be offered for kindreds with familial autosomal dominant thrombocytopenia. Thorough family histories should be recorded in patients carrying the diagnosis of childhood chronic ITP and in patients with myelogenous leukemia to identify additional families with FPD-AML with its risk of AML so that these families can be monitored for the risk of developing myelodysplasia or AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal