Abstract
This study investigated the release of erythrocyte-derived iron from purified human monocytes obtained from healthy volunteers and hereditary hemochromatosis (HH) patients. After erythrophagocytosis of59Fe-labeled erythrocytes, a complete transfer of iron from hemoglobin (Hb) to ferritin was observed within 24 hours in both control and HH monocytes. The iron was released from the monocytes in the form of ferritin, Hb, and as nonprotein bound low molecular weight iron (LMW-Fe). During the initial rapid phase (<1.5 hours), iron release mostly consisted of Hb and LMW-Fe, while in the later phase (>1.5 hours), it was composed of ferritin and LMW-Fe. The kinetics of iron release were identical for HH monocytes. A high percentage of the total amount of iron was released as Hb both by viable normal and HH monocytes, suggesting that iron release as Hb is a physiologic process, which may occur whenever the erythrocyte-processing capacity of macrophages is exceeded. Most remarkably, HH monocytes released twice as much iron in a LMW form as control cells. Iron released in the form of LMW-Fe readily binds to plasma transferrin and may contribute to the high transferrin saturation and the occurrence of circulating nontransferrin-bound iron observed in HH patients.
AFTER UPTAKE by macrophages of the mononuclear phagocytic system (MPS), red blood cells are destroyed and iron is released from hemoglobin (Hb) to be reused for the production of heme-, iron-sulphur-, and other proteins. Little is understood about the intracellular pathways responsible for transport of iron to the cell surface and the form in which iron is released after erythrophagocytosis. It is known that iron storage in macrophages differs depending on the cause of iron accumulation. In secondary iron overload, as a result of dyserythropoiesis, hemolysis, or transfusions, macrophages are heavily loaded with iron. In contrast, in hereditary hemochromatosis (HH), little iron is seen in the Kupffer cells and other macrophages, while hepatocytes already suffer from iron overload. In both forms of iron overload, low-molecular weight complexes of iron are observed in plasma.1 Iron overload of macrophages in secondary hemochromatosis can be explained partially by the fact that these cells must process larger quantities of (immature) erythrocytes, while the primary cause of iron overload in HH is an inappropriately increased absorption of iron. However, defects in erythrophagocytosis or differences in cellular iron processing and iron release may contribute to the low amounts of iron in the MPS of patients with HH.
Previous in vitro studies of human monocytes loaded with iron by erythrophagocytosis demonstrated iron in the form of Hb and ferritin inside the cell and in the culture supernatant, suggesting that iron was released in these macromolecular forms.2 Studies using rat peritoneal macrophages and Kupffer cells detected iron as ferritin and in a low molecular weight form that readily bound to apotransferrin or to desferrioxamine.3-5 In some of these experiments, Hb was also recovered, but this was considered to be artifactual.4
To investigate the normal physiology and the differences in iron metabolism between monocytes obtained from HH patients and healthy volunteers, monocytes were loaded with 59Fe-labeled erythrocytes by antibody-mediated phagocytosis. Distribution of iron within the cellular compartments and iron release was studied for a period of 48 hours after erythrophagocytosis. Size-exclusion high performance liquid chromatography (SE-HPLC) was used for analyzing the forms of intracellular and released iron. Comparison of normal monocytes with monocytes derived from patients with HH showed identical kinetics of iron release. Both control and HH monocytes released iron in the forms of ferritin, Hb, and as nonprotein-bound low molecular weight iron (LMW-Fe) complexes. Iron release as Hb is probably a physiologic process occurring whenever the erythrocyte catabolizing capacity of macrophages is exceeded. Our previous studies demonstrated that HH monocytes have a decreased ability to phagocytose opsonized rabbit erythrocytes.6 Here we present evidence indicating that after erythrophagocytosis, iron release is also different between these two cell types. Remarkably, HH monocytes released twice as much iron in the form of LMW-Fe complexes than control monocytes. Our finding of increased release of LMW-Fe in HH might explain the high transferrin saturation and nontransferrin-bound iron in HH.
MATERIALS AND METHODS
Subjects
Monocytes were obtained from healthy volunteers and from six unrelated patients with HH. After informed consent, blood from therapeutic phlebotomies of patients with HH (University Hospital Utrecht) was used. Patients were characterized for HH by standard biochemical parameters and by mutations in the HFE gene. Hb concentration (Hb), mean corpuscular volume (MCV), serum iron (SI), transferrin saturation (FeSat), serum ferritin concentration, alanine amino transferase (ALAT), and γ-glutamyl transpeptidase (γ-GT) were evaluated using routine laboratory methods. Liver biopsy and liver histology were performed in five of the six patients. For light microscopy, liver biopsies were stained with hematoxylin and with Perls' Prussian blue. HFE gene mutation was assessed as described elsewhere.7 Results for the clinical data of the patients at the time of the experiment are shown in Table 1: two patients had been treated by depletion of iron stores and the other four were on intensive phlebotomy treatment. One patient, patient 1, was studied twice during the intensive phlebotomy treatment. Intensive treatment of HH consisted of weekly removal of 400 to 500 mL of blood until iron stores were depleted. Iron depletion was considered to have been achieved when the following criteria were met: (1) serum ferritin decrease to values lower than 100 ng/mL and (2) urinary iron excretion after 1,000 mg intramuscular desferrioxamine fell to below 20 μmol/24 hours. The total iron removed after completion of intensive treatment is also shown in Table 1. All patients were judged clinically to have the manifestations of homozygous HH. Genetically, four were homozygous for the Cys282Tyr (C282Y) mutation in the HFE gene, one was heterozygous (patient 5), and one did not carry any of the described mutations of this gene (patient 1).
Clinical Data From Patients With HH at the Time of the Experiment and Phagocytic Capacity of Monocytes as Compared With Healthy Donors
| Patient No. . | Sex/Age . | PI-150 . | Pld/Plp-151 . | Fe Removed (g) . | Hb (mmol/L) 8.6-10.7-152 . | Serum Ferritin (μg/L) 15-150-152 . | Serum Iron (μmol/L) 14-32-152 . | Fe Saturation 0.25-0.60-152 . | ALAT IU 10-50-152 . | g-GT IU 15-70-152 . | Liver . | HFE-153 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/31 | .57 | 2.63 | 16 | 9.1 | 2,970 | 46 | 0.73 | 62 | 31 | SF | −/− |
| 1 | M/31 | .61 | 2.84 | 10.1 | 2,160 | 54 | 0.81 | 49 | 30 | |||
| 2 | M/30 | .6 | 1.95 | 4.4 | 9.8 | 271 | 39 | 0.74 | 43 | 47 | N | +/+ |
| 3 | M/66 | .61 | 3.2 | 5.5 | 7.8 | 30 | 7 | 0.1 | 52 | 230 | C | +/+ |
| 4 | M/65 | 1.13 | 1.95 | 7 | 9.1 | 1,179 | 31 | 0.76 | 41 | 21 | N | +/+ |
| 5 | M/66 | .73 | 3 | 9.5 | 31 | 21 | 0.43 | 26 | 24 | −/+ | ||
| 6 | M/52 | 1.17 | 1.48 | 6 | 8.9 | 409 | 36 | 0.79 | 9 | 16 | LF | +/+ |
| Patient No. . | Sex/Age . | PI-150 . | Pld/Plp-151 . | Fe Removed (g) . | Hb (mmol/L) 8.6-10.7-152 . | Serum Ferritin (μg/L) 15-150-152 . | Serum Iron (μmol/L) 14-32-152 . | Fe Saturation 0.25-0.60-152 . | ALAT IU 10-50-152 . | g-GT IU 15-70-152 . | Liver . | HFE-153 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/31 | .57 | 2.63 | 16 | 9.1 | 2,970 | 46 | 0.73 | 62 | 31 | SF | −/− |
| 1 | M/31 | .61 | 2.84 | 10.1 | 2,160 | 54 | 0.81 | 49 | 30 | |||
| 2 | M/30 | .6 | 1.95 | 4.4 | 9.8 | 271 | 39 | 0.74 | 43 | 47 | N | +/+ |
| 3 | M/66 | .61 | 3.2 | 5.5 | 7.8 | 30 | 7 | 0.1 | 52 | 230 | C | +/+ |
| 4 | M/65 | 1.13 | 1.95 | 7 | 9.1 | 1,179 | 31 | 0.76 | 41 | 21 | N | +/+ |
| 5 | M/66 | .73 | 3 | 9.5 | 31 | 21 | 0.43 | 26 | 24 | −/+ | ||
| 6 | M/52 | 1.17 | 1.48 | 6 | 8.9 | 409 | 36 | 0.79 | 9 | 16 | LF | +/+ |
Abbreviations: Liver damage: N, no damage; LF, light fibrosis; SF, severe fibrosis; C, cirrhosis.
Phagocytic index (PI) = total number of erythrocytes/total number of cells.
Ratio PI donor (Pld) versus PI patient (Plp).
Normal values.
C282Y mutation in the HFE gene.
Monocyte Isolation and Purification
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats by fractionation on Ficoll-Paque density centrifugation at room temperature (Ficoll-Paque, p = 1.077 g/mL; Pharmacia, Uppsala, Sweden). Monocytes were purified from PBMC by monocyte clumping as described by Shen et al,8 followed by adherence to plastic. The combination of the two techniques was used to ensure a monocyte purity higher than 85%. Monocytes were finally resuspended in RPMI 1640 (GIBCO-BRL, Breda, The Netherlands) supplemented with 5 mmol/L HEPES, 19 mmol/L sodium bicarbonate, 10 μg/mL gentamicin and 10% fetal bovine serum (FBS) at a concentration of 1 × 107 cells/mL, seeded on tissue culture flasks (Costar Europe Ltd, Badhoevedorp, The Netherlands), and incubated overnight before erythrophagocytosis. The purity of the isolated monocytes was evaluated on cytospins stained with May-Grünwald-Giemsa (Dade Diff-Quick solutions; Baxter, Düdingen, Switzerland) and by fluorescence-activated cell sorting (FACS) analysis using anti-CD14–fluorescein isothiocyanate (FITC)-labeled monoclonal antibody (MoAb).
Erythrophagocytosis Assay
59Fe in vivo erythrocyte labeling.
To produce 59Fe-labeled red blood cells, 9.5 MBq59Fe-citrate (specific activity, 0.15 to 0.44 MBq/μg iron; Amersham, Den Bosch, The Netherlands) was injected intravenously into a chinchilla rabbit (weight 4,000 g). A total of 6 to 19 mL of blood was collected 1 week after injection. When required, blood could be withdrawn every 2 weeks. To compensate for the 59Fe decay, 59Fe-citrate was injected after each bleeding.9
Preopsonization of the erythrocytes.
Rabbit red blood cells (RRBC) were washed four times and suspended in RPMI at a concentration of 1 × 109cells/mL. RRBC were incubated with heat-inactivated mouse antirabbit erythrocyte serum at a dilution of 1:2,000 at 37°C in a shaking water-bath. The mouse serum was kindly provided by Prof H. van Dijk (Eijkman-Winkler Institute, University Hospital Utrecht, The Netherlands). After 30 minutes, the erythrocyte suspension was washed and resuspended in RPMI at a concentration of 1 × 109cells/mL.
Phagocytosis.
Opsonized erythrocytes were added at a ratio of monocytes:RRBC of 1:50 for 2 hours. Noningested RRBC were removed by performing hypotonic lysis twice. The extent of phagocytosis was evaluated by light microscopy on Giemsa-stained cytospins. In addition, iron uptake was assessed by counting 59Fe activity in the cells in an automatic γ-counter (PW4800; Phillips, Almelo, The Netherlands). Erythrophagocytosis was expressed as phagocytic index (PI), ie, the number of erythrocytes taken up per monocyte. No radioactivity was measured in the monocytes after these cells were cocultured for 24 hours with the same amount of 59Fe-Hb present in the RRBC used in a standard erythrophagocytosis experiment. To exclude the possibility that 59Fe-Hb detected in the supernatant was the result of nonspecific adherence of erythrocytes to monocytes, which were not removed by the hypotonic lysis, the following control experiment was conducted. Monocytes and erythrocytes were incubated for 2 hours at 4°C, which prevents phagocytosis and results in nonspecific adherence of RRBC to monocytes. Erythrocytes were then removed by performing hypotonic lysis twice, and a standard release experiment was conducted. No radioactivity was recovered from the monocyte cultures. Therefore, the radioactivity measured after erythrophagocytosis is due to uptake of RRBC and cannot be ascribed to uptake of 59Fe-Hb from lysed erythrocytes or from nonspecific adsorption of 59Fe-Hb or RRBC to the monocytes.
Iron Release by Monocytes
After erythrophagocytosis, monocytes were resuspended in RPMI supplemented with 5 mmol/L HEPES, 19 mmol/L sodium bicarbonate, and 10 μg/mL gentamicin at a concentration of 1 × 106/mL. No protein was added. Monocytes were seeded in tissue culture flasks (Costar Europe, Lda, Badhoevedorp, The Netherlands) and incubated at 37°C in the presence of 7% CO2 and at 95% humidity. At time points 0, 1.5, 4, 24, and 48 hours during the release experiment, cells and supernatants were collected separately and59Fe was measured in both samples. Iron activity in the supernatant or cell fractions is expressed as percentage of the total iron present in the system (cells + supernatant). Erythrophagocytosis and iron release of a patient and a healthy donor were always performed on the same day. Cell viability was determined by trypan blue dye exclusion after each cell isolation, after erythrophagocytosis, and during iron release. In seven experiments, cell viability was also assessed by the 51Cr-release method.10 In brief, monocytes were incubated with 51Cr before the addition of nonlabeled erythrocytes. At the same time points after erythrocyte uptake as for the release experiment, 51Cr activity was measured in supernatants from monocytes ingesting erythrocytes and was compared with that of supernatants from monocyte cultures that were incubated with 51Cr, but were not allowed to phagocytose erythrocytes.
Preparation of Samples for SE-HPLC
Analysis of the molecular forms in which radioactive iron was present was performed on cell and supernatant fractions at 0, 1.5, 4, 24, and 48 hours. Cells were harvested and pelleted at 390g for 10 minutes at 4°C. Adherent cells were detached with a trypsin solution (2 g/L trypsin/phosphate-buffered saline [PBS]) (GIBCO-BRL) for 5 minutes at 4°C and subsequently gently removed with a cell scraper. The supernatants were collected (S fractions) and the cell pellets were suspended in ice-cold double-distilled deionized water (ddH2O). Cells were lysed by three cycles of freezing (−20°C) and thawing. Cell membranes and nuclei were pelleted by centrifugation in the cold for 30 minutes at 10,000g (Cm fractions). The supernatant (cell cytosol) was transferred into a different tube (Cc fractions). Cc and S fractions were frozen (-20°C) and subsequently dried in a speed vacuum concentration system (Speed vacuum HS-1-110; Hetto Lab Equipment, Radiometer Nederland, Zoetermeer, The Netherlands). 59Fe activity of all the fractions (Cc, Cm, and S fractions) was measured. Total activity of the samples, PI, and iron release curves were calculated based on the radioactivity measured. The samples were then stored at −20°C until analysis was performed. Before analysis, Cc and S fractions were reconstituted with ddH2O. One hundred microliters of water was added per 1 × 106cells-equivalent of dried sample. Before injection into the HPLC system, samples were filtered twice through low-protein binding 0.45 μm filters (a 0.45-μm nonsterile Spin-X from Costar, Cambridge, MA, followed by a 0.45-μm type VI filter from Nihon Millipore Lda, Yonezawa, Japan).
Analysis of Iron Forms by SE-HPLC
SE-HPLC was performed using an LKB high performance liquid chromatography system consisting of a 2150 HPLC pump, a 2152 HPLC controller, and a manual Syringe Loading Sample Injector (Pharmacia LKB Biotechnology, Uppsala, Sweden), and using a Bio-Sil Sec-250 Column (BioRad, Hercules, CA). The fractionation range of this column for proteins is from 10,000 to 300,000 d. Separations were performed at a rate of 0.75 mL/min with HEPES buffer (HEPES 50 mmol/L, NaCl 100 mmol/L, NaN3 10 mmol/L, pH 7.4). The eluate was monitored by a 2151 variable wavelength detector with absorbency detector at 280 nm and recorded on a 2210 2-channel recorder. To remove nonspecifically bound iron, the column was finally perfused with formic acid (formic acid 1N, pH 2.8),11 and the pH was restored with HEPES buffer before each new fractionation. 59Fe activity was assessed in the eluted fractions.
Preparation of Standards and Calibration of the SE-HPLC Column
To determine in which fractions ferritin, Hb, transferrin, and LMW-Fe would elute, human liver ferritin, human 59Fe-transferrin, rabbit 59Fe-Hb, and adenosine triphosphate (ATP)-59Fe were prepared and injected separately into the column. These references were prepared as described below.
Ferritin.
Ferritin type V from human spleen was purchased from Sigma (Sigma Chemie, Bornem, Belgium) and 2 μg ferritin was reconstituted with 200 μL H2O (final concentration 10 μg/mL). Ferritin (MW 450,000 to 600,000) was determined in the eluted fractions by a standard method (automated chemiluminescence system-immunoluminometric assay [ILMA], on a ACS-180, from Chiron, Emeryville, CA).
59Fe-transferrin.
59Fe-labeled transferrin was prepared as follows. A 6.25-μmol/L human apotransferrin solution, (approximately 98% pure, MW 76,000 to 81,000; Sigma Chemie) was prepared in H2O. To a 1-mL solution, 0.1 MBq 59Fe citrate (specific activity 0.24 MBq/μg iron) was added and the iron was allowed to bind to the apotransferrin by rotating the solution slowly at 4°C (molar ratio Tf:Fe ≈ 1:1). To saturate the Tf binding sites, excess cold Fe-citrate was added and the solution was further incubated overnight at 4°C. Nonbound 59Fe was removed by gel permeation chromatography. After fractionation, radioactivity was assessed on the eluted fractions. Transferrin was completely saturated (Tf: Fe ratio was 1:2).
59Fe-rabbit Hb.
Washed 59Fe-labeled RRBC (1 × 109) were pelleted and lysed in 1 mL ice cold H2O. Membranes and nonlysed erythrocytes were pelleted at 10,000g for 10 minutes at 4°C. The Hb solution was filtered through a 0.45-μm filter and an amount of Hb equivalent to 2 × 108 RRBC was injected into the column. The Hb solution (MW ca 68,000) was freshly prepared. Radioactivity of the collected fractions was measured.
ATP-59Fe.
ATP-59Fe complex solution was prepared as described elsewhere.11 5′-ATP was dissolved in 0.1 mol/L NaCl-0.1 mol/L HEPES, pH 7.0. To 10 mL ATP solution, 50 KBq59Fe-citrate (specific activity, 0.3 MBq/μg iron) was added. Next, a solution of cold FeCl3 in 1 mol/L HCl was slowly added with rapid stirring and 1 mol/L NaOH was added to maintain the pH at 7.0. The final complex had a fourfold molar excess of ATP (5′-ATP 16 mmol/L, FeCl3 4 mmol/L). This ratio ensures a complex free of polynuclear iron.11 12 ATP-Fe complex solution was freshly prepared. A total of 40 μL of the ATP-59Fe was injected into the column. Radioactivity of the collected fractions was measured.
Calibration of the SE-HPLC column.
Ferritin eluted as a single peak (fractions 9 to 11). Transferrin was detected as one major peak in fractions 11 to 13 and one small peak in fractions 14 to 16, the latter probably as a result of a contamination. Hb eluted as a single peak in fractions 13 to 15. For ATP-Fe, a single major peak was recovered in fractions 27 to 29.
Chemical analysis of the pooled fractions.
Ferritin, transferrin, and Hb standards eluted from the HPLC column in fractions 9 to 11, 11 to 13, and 13 to 15, respectively. Fractions from three different release experiments were pooled, dialyzed against ddH2O, and lyophilized. The lyophilized material was reconstituted in H2O and analyzed for the presence of ferritin, transferrin, Hb and iron by standard chemical methods.13-15 Ferritin was measured by automated chemiluminescence - ILMA; Hb was measured as free Hb by spectrophotometry; iron was measured by dry chemistry in a Ektachem 950 from Johnson & Johnson (Clinical Diagnostics, Amersfoort, The Netherlands); transferrin was measured by immunonephelometry in a Hitachi 911 from Behring (Behringwerke, AG, Marburg, Germany). Ferritin and Hb were mainly detected in those pooled fractions as predicted by the elution patterns of the standards (ferritin, fractions 9 to 11 and Hb, fractions 13 to 15). Iron was detected in all pooled fractions. No transferrin was detected in any of the pooled fractions (detection limit of the analysis method used for transferrin was 2.5 mg/L ≈ 36 nmol/L).
Measurement of Heme Oxygenase Activity in Control and HH Monocytes
Monocytes were purified from healthy donors and from HH patients. Because heme oxygenase activity in monocytes was too low to measure, the following procedure was used: (1) heme oxygenase was induced in the cells by incubation with hemin for 16 hours; (2) microsomal fractions were isolated,; and (3) for each measurement microsomal fractions of either six controls or six patients were pooled.16 Because the microsomal fractions did not contain biliverdin reductase, this enzyme was isolated from rat livers.17 As a positive control, heme oxygenase was isolated from rat livers and spleens.17 After induction, the viability of the cells was higher than 90%. Although the induction of heme oxygenase is complete after 6 hours,16 for convenience, it was performed overnight. The protein concentrations in the microsomal and the biliverdin reductase fractions were determined by the microtiter Pierce BCA assay (Pierce, Rockford, IL).18 The microsomal fractions of spleen, liver, and monocytes were supplemented with the isolated liver biliverdin reductase and incubated with hemin.19 During this incubation, the hemin is converted to bilirubin. Samples were taken at several time intervals and the bilirubin concentration was determined.20 In the assay, bilirubin is converted to azopigments. The concentration of these azopigments was determined by spectrophotometry (Bio-Rad micro plate reader 3550, BioRad Laboratories BV, Veenendaal, The Netherlands).
Heme oxygenase activity was calculated as the amount of bilirubin formed per 10 minutes per mg of protein in the microsomal fraction. For this calculation the steepest part of the curve was used.
Statistical Analysis
Statistical analysis was performed using the Student's t-test assuming equal variance for the means. Relations between phagocytic index and 51Cr release were evaluated by correlation analysis.21 A P < .05 was considered significant.
RESULTS
Iron Release by Monocytes
After erythrophagocytosis for 2 hours, erythrocyte uptake was assessed either by light microscopy or by measuring 59Fe. The correlation between the two methods was highly significant (r = .92, n = 21, P < .001). HH monocytes phagocytosed less than half the number of erythrocytes taken up by healthy donor monocytes (PI for controls = 1.3 ± 0.3 [n = 9]; PI for HH patients = 0.6 ± 0.2 [n = 10 P < .0001]). Table1 shows the individual values of the PI for the patients. To compare values from different experiments, the ratio of the PI from HH patients to control performed on the same day (PId/PIp) is also shown. These results confirm previous data of our group demonstrating a decreased phagocytic ability of HH monocytes.6 22
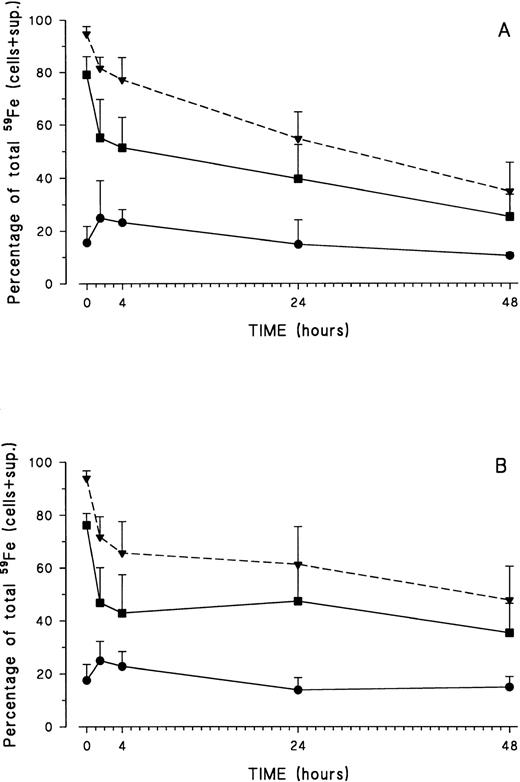
Iron release was monitored at 37°C from 0 minutes up to 48 hours. Iron release at 37°C was identical in control and HH monocytes and displayed a biphasic pattern: a fast release phase during the first 1.5 hours, followed by a much slower release during the remainder of the experiment (Fig 1).
Iron release by healthy volunteer (○) and HH patient (•) monocytes. After erythrophagocytosis, monocytes were incubated in RPMI from 0 to 48 hours. At a given time point, cells and supernatants were collected and 59Fe activity measured in both fractions. Results are expressed as percentage of the total iron in the system (mean ± standard deviation [SD] of six experiments).
Iron release by healthy volunteer (○) and HH patient (•) monocytes. After erythrophagocytosis, monocytes were incubated in RPMI from 0 to 48 hours. At a given time point, cells and supernatants were collected and 59Fe activity measured in both fractions. Results are expressed as percentage of the total iron in the system (mean ± standard deviation [SD] of six experiments).
If uptake of erythrocytes is too high, this can influence cell viability, thereby affecting release patterns.4 As assessed by trypan blue, cell viability of both donor and HH monocytes exceeded 90% throughout the experiments. 51Cr release experiments gave identical results. For both normal and HH monocytes, no significant differences in 51Cr release were found between monocytes ingesting erythrocytes and those not doing so. There was no difference in 51Cr release between monocytes + RRBC and control monocytes at 1.5 hours (P = .76, n = 7), at 24 hours (P = .33, n = 7), and at 48 hours (P = .32, n = 3). Furthermore, no correlation was observed between the number of erythrocytes taken up by monocytes (PI ≤ 1.5) and 51Cr release, demonstrating that the number of erythrocytes taken up in our experiments was not toxic for the cells (r = .123, P = .88).
Characterization of the Intracellular Forms of Iron in Monocytes After Erythrophagocytosis
At different time points during iron release, monocytes were lysed by freeze-thawing and cell membranes and nuclei were removed by centrifugation. Radioactivity present in both fractions was measured. Most of the radioactive iron was detected in the cytosol fraction, whereas only a low, but constant, percentage of iron was associated with the cell membranes (Fig 2). These results were identical for control (Fig 2A) and HH monocytes (Fig 2B).
Distribution of iron inside healthy volunteer (A) and HH (B) monocytes after erythrophagocytosis and during the release experiment. At different time points during iron release, monocytes were lysed by freeze-thawing, and cell membranes and nuclei were removed by centrifugation. Radioactivity was measured in the pellet-membrane fraction (•) and in the supernatant-cytosol fraction (▪). The total radioactivity present in the cell at any given time is also shown (▾ and dashed line). Results are expressed as percentage (mean ± SD) of total iron present in the system (= cells + supernatant) of five control and six HH experiments.
Distribution of iron inside healthy volunteer (A) and HH (B) monocytes after erythrophagocytosis and during the release experiment. At different time points during iron release, monocytes were lysed by freeze-thawing, and cell membranes and nuclei were removed by centrifugation. Radioactivity was measured in the pellet-membrane fraction (•) and in the supernatant-cytosol fraction (▪). The total radioactivity present in the cell at any given time is also shown (▾ and dashed line). Results are expressed as percentage (mean ± SD) of total iron present in the system (= cells + supernatant) of five control and six HH experiments.
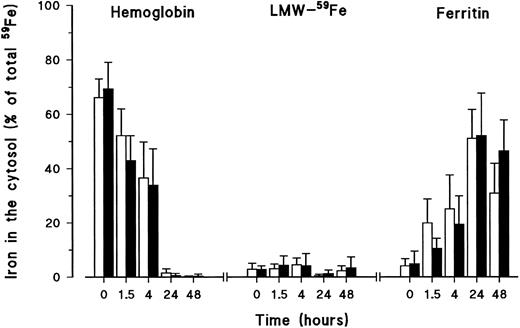
The forms in which iron was present in the cytosol fractions were analyzed by SE-HPLC. After control monocytes had been allowed to phagocytose for 2 hours, most of the 59Fe inside the monocytes was present as Hb (time point 0 of release experiment) (Fig 3). This Hb then progressively decreased so that it was barely detectable after 24 hours. At the beginning of the release experiment, some 59Fe could already be detected in the form of ferritin, apparently because the release experiment was started after 2 hours of erythrophagocytosis. In this time period, some degradation of Hb and transfer of iron to ferritin had already taken place. Ferritin steadily increased during the experiment and at 24 hours, almost all of the 59Fe in the cytosol could be identified as ferritin. Only a very low amount of iron in the cytosol was recovered as LMW-Fe. This amount was constant during the experiment.
Iron forms in the cytosol of healthy volunteer monocytes (□) and HH monocytes (▪) after erythrophagocytosis and during the release experiment. At different time points during iron release, monocytes were lysed by freeze-thawing and cell membranes and nuclei removed by centrifugation. Cytosol samples were frozen, dried, reconstituted with bidistilled deionized water, filtered, and fractionated on SE-HPLC. Radioactive iron was measured again in the eluted fractions. Iron was recovered as Hb, LMW-Fe, and ferritin. The results are expressed as percentage (mean ± SD) of the total iron present in the system (= cells + supernatant) of five experiments. Statistical analysis was by Student's t-test. Results of controls and patients were not statistically different.
Iron forms in the cytosol of healthy volunteer monocytes (□) and HH monocytes (▪) after erythrophagocytosis and during the release experiment. At different time points during iron release, monocytes were lysed by freeze-thawing and cell membranes and nuclei removed by centrifugation. Cytosol samples were frozen, dried, reconstituted with bidistilled deionized water, filtered, and fractionated on SE-HPLC. Radioactive iron was measured again in the eluted fractions. Iron was recovered as Hb, LMW-Fe, and ferritin. The results are expressed as percentage (mean ± SD) of the total iron present in the system (= cells + supernatant) of five experiments. Statistical analysis was by Student's t-test. Results of controls and patients were not statistically different.
Identical results were obtained for HH monocytes. Although the relative percentage of ferritin detected in the cytosol of HH monocytes was lower at 1.5 and 4 hours, these differences between control and HH monocytes were not statistically significant (P = .069 at 1.5 hours and P = .13 at 4 hours). In addition, the relative percentage of ferritin in the cytosol of HH monocytes was higher at 48 hours. But again, this difference was not statistically significant (P = .159).
Characterization of Forms of Iron Released by Control and HH Monocytes After Erythrophagocytosis
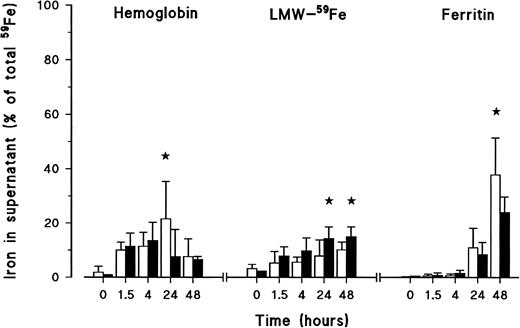
At different time points during iron release, supernatants from control and HH samples were collected and fractionated by SE-HPLC (Fig 4). In the first 4 hours, most of the activity in the supernatants was either in a LMW-form or detected as Hb. At 24 hours and 48 hours, additional release of iron was mainly in the form of LMW-Fe and ferritin. Despite a lower rate of erythrophagocytosis, HH monocytes released twice as much iron in a LMW-form as compared with control cells (P < .05 at 24 hours and P < .03 at 48 hours). When calculated as percentage of the total 59Fe released at 24 hours, HH monocytes released 42% ± 6% of the iron as LMW-Fe, while only 16% ± 13% iron was released in this form by control monocytes in the same period of time. In addition, control monocytes released significantly more iron as Hb at 24 hours (iron released as Hb was 21% ± 14% and 7.7% ± 10% for control and HH monocytes, respectively, P < .05). Significantly more iron was released as ferritin by control monocytes at 48 hours (37.8% ± 13.6% and 24.2% ± 5.6%, for control and HH monocytes, P < .05). No radioactive peak was recovered at the place were the transferrin standard eluted.
Iron forms in the supernatants of healthy volunteer monocytes (□) and HH monocytes (▪) after erythrophagocytosis and during the release experiment. At different time points during iron release, supernatants were collected, frozen, dried, reconstituted with ddH2O, and fractionated on SE-HPLC. Radioactive iron was measured again in the eluted fractions. Iron was recovered as Hb, LMW-Fe, and ferritin. The results are expressed as percentage (mean ± SD) of the total iron present in the system (= cells + supernatant) of six experiments. Statistical analysis was by Student'st-test (★, P < .05).
Iron forms in the supernatants of healthy volunteer monocytes (□) and HH monocytes (▪) after erythrophagocytosis and during the release experiment. At different time points during iron release, supernatants were collected, frozen, dried, reconstituted with ddH2O, and fractionated on SE-HPLC. Radioactive iron was measured again in the eluted fractions. Iron was recovered as Hb, LMW-Fe, and ferritin. The results are expressed as percentage (mean ± SD) of the total iron present in the system (= cells + supernatant) of six experiments. Statistical analysis was by Student'st-test (★, P < .05).
Measurement of Heme Oxygenase Activity in Control and HH Monocytes
Control and HH monocytes released a substantial part of their iron in the form of Hb over a 24-hour period. Because heme oxygenase is the limiting enzyme in the breakdown of Hb and monocytes are immature macrophages, we investigated whether this finding could be ascribed to a lack of this enzyme in monocytes, or to the level that this enzyme can be induced in these cells. Heme oxygenase levels are very low in monocytes and high numbers of cells are required to detect its activity (> 200 × 106). However, heme oxygenase can be induced in cells by incubation with hemin. Therefore, monocytes from controls and patients were incubated overnight with hemin and the microsomal fractions of six controls and six patients were pooled before measuring heme oxygenase activity. Heme oxygenase activity was assessed by measuring bilirubin formation. As positive controls, heme oxygenase activity of rat liver and rat spleen cells were measured as well. Both control and HH monocytes had similar inducible heme oxygenase activity (2.13 and 1.96 nmol bilirubin formed/10 min/mg protein for control and HH monocytes, respectively). The heme-oxygenase activity detected was similar to the activity present in rat spleen cells (2.25 nmol bilirubin formed/10 min/mg protein).
DISCUSSION
To study iron release by monocytes and macrophages, we chose to load monocytes with iron by erythrophagocytosis, as in vivo this method of processing senescent erythrocytes is how the mononuclear-phagocyte system recycles most of the iron. Calculations of the rate of processing of erythrocytes in vivo suggest that, under normal conditions, splenic macrophages process one erythrocyte per macrophage per hour.23 Kondo et al4 demonstrated a strong decrease in cell viability after 24 hours when the average number of erythrocytes per Kupffer cell was higher than two. To resemble the in vivo situation in our experiments, monocytes were loaded with RRBC under such conditions that as many monocytes as possible contained RRBC, but with an average number of erythrocytes per phagocyte not exceeding 1.5.
Our experiments required viable, intact, and metabolically active monocytes. The cell viability as assessed by trypan blue exclusion exceeded 90% throughout the whole experiment for both donor and HH monocytes and no significant differences were observed in the51Cr release by monocytes ingesting erythrocytes as compared with control monocytes. Furthermore, the progressive shift of59Fe activity from Hb to ferritin inside the cells demonstrates the presence of metabolically active and intact cells (Fig3). Therefore, the experimental conditions chosen neither affected the cell viability and metabolic activity, nor resulted in cell leakage.
As expected from erythrocyte catabolism, erythrocyte degradation resulted in progressive transfer in the cytosol of 59Fe from Hb to ferritin (Fig 3). In this compartment, only very little iron was recovered as a nonprotein bound form. This might, however, be an underestimation of the actual amount, because during the whole release experiment, about 20% of the iron was found in the membrane fraction, which could not be analyzed by SE-HPLC (Fig 2). In contrast to Custer et al,2 we were not able to detect transferrin inside the monocytes. Our results are in agreement with previous studies showing that human macrophages, in contrast to mouse macrophages, do not synthesize transferrin.24 The rate of iron transfer to ferritin in the beginning of the experiment (0 to 4 hours) was apparently slower for HH monocytes, but these differences were not statistically significant (Fig 3). No other differences in the rate of erythrocyte catabolism were observed between donors and patients, suggesting that the metabolic activity of the monocytes obtained was similar for both groups.
The erythrophagocytic ability of HH monocytes was about half that of control monocytes, as described previously.6 Iron release showed a biphasic pattern: an early rapid phase, followed by a slower late phase. Although the absolute amount of iron loaded in control and patient monocytes was different, the relative percentage of iron released, as well as the kinetics of iron release, were identical (Fig1). Iron release has previously been studied in vitro using rat peritoneal macrophages performing erythrophagocytosis, rat Kupffer cells, and human monocytes.2-4 It has also been studied in vivo in humans.25 In all cases, similar iron release kinetics were described and a biphasic pattern for the iron release was demonstrated. In addition, Fillet et al25 also studied iron release from the MPS in HH patients in vivo. In these patients, the early appearance of 59Fe (fast phase) was similar to that of healthy individuals, but in contrast to our results, the late release phase was delayed as compared with controls.
In our study, iron released after erythrophagocytosis was recovered in three different molecular forms: ferritin, Hb, and nonprotein-bound LMW-Fe complexes. The fast release phase (0 to 1.5 hours) consisted mainly of Hb and LMW-Fe. Additional release (after 1.5 hours) was mostly in the form of ferritin and LMW-Fe (Fig 4). The most striking finding was the observation that HH monocytes released more than twice as much iron in the LMW form as compared with control monocytes (42% and 16%, respectively).
Transfer of iron to ferritin after erythrophagocytosis has previously been demonstrated in human monocytes2 and rat Kupffer cells.4 In the latter study, the addition of an intracellular iron ligand (desferrioxamine) resulted in the binding of iron to this chelator, suggesting the existence of a transient LMW-Fe pool inside the cell.4 In the same study, iron was found to be released as ferritin and as a form readily binding to transferrin in the medium. Custer et al2 demonstrated iron release in the form of ferritin and transferrin, but the presence of LMW-Fe was not studied. The detection of transferrin in these studies might be due to the presence of serum in the media used during the release experiments.
Part of the iron released in our experiments was in the form of Hb. This finding has been described by others previously. While Kondo et al4 considered this finding to be a consequence of loading the phagocytes with too many erythrocytes (number of erythrocytes per monocyte > 2.0), Custer et al2 has postulated that this Hb release may represent a normal physiologic process in vivo. Although the uptake of too many erythrocytes by phagocytes is toxic and could result in cell damage and release of Hb, our experimental conditions ensured a phagocytic index below 1.5 and that the monocytes used in the experiments were viable and metabolically active (see Fig 3).
Monocytes contain only limited amounts of heme oxygenase, the rate limiting enzyme of Hb degradation.26 Because the initial release of Hb might be due to insufficient amounts of (inducible) heme oxygenase, the presence of inducible heme oxygenase was studied in monocytes obtained from donors and HH patients. Heme oxygenase activity was readily induced in monocytes from both groups and in similar amounts as the heme oxygenase activity present in rat spleen cell suspensions. In this study, the heme oxygenase activity was measured after overnight incubation with hemin, whereas in our experiments, erythrophagocytosis was stopped after 2 hours. Previous studies performed with rat peritoneal macrophages showed that the induction of heme oxygenase was complete 5 hours after the beginning of erythrophagocytosis.24 Iron release, however, was quite constant in all our experiments and not only observed with monocytes, but also with in vitro differentiated monocyte-derived macrophages. Moreover, monocytes preincubated with low numbers of erythrocytes to induce heme oxygenase still released similar amounts of Hb as compared with monocytes, which were not preincubated with erythrocytes (data not shown). Although it cannot be excluded that the early release of Hb is in part due to the lack of heme oxygenase in the first hours of the experiments, we feel that iron release in the form of Hb is a normal physiologic process, not only occurring after intravascular hemolysis, but also after normal erythrophagocytosis. Furthermore, when control monocytes were loaded with fewer RRBC, less iron was released as Hb (results not shown). Therefore, the differences in iron release as Hb by control and HH monocytes may probably reflect the higher uptake of erythrocytes by control monocytes.
An argument against the physiologic role of iron release from monocytes as Hb may be the presence of only low concentrations of free or haptoglobin-bound Hb in plasma (normal values < 40 mg Hb/L plasma).27 It has been calculated that, in humans, 360 × 109 red blood cells are processed per day.23 Assuming that all erythrocytes would be processed in the spleen and that, like in our experiments, from all these erythrocytes 22% of the Hb would be released into the portal system (control monocytes at 24 hours), then the amount of Hb released would be approximately 22 nmol/L/min. Hb or heme in the plasma will bind to haptoglobin and to albumin or hemopexin, respectively.28Hepatocytes possess receptors for complexed and free Hb and heme. It has been estimated that in humans 113 to 157 nmol (21.6 to 30 mg) of haptoglobin-Hb complexes are cleared per liter per minute (T1/2 haptoglobin-Hb in the rat is 7 minutes).28 29 As a result, the amount of circulating Hb will be even lower. Therefore, if Hb is released in similar amounts from splenic macrophages as the monocytes in our studies, a maximum of 22 nmol/L/min would be released in the blood stream, which is below the detection limit of the currently used methods of detection of free Hb (6 μmol/L). Therefore, the release of Hb from monocytes and macrophages is quite plausible as a mechanism of iron release from the MPS.
The following model for iron metabolism in the MPS system can thus be proposed. After erythrophagocytosis, Hb is degraded by heme oxygenase. Whenever the capacity to handle erythrocytes or heme is exceeded, Hb or heme are released into the circulation where they bind to haptoglobin, and to hemopexin and albumin, respectively, and are taken up by hepatocytes. In the macrophage, iron that is freed from the Hb is either released as LMW-Fe (fast, early release phase) and binds to circulating apotransferrin, or is incorporated into ferritin, part of which is released during a second, slower phase. As a consequence, LMW and transferrin-bound iron in plasma can originate directly from the MPS, but also from hepatocytes, following further catabolism of MPS-derived Hb. The molecular mechanisms of iron release from the MPS, as LMW-Fe, Hb, and ferritin, remain obscure.
A hallmark of HH is the inappropriately high level of iron absorption in the gut despite the presence of iron overload.30-32Untreated adult HH patients, however, strongly differ in their level of iron absorption with values ranging from around 10%, which are also found in healthy individuals, to almost 100%31,33 (E. Moura, PhD thesis, University Utrecht, The Netherlands, 1997). All HH patients have a high plasma iron and transferrin saturation, even in the group with normal iron absorption. Increased iron absorption alone, therefore, cannot explain the high transferrin iron saturation in HH patients. Because HH monocytes release more iron in the LMW-Fe form, this might contribute to the higher transferrin saturation and the presence of nontransferrin-bound iron encountered in these patients. This happens despite normal erythropoietic activity, in contrast to severe hemolysis or dyserythropoiesis, in which patients have a very high plasma iron turn-over and also have nontransferrin-bound iron in their plasma.34 Another important characteristic of HH is the absence of iron in MPS until later stages of the disease. In HH patients, more iron is released as LMW-Fe, leading to increased transferrin saturation and iron circulation in a LMW-form, probably as Fe-citrate or Fe-acetate.33 LMW-Fe can then rapidly be taken up by hepatocytes,35 explaining the preferential iron storage in the hepatocytes.
Iron released as ferritin was also different when control and HH monocytes were compared. At 48 hours, control monocytes released significantly more iron as ferritin (Fig 4). In a follow-up period of 2 weeks, Fillet et al25 observed a delayed late phase of iron release in HH patients. The form of iron released in that study was not characterized. In the present work, iron release by HH monocytes as ferritin over a 48-hour period is lower than in control monocytes, while the intracellular ferritin is increased. These findings suggest that the form in which iron was released during the slower phase of iron release, observed in HH patients by Fillet et al, may be in the form of ferritin.
The genetic basis of HH in the majority of cases in Caucasians has been clarified by the identification of the C282Y mutation in the HFE gene. However, the pathogenesis of abnormal iron accumulation in HH remains obscure. The demonstration of an increased release of LMW-Fe from HH monocytes may represent a significant advance in the clarification of HH pathophysiology. It may signify a basic abnormality in the retention of iron in macrophages and probably from intestinal mucosal cells, as well. This assumption is supported by recent work of Cairo et al,36 who demonstrated an inappropriately high iron regulatory protein activity in monocytes obtained from HH patients, indicating the low levels of labile iron pool in these cells. Recently, it was demonstrated in human embryonic kidney cells that the HFE gene protein product associates with the transferrin receptor (TfR), resulting in a decreased affinity of the transferrin receptor for transferrin.37 The C282Y mutation almost completely eliminated this association between HFE-protein and the transferrin receptor. Iron uptake from transferrin, however, is limited in macrophages, occurring mainly during cell diferentiation and activation, but is latent in resident macrophages.38 We have previously reported that HH monocytes and monocyte-derived macrophages have a decreased ability to phagocytize opsonized erythrocytes (Fc receptor-mediated process), not attributable to differential expression of Fcγ or complement receptors.6,39 In addition, the level of expression of the CD14 molecules was significantly lower in HH monocytes (unpublished observation), which might be related to the decreased ability of HH monocytes to produce tumor necrosis factor-α (TNF-α) on stimulation with lipopolysaccharide (LPS), as has been reported by Gordeuk et al.40 Taken together, these observations may suggest that, besides binding to the TfR, the HFE protein also associates with other surface proteins, thereby promoting or stabilizing their expression on the outer-cell membrane or influencing their function.
The molecular mechanisms of iron release from the MPS, as LMW-Fe, Hb, and ferritin remains obscure. However, the remarkable finding of the high proportion of iron released from the monocytes, as Hb and ferritin ought to be incorporated into models of iron metabolism and ferrokinetics. Our finding of increased release of LMW-Fe in HH may explain the high transferrin saturation and nontransferrin-bound iron in HH, especially in patients with low iron absorption.
Supported by Grant No. BD 1656-91 from Junta Nacional de Investigaçäo Cientı́fica e Tecnológica (Portugal).
Address reprint requests to Joannes J.M. Marx, MD, PhD, University Hospital Utrecht, Department of Internal Medicine, Postnr. G02.228, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail: J.Marx@digd.azu.nl.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Iron release by healthy volunteer (○) and HH patient (•) monocytes. After erythrophagocytosis, monocytes were incubated in RPMI from 0 to 48 hours. At a given time point, cells and supernatants were collected and 59Fe activity measured in both fractions. Results are expressed as percentage of the total iron in the system (mean ± standard deviation [SD] of six experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2511/3/m_blod41906001x.jpeg?Expires=1769139230&Signature=VpCmF4JN7YsiqGCYblPqCmrod-hIpNdc9nVSHVIY53wnuiznWqhSQt8dKLrQ5p4jzU-OVX6WVDA2FLPZJFuMk~lED38-mcMilnRhnPnuziOLxW9G2V6I7p-2HcZQAYGKUF3oiWoTnRZHQ26hvs7H9Rx6MrdqstUzY8iTGe2iWJ9hnvSBZHPS2ioXEvh7OyMAIkB9cbSDnabwEokBdHvV4Jo9FbyooeAqwoXfDpARVfHd3YPNNJTCcOM9hGLc9aShk2FEr96l79RRJrRyKlBbfaabY7iPEfXJTXh~vOk6RxXkXlt4C25~nWmGd6IuV0F8TCvtLEKgfO2E43eDom~79A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal