Abstract
Fibrinogen is a plasma protein that interacts with integrin αIIbβ3 to mediate a variety of platelet responses including adhesion, aggregation, and clot retraction. Three sites on fibrinogen have been hypothesized to be critical for these interactions: the Ala-Gly-Asp-Val (AGDV) sequence at the C-terminus of the γ chain and two Arg-Gly-Asp (RGD) sequences in the Aα chain. Recent data showed that AGDV is critical for platelet adhesion and aggregation, but not retraction, suggesting that either one or both of the RGD sequences are involved in clot retraction. Here we provide evidence, using engineered recombinant fibrinogen, that no one of these sites is critical for clot retraction; fibrinogen lacking all three sites still sustains a relatively normal, albeit delayed, retraction response. Three fibrinogen variants with the following mutations were examined: a substitution of RGE for RGD at position Aα 95-97, a substitution of RGE for RGD at position Aα 572-574, and a triple substitution of RGE for RGD at both Aα positions and deletion of AGDV from the γ chain. Retraction rates and final clot sizes after a 20-minute incubation were indistinguishable when comparing the Aα D97E fibrinogen or Aα D574E fibrinogen with normal recombinant fibrinogen. However, with the triple mutant fibrinogen, clot retraction was delayed compared with normal recombinant fibrinogen. Nevertheless, the final clot size measured after 20 minutes was the same size as a clot formed with normal recombinant fibrinogen. Similar results were observed using platelets isolated from an afibrinogenemic patient, eliminating the possibility that the retraction was dependent on secretion of plasma fibrinogen from platelet α-granules. These findings indicate that clot retraction is a two-step process, such that one or more of the three putative platelet binding sites are important for an initial step in clot retraction, but not for a subsequent step. With the triple mutant fibrinogen, the second step of clot retraction, possibly the development of clot tension, proceeds with a rate similar to that observed with normal recombinant fibrinogen. These results are consistent with a mechanism where a novel site on fibrin is involved in the second step of clot retraction.
CLOT RETRACTION IS believed to be a primary step in the clearance of a thrombus, which initiates wound healing. The process of clot retraction is modeled in vitro using three blood components: fibrinogen, platelets, and thrombin. Fibrinogen is a large (340 kD) glycoprotein composed of six polypeptide chains: two each of Aα, Bβ, and γ. Platelets are small, anuclear cells that contain a variety of inflammatory agents and are responsible for formation of an initial hemostatic plug at sites of injury. Thrombin, a serine protease, converts soluble fibrinogen to fibrin monomers that spontaneously polymerize in an ordered fashion to form fibrin fibers. These fibers serve as the lattice of a clot and function as the ligand for platelets during clot retraction. Thrombin, in addition, is a platelet agonist that activates platelets by cleaving a specific receptor.
Platelets express a specific receptor for fibrinogen and fibrin on their surface in a high-copy number, approximately 45,000 copies per cell.1 This receptor binds ligand after platelet activation by an agonist. The receptor is a member of the integrin superfamily of receptors and is composed of an α and a β subunit, specifically αIIbβ3.2αIIbβ3 is a receptor found on platelets and their parental cells, megakaryocytes. Previous experiments using antibodies directed against either αIIb, β3, or the receptor complex have shown that this receptor is required for platelet adhesion, platelet aggregation, and platelet-mediated clot retraction.3-5 These results were confirmed using platelets isolated from patients diagnosed with Glanzmann's thrombasthenia, a condition in which functional αIIbβ3 is not expressed on the surface of platelets. These platelets do not adhere to immobilized fibrinogen, do not aggregate in the presence of soluble fibrinogen and agonist, and do not support clot retraction.6-8
Three sites on fibrinogen have been hypothesized to bind to αIIbβ3 during interactions with platelets: two Arg-Gly-Asp (RGD) sites and the C-terminal portion of the γ chain. RGD is a consensus binding sequence for integrins and was identified using synthetic peptides9; the two RGD sequences in fibrinogen are both located in the Aα chain at residues 95-97 and 572-574.10 The third site is specific for fibrinogen and is composed of the 12 C-terminal residues of the γ chain (H12: HHLGGAKQAGDV).11,12 The role of each of these sites in the interactions of fibrinogen with platelets has been probed in a variety of ways. Initially, these sites were identified because peptides containing the RGD or H12 sequence inhibit fibrinogen binding to platelets, a prerequisite for aggregation.12,13 Subsequent experiments using antibodies directed against either H12 or the Aα RGD at position 95-97 implicated both sites as players in fibrinogen binding to αIIbβ3 and in platelet aggregation.14 In addition, experiments using an RGDS-containing peptide, the sequence corresponding to the Aα 572-575 putative binding site, suggested that this C-terminal site in the Aα chain interacts with the fibrinogen receptor on activated platelets.15 Direct observation of αIIbβ3-fibrinogen complexes by electron microscopy supported the conclusion that the C-terminal γ chain domain is the primary interaction site.16 Genetically engineered fibrinogen variants have since shown that the Aα chain RGD sequences are not required for binding of αIIbβ3 to soluble fibrinogen or for platelet aggregation. Instead, the γ chain dodecapeptide, specifically residues Ala-Gly-Asp-Val (AGDV), seems to be the important sequence on fibrinogen for αIIbβ3 binding and platelet aggregation.17-19
In contrast, the γ chain binding site is not required for clot retraction in either human or mouse systems.19 20Therefore, it has been suggested that the RGD sites in the Aα chain mediate clot retraction. We tested this hypothesis using three variant fibrinogens, two with substitutions of RGE for RGD at either Aα 95-97 or Aα 572-574, and a third variant with both RGD sites changed to RGE and the AGDV residues of the γ chain H12 sequence deleted. Our results indicate that loss of all three hypothesized platelet binding sites on fibrinogen only influence the initial phase of clot retraction and are consistent with the presence of an additional, unknown site on fibrin that is involved in clot retraction.
MATERIALS AND METHODS
Materials.
The Chinese hamster ovary (CHO) cells; culture media; and vectors pMLP-Aα, pMLP-Bβ, and pMLP-γ; encoding for the three fibrinogen cDNAs; have been described.21 Restriction enzymes were purchased from New England Biolabs (Beverly, MA). Monoclonal antibody IF-1 was kindly provided by Dr Michio Matsuda (Institute of Hematology, Jichi Medical School, Tochigi-Ken, Japan). Human α-thrombin was a generous gift of Dr Frank Church (University of North Carolina at Chapel Hill).
Construction of mutant expression vectors.
A mammalian vector encoding for the deletion of residues AGDV from the C-terminus of the γ chain (pMLP-γ 407) has been described.22 Likewise, baby hamster kidney (BHK) cells expressing recombinant fibrinogen with RGE substituted for RGD at either position Aα 95-97 or Aα 572-574 were previously constructed and cells grown as described.17 The proteins expressed by these cells are referred to as Aα D97E and Aα D574E, respectively, and were purified as outlined below.
A plasmid encoding for RGD to RGE mutations at both sites in the Aα chain (pMLP-Aα DE2) was constructed using plasmid pMLP-Aα and the Clontech Transformer Site Directed Mutagenesis kit (Palo Alto, CA), as described below. The mutagenic primers have the following sequences: Aα D97E-TTG AGA GGA# GAG* TTT TCC TCA GC and Aα D574E-AGA GGA GAG* TCA° ACG° TTT GAA AGC, where * indicates a base substitution resulting in a D to E mutation,# indicates the silent introduction of a BseRI site, and ° marks the base changes required to introduce a silentPsp1406I site. The selection primer has the sequence TCT AGG GCC CAG GCT TGT TTC C, and encodes for the deletion of a uniqueHindIII site located in the vector. The parent plasmid was heated to 100°C in the presence of 5′-phosphorylated selection and mutagenic oligonucleotides and allowed to anneal on ice. T4 DNA polymerase and T4 DNA ligase were added and the mutant DNA strand was synthesized at 37°C. The parental plasmid DNA was linearized withHindIII and the plasmid mixture used to transform BMH 71-18mut S bacteria (Clontech) by electroporation using a Gene Pulser (Bio-rad, Hercules, CA). Bacteria were grown in culture overnight and the plasmid pool was isolated using a Qiagen Plasmid Kit (Chatsworth, CA). Purified plasmid pools were linearized with HindIII and the products were used to transform DH5αF′ bacteria by electroporation using a Gene Pulser (Bio-rad). The bacteria were selected by growth on ampicillin-containing media and plasmids were isolated from resistant colonies using the Qiagen Plasmid Kit. The introduction of both RGD to RGE mutations was confirmed by digestion with BseRI and Psp1406I followed by sequencing the entire fibrinogen Aα chain cDNA using an Applied Biosystems automated sequencer (Foster City, CA). The positively identified plasmid is referred to as pMLP-Aα DE2.
Synthesis of recombinant fibrinogens.
The preparation of a CHO line expressing recombinant fibrinogen with the desired alterations was performed essentially as described.21 Briefly, plasmids pMLP-Aα DE2, pMLP-γ 407, and pMSVhis were used to transfect CHO cells containing pMLP-Bβ and pRSVneo. Colonies that grew in the presence of histidinol (Sigma Chemical Co, St Louis, MO) and G418 (Life Technologies, Inc, Grand Island, NY) were selected and screened for fibrinogen expression by enzyme-linked immunoassay (ELISA). The clone with the highest expression level was used to seed roller bottles. Each week all 200 mL of serum-free media from roller bottles containing CHO cells was collected and pooled, and phenylmethylsulfonyl fluoride was added to 150 μmol/L, and stored at −70°C until the fibrinogen was purified. Fresh media were added to the cultures and fibrinogen production continued for 3 months. The fibrinogen purified from the media obtained from these clones is designated Aα DE2/γ 407. Normal recombinant fibrinogen was grown in roller bottles and media collected as described.20
Purification and characterization of recombinant fibrinogens.
All of the recombinant fibrinogens were purified from pooled serum-free media collected from CHO or BHK cells as described.23Briefly, fibrinogen was precipitated from the media by adding ammonium sulfate to 40% final concentration. The precipitate was collected by centrifugation at 16,000g at 4°C and resuspended in Tris-buffered saline, pH 7.4 (TBS; 20 mmol/L Tris-HCl and 150 mmol/L NaCl) with 1 mmol/L CaCl2. The resuspended precipitate was clarified by centrifuging at 27,000g before loading onto an immunoaffinity column equilibrated in the same buffer. The column was washed consecutively with high salt (1 mol/L NaCl) and low pH (6.0) buffers followed by TBS with 10 mmol/L EDTA. The antibody (IF-1) is calcium dependent, binding fibrinogen in the presence of calcium and failing to bind fibrinogen in the absence of calcium.24 Therefore, fibrinogen was eluted with EDTA-containing buffer. The protein was then dialyzed for 3 hours at 4°C against 1 L TBS with 1 mmol/L added calcium followed by extensive dialysis against TBS without added calcium. The dialyzed fibrinogen was aliquoted and stored at −70°C until it was used. Except for the dialysis steps, every step of the purification procedure was performed in the presence of a cocktail of protease inhibitors, as previously described.23 The purity of the recombinant fibrinogens was monitored using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), according to the method of Laemmli.25
Preparation of platelets.
Platelets were prepared from a normal donor's blood as described.19 Platelets prepared from a patient diagnosed with afibrinogenemia were purified with the following modifications, as described.26 Human blood (60 mL) from the patient, who had abstained from aspirin, was collected in tubes containing one-tenth volume of 3.4% acid/citrate/dextrose (ACD) anticoagulant. Platelet-rich plasma was obtained by centrifugation at 150g for 10 minutes at room temperature. ACD was added to 10% final concentration before applying the platelet-rich plasma to a Sepharose CL-2B column (Sigma) equilibrated with Tyrodes buffer, pH 7.2 (10 mmol/L Hepes, 135 mmol/L NaCl, 2.7 mmol/L KCl, 12 mmol/L NaHCO3, 5.5 mmol/L glucose, 2% bovine serum albumin [Fr V, pH 7.0; Bayer Corp, Kankakee, IL]). The platelets were eluted with Tyrodes and ACD added to 10% final concentration before centrifuging the platelet suspension at 700g for 10 minutes at ambient temperature. The platelet pellet was resuspended in Tyrodes buffer, counted with a Cell-Dyn 1600 (Abbott Diagnostics, Abbott Park, IL) and adjusted to a final concentration of 4 × 108 platelets/mL with 1 mmol/L calcium and 2 mmol/L magnesium.
Platelet aggregation.
Aggregation experiments with gel-filtered platelets were performed as described.19 Fibrinogen was added to platelets to give final concentrations of 250 nmol/L and 2 × 108platelets/mL, respectively. The sample was placed in an aggregometer (Chrono-Log Corp, Haverton, PA) with stirring at 37°C, using Tyrodes buffer as the reference solution. Adenosine diphosphate (ADP; Chrono-Log Corp) was added to a final concentration of 10 μmol/L and the change in light transmission was recorded versus time.
Clot retraction.
Platelet-mediated clot retraction experiments were performed similarly to Tuszynski et al27 and as described.19 Briefly, fibrinogen (final concentration 300 nmol/L) was preincubated with platelets (final concentration 2 × 108 platelets/mL) at 37°C for 10 minutes before adding human α-thrombin to a final concentration of 0.5 U/mL. The tubes were inverted several times and allowed to incubate at 37°C for 20 minutes. During this period the length and width of the clots were measured with a ruler and were used to calculate clot area. For the experiment involving platelets isolated from an afibrinogenemic patient, clot area was calculated by measuring the length and width of computer-generated images of the clot at 2-minute intervals. The clot areas were then used to calculate percent of retraction using the following equation:
After the appropriate incubation time, the tubes were placed on ice until a photograph was taken.
RESULTS
Production and characterization of recombinant fibrinogens.
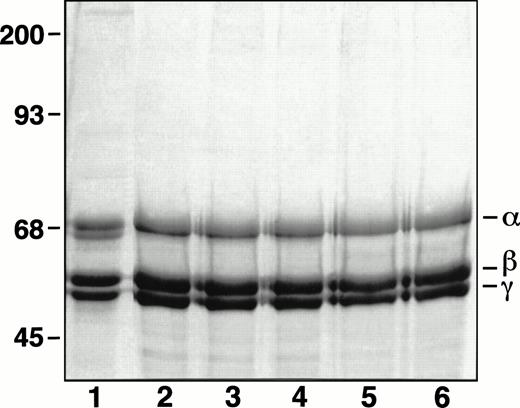
Oligonucleotide-directed mutagenesis was used to introduce RGD to RGE mutations at both positions 95-97 and 572-574 in the fibrinogen Aα chain. The mutations made in the expression vector pMLP-Aα were confirmed by restriction digests with BseRI andPsp1406I and by sequence analysis of the complete cDNA (data not shown). The sequence data ensured that there were no unanticipated codon changes. The altered expression plasmid was called pMLP-Aα DE2. After cotransformation of pMLP-Aα DE2and pMLP-γ 407 into CHO cells containing pMLP-Bβ, the media were screened for fibrinogen by ELISA. Of 17 colonies tested, 4 were positive. The clone with the highest expression level (0.6 μg/mL) was expanded and grown in roller bottles for 3 months, essentially as previously described.21 Likewise, two previously described BHK clones expressing fibrinogen with either Aα D97E or Aα D574E mutations were used to synthesize these two variant proteins.17 The different variant fibrinogens were all purified as described.23 When analyzed by SDS-PAGE under reduced conditions, the different fibrinogen variants all showed three major bands with molecular weights corresponding to the α, β, and γ chains (Fig 1).
SDS-PAGE analysis of purified recombinant fibrinogens. Recombinant fibrinogens in Laemmli25 sample buffer with SDS were separated on an 8% gel run under reduced conditions and stained with Coomassie Brilliant Blue R-250. Lanes: 1, plasma fibrinogen; 2, normal recombinant fibrinogen; 3, recombinant Aα D97E fibrinogen; 4, recombinant Aα D574E fibrinogen; 5, recombinant Aα DE2/γ 407 fibrinogen; 6, recombinant γ 407 fibrinogen.
SDS-PAGE analysis of purified recombinant fibrinogens. Recombinant fibrinogens in Laemmli25 sample buffer with SDS were separated on an 8% gel run under reduced conditions and stained with Coomassie Brilliant Blue R-250. Lanes: 1, plasma fibrinogen; 2, normal recombinant fibrinogen; 3, recombinant Aα D97E fibrinogen; 4, recombinant Aα D574E fibrinogen; 5, recombinant Aα DE2/γ 407 fibrinogen; 6, recombinant γ 407 fibrinogen.
Aggregation of ADP-stimulated platelets.
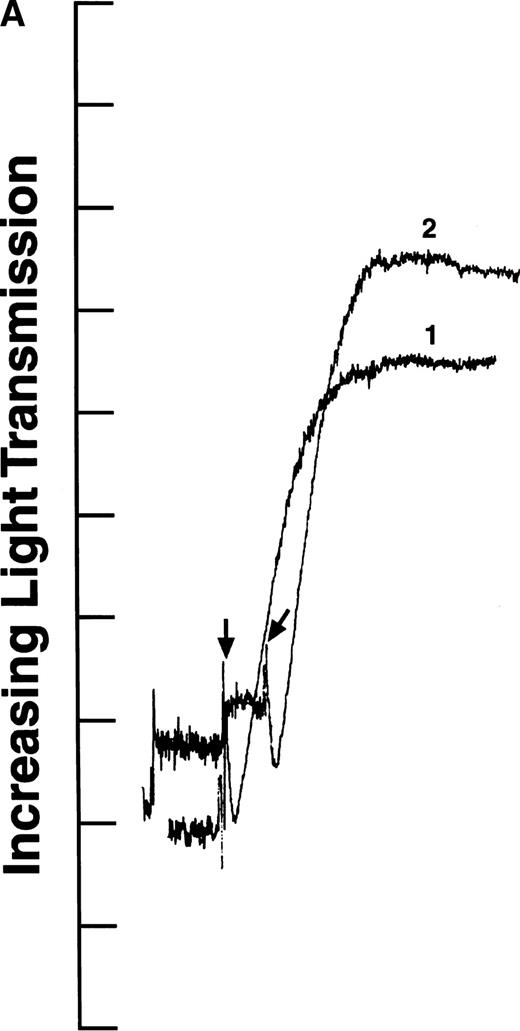
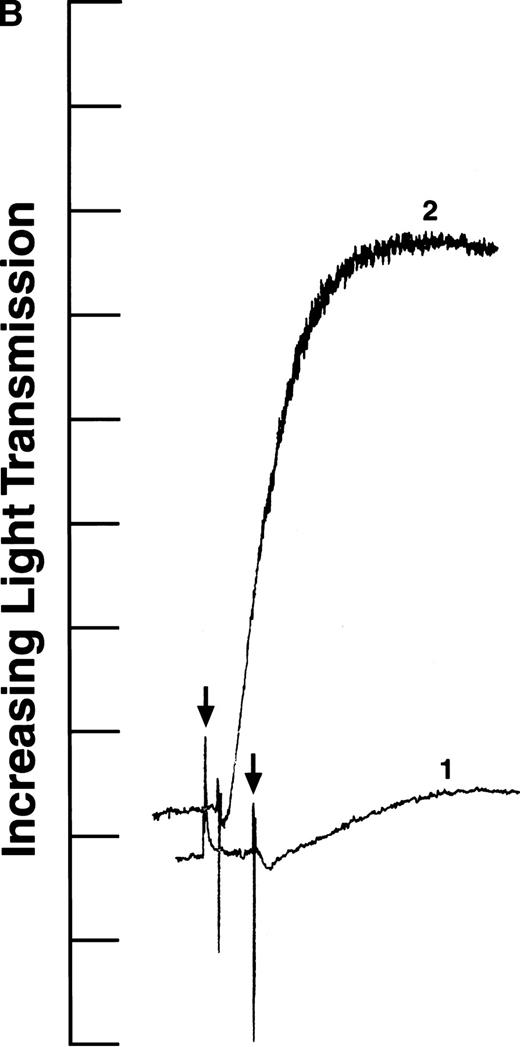
We tested the variants in aggregation experiments using ADP-stimulated platelets. Platelet aggregation was measured by monitoring the change in light transmission versus time.28 As shown in Fig2, all four aggregation curves had an initial decrease in light transmission after ADP addition (indicated by arrows), as expected for agonist-induced platelet activation.29 In addition, the aggregation curves generated with recombinant fibrinogens containing intact γ chains (normal recombinant fibrinogen: Fig 2A, curve 1; Aα D97E fibrinogen: Fig 2A, curve 2; and Aα D574E fibrinogen: Fig 2B, curve 2) showed a rapid increase in light transmission characteristic of platelet aggregation. In contrast, in the presence of fibrinogen Aα DE2/γ 407 no change in light transmission was observed (Fig 2B, curve 1). This indicates that the platelets did not aggregate in the presence of fibrinogen Aα DE2/γ 407, which is consistent with previous results showing that residues AGDV of the γ chain are required for platelet aggregation.19
ADP-induced platelet aggregation. Platelets (2 × 108 mL−1 final concentration) were preincubated at 37°C with 250 nmol/L final concentration of the indicated fibrinogen before adding ADP to 10 μmol/L final concentration (indicated by arrows). The increase in light transmission is plotted versus time. Shown are representative curves, with each experiment performed at least four times. (A) Curve 1, normal recombinant fibrinogen; curve 2, recombinant Aα D97E fibrinogen. (B) Curve 1, recombinant Aα DE2/γ 407 fibrinogen; curve 2, recombinant Aα D574E fibrinogen.
ADP-induced platelet aggregation. Platelets (2 × 108 mL−1 final concentration) were preincubated at 37°C with 250 nmol/L final concentration of the indicated fibrinogen before adding ADP to 10 μmol/L final concentration (indicated by arrows). The increase in light transmission is plotted versus time. Shown are representative curves, with each experiment performed at least four times. (A) Curve 1, normal recombinant fibrinogen; curve 2, recombinant Aα D97E fibrinogen. (B) Curve 1, recombinant Aα DE2/γ 407 fibrinogen; curve 2, recombinant Aα D574E fibrinogen.
Platelet-mediated clot retraction.
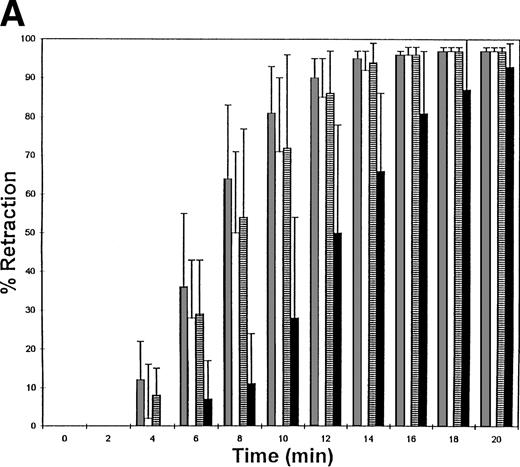
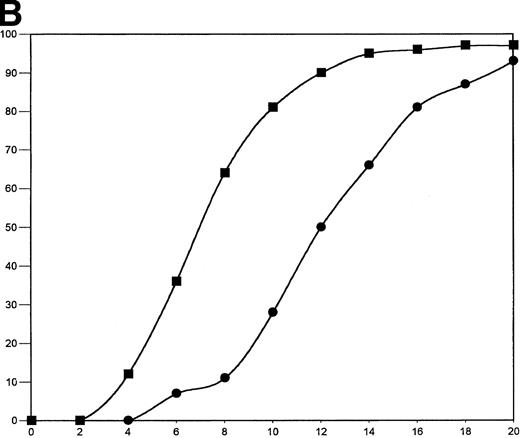
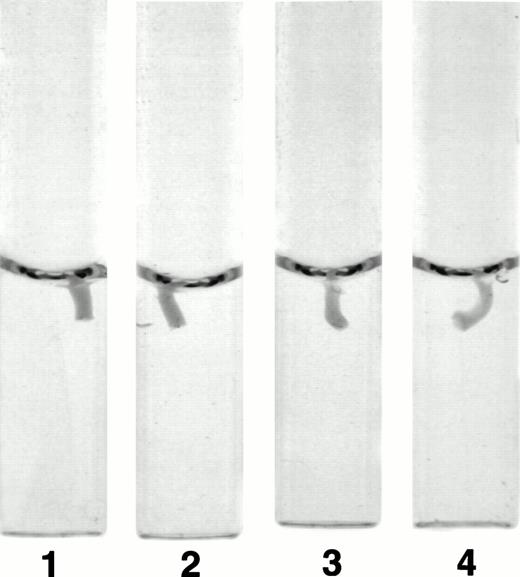
The fibrinogen variants were tested for their ability to retract a clot formed by adding thrombin to platelets in the presence of fibrinogen. Figure 3A shows the average (n = 4) change in clot area with time for the normal recombinant and three variant fibrinogens. Both fibrinogen Aα D97E and fibrinogen Aα D574E showed a slightly lower retraction rate relative to normal recombinant fibrinogen; however, this difference was within the large systematic error inherent in this semiquantitative method of calculating clot area and therefore was not statistically significant. On the other hand, Aα DE2/γ 407 fibrinogen showed a marked delay in retraction. After the delay, the retraction rate of Aα DE2/γ407 fibrinogen seemed similar to normal recombinant fibrinogen. When the data were displayed as shown in Fig 3B the rate of Aα DE2/γ407 fibrinogen retraction approximated the rate of retraction of normal recombinant fibrinogen, as indicated by the similarities in the slopes of the curves. The final size of all four clots was indistinguishable as shown in the representative photograph taken after a 20-minute incubation at 37°C (Fig 4). Two control experiments were performed to ensure that clot retraction was dependent on both platelets and exogenously added fibrinogen. Fibrinogen, in the absence of platelets, formed a clot that failed to retract, whereas platelets treated with thrombin in the absence of added fibrinogen failed to form a clot (data not shown).
Clot retraction kinetics. Platelets (2 × 108 mL−1 final concentration) were preincubated for 10 minutes at 37°C with 300 nmol/L final concentration of the indicated fibrinogen before adding human α-thrombin to 0.5 U/mL final concentration. The length and width of the clot were measured with a ruler every 2 minutes and used to calculate clot area, which was then used to calculate % retraction. (A) Average % retraction, expressed as mean ± standard error from four experiments, is plotted versus time as a bar graph for the various recombinant fibrinogens, which are indicated in the legend. (▧), Normal recombinant; (□), Aα D97E fibrinogen; (▤), Aα D574E fibrinogen; (▪), Aα DE2/γ 407 fibrinogen. (B) Curves generated by plotting average % retraction versus time for normal recombinant and Aα DE2/γ407. (▪), Normal recombinant; (•), Aα DE2/γ 407 fibrinogen.
Clot retraction kinetics. Platelets (2 × 108 mL−1 final concentration) were preincubated for 10 minutes at 37°C with 300 nmol/L final concentration of the indicated fibrinogen before adding human α-thrombin to 0.5 U/mL final concentration. The length and width of the clot were measured with a ruler every 2 minutes and used to calculate clot area, which was then used to calculate % retraction. (A) Average % retraction, expressed as mean ± standard error from four experiments, is plotted versus time as a bar graph for the various recombinant fibrinogens, which are indicated in the legend. (▧), Normal recombinant; (□), Aα D97E fibrinogen; (▤), Aα D574E fibrinogen; (▪), Aα DE2/γ 407 fibrinogen. (B) Curves generated by plotting average % retraction versus time for normal recombinant and Aα DE2/γ407. (▪), Normal recombinant; (•), Aα DE2/γ 407 fibrinogen.
Photograph of clots after 20 minutes. Clot retraction experiments were performed as described in Fig 3A and a photograph of the tubes containing the clots was taken after 20 minutes. The photograph is representative of the results observed in all four experiments performed. Tubes: 1, normal recombinant fibrinogen; 2, recombinant Aα D97E fibrinogen; 3, recombinant Aα D574E fibrinogen; 4, recombinant Aα DE2/γ 407 fibrinogen.
Photograph of clots after 20 minutes. Clot retraction experiments were performed as described in Fig 3A and a photograph of the tubes containing the clots was taken after 20 minutes. The photograph is representative of the results observed in all four experiments performed. Tubes: 1, normal recombinant fibrinogen; 2, recombinant Aα D97E fibrinogen; 3, recombinant Aα D574E fibrinogen; 4, recombinant Aα DE2/γ 407 fibrinogen.
To exclude the possible involvement of fibrinogen from platelet α-granules, clot retraction experiments were performed in an identical manner with platelets isolated from an afibrinogenemic patient. These platelets contain minute levels of fibrinogen in their α-granules (10 μg/109 platelets) as determined by ELISA with a peroxidase-conjugated rabbit anti-human fibrinogen antibody.30 Platelets isolated from an afibrinogenemic patient were able to retract clots formed with both normal recombinant and Aα DE2/γ 407 fibrinogen. As with experiments using platelets isolated from normal donors, clots formed with added Aα DE2/γ 407 fibrinogen showed delayed retraction when compared with clots formed with normal recombinant fibrinogen (Fig5). In addition, the retraction of clots formed with normal recombinant fibrinogen and platelets from the afibrinogenemic patients was delayed relative to retraction using platelets from normal donors (compare Figs 3 and 5). The retraction rates were different even though the ADP-induced aggregations were comparable (data not shown).
Clot retraction kinetics using platelets from an afibrinogenemic patient. Platelets (1.6 × 108mL−1 final concentration) were preincubated for 10 minutes at 37°C with 300 nmol/L final concentration of the indicated fibrinogen before adding human α-thrombin to 0.5 U/mL final concentration. The retraction was filmed on videotape, which was used to generate computer images of the clots at 2-minute intervals. The length and width of the computer images were measured with a ruler and used to calculate clot area, which was then used to calculate % retraction. Percent retraction is plotted versus time for the various recombinant fibrinogens, which are indicated in the legend (N = 1). (□), Normal recombinant; (▪), Aα DE2/γ407 fibrinogen.
Clot retraction kinetics using platelets from an afibrinogenemic patient. Platelets (1.6 × 108mL−1 final concentration) were preincubated for 10 minutes at 37°C with 300 nmol/L final concentration of the indicated fibrinogen before adding human α-thrombin to 0.5 U/mL final concentration. The retraction was filmed on videotape, which was used to generate computer images of the clots at 2-minute intervals. The length and width of the computer images were measured with a ruler and used to calculate clot area, which was then used to calculate % retraction. Percent retraction is plotted versus time for the various recombinant fibrinogens, which are indicated in the legend (N = 1). (□), Normal recombinant; (▪), Aα DE2/γ407 fibrinogen.
DISCUSSION
The use of recombinant technology has contributed to our understanding of the role of the two RGD sequences in both platelet adhesion and aggregation. Farrell et al17,18 found that neither RGD site is required for platelet aggregation or adhesion of stimulated or unstimulated platelets. Furthermore, recombinant fibrinogens with alterations to the H12 sequence were found to have a reduced ability to support platelet aggregation and adhesion when compared with normal recombinant fibrinogen.17-19 Similarly, recombinant technology has clarified the role of the three putative binding sites on fibrinogen during interactions with endothelial cells. The importance of the C-terminal RGD sequence in the Aα chain of fibrinogen in endothelial adhesion by integrin αvβ3 was identified by replacement of Asp with Glu at residue 574; this mutation substantially reduced endothelial cell adhesion to fibrinogen via integrin αvβ3.31 In addition, γ407 fibrinogen, with deletion of AGDV from the γ chain, and proteolytic fragments of plasma fibrinogen were used in a study of endothelial cell-mediated clot retraction. These experiments showed that neither the RGD at residues 572-574 of the Aα chain nor the AGDV residues of the γ chain were required for endothelial cell αvβ3-mediated clot retraction.32 However, the role of these sites in platelet-mediated clot retraction was not addressed.
This communication reports on the results of a study of platelet-mediated clot retraction using similar recombinant technology. As previously shown, both Aα D97E fibrinogen and Aα D574E fibrinogen could support platelet aggregation to the same extent as normal recombinant fibrinogen.17 Moreover, the results of this study indicate that both the rate of clot retraction and the final size of the clots were indistinguishable when using normal recombinant fibrinogen, Aα D97E fibrinogen, or Aα D574E fibrinogen (Figs 3 and4). Combining these results with previous data indicating that the γ chain AGDV residues are not required for clot retraction19shows that no one of the three proposed αIIbβ3 binding sites on fibrin is critical for platelet-mediated clot retraction.
It remained possible that each of the platelet binding sites could support clot retraction independently of one another such that elimination of a single site would show no adverse effects, as one or both of the other two sites would compensate for the loss. To test this possibility we constructed a third variant fibrinogen, called Aα DE2/γ 407, where both RGD sequences in the Aα chain were changed to RGE and the AGDV residues of the γ chain were deleted. Although this variant did not support platelet aggregation (Fig 2), it did support clot retraction. However, the initiation of retraction was delayed when compared with normal recombinant fibrinogen (Fig 3A). This result suggests that clot retraction occurs in two distinct phases. The first phase would involve one or more of the putative fibrinogen sites, whereas the second phase would be independent from these sites. Several mechanisms are consistent with such a two-step process. We favor a model where in the first phase platelets are recruited to the clot, either by interactions between the platelets and fibrinogen or between the platelets and polymerizing fibrin. After recruitment of the platelets to the clot, the second phase of clot retraction involves the transmission of the contractile force from the platelets to the fibrin strands. The result of this second phase is the collapse of the fibrin clot. This two-step mechanism would predict that platelets cannot interact with fully polymerized fibrin, as has been observed.33 34
In our experiments with Aα DE2/γ407 fibrinogen, loss of all three sites affected the initial phase of clot retraction, suggesting that one or more of the three putative binding sites on fibrinogen or polymerizing fibrin play a role in the initial recruitment of platelets to the clot. In contrast to Aα DE2/γ407 fibrinogen, retraction with three fibrinogens that lack only one of the binding sites was indistinguishable from normal recombinant fibrinogen (Fig 3A).19 This suggests that the loss of one site can be compensated by either one or both of the other two sites. Once a sufficient number of platelets are incorporated into the clot formed with the Aα DE2/γ407 fibrinogen, retraction proceeded to completion at a normal rate, as shown by the similarities in the retraction rate curves for Aα DE2/γ407 and normal recombinant fibrinogen (Fig 3B). This finding indicates that the loss of the three hypothesized binding sites has no bearing on the force generation phase of retraction. Therefore, the second phase of clot retraction seems to involve a novel site on fibrin. This introduces the possibility that distinct platelet receptors are involved in the two phases of clot retraction. This possibility is consistent with the finding of Cohen et al35that the receptor necessary for clot tension may be another glycoprotein and/or domain of αIIbβ3 not involved in fibrinogen binding. Perhaps αIIbβ3 mediates the interactions between platelets and fibrinogen or polymerizing fibrin during the recruitment phase and a second, unidentified receptor and/or domain of αIIbβ3 mediates the contractile phase.
Alternatively, platelet α-granule fibrinogen, which contains all three binding sites, may be required for platelet adhesion and subsequent clot retraction using Aα DE2/γ 407 fibrinogen. The observed delay would then arise from the time required for α-granule release and the incorporation of the platelet fibrinogen into the fibrin fibers. This possibility prompted us to examine clot retraction using platelets isolated from an afibrinogenemic patient. In this experiment, the delay in retraction of clots formed with Aα DE2/γ 407 compared with normal recombinant fibrinogen was similar to that observed using platelets from donors with normal fibrinogen levels, even though the afibrinogenemic platelets have α-granule fibrinogen levels of less than 10 μg/109 platelets.30 This is in contrast to the normal fibrinogen levels in platelet α-granules, reported to be between 63 to 140 μg/109platelets.36-38 In the experiments described here, the exogenous fibrinogen concentration (51 μg) is approximately 63 times greater than the fibrinogen released by the afibrinogenemic platelets (0.8 μg from 500 μL of 1.6 × 108 platelets/mL). Therefore, these results suggest that either α-granule fibrinogen is not required for retraction of clots formed from Aα DE2/γ407 fibrinogen or minute quantities are sufficient. These results do not eliminate the possibility that other α-granule proteins, most notably von Willebrand factor, vitronectin, fibronectin, and thrombospondin, may bind to fibrin and participate in clot retraction when fibrinogen lacking all three binding sites is used to form the clot.
We observed a difference in the retraction rates of clots formed with platelets from normal donors versus platelets from the afibrinogenemic patient using both normal recombinant fibrinogen and Aα DE2/γ 407 fibrinogen. The difference may result from a variety of factors, including the normal donor-to-donor variation in platelet responsiveness and the differences in the platelet purification procedures for the two experiments. In addition, whereas the normal platelet data is the average from four experiments, the afibrinogenemic experiment was performed only once because of the limited availability of these platelets. Because of these differences, our data are not sufficient to establish whether platelet α-granule fibrinogen participates in clot retraction. However, we can conclude that platelet fibrinogen does not have a critical role in retraction of clots formed with Aα DE2/γ407, as the relative delay of Aα DE2/γ407 fibrinogen versus normal recombinant fibrinogen with afibrinogenemic platelets is similar to the relative delay with normal platelets (Figs 3 and 5).
Recombinant technology has allowed us to eliminate a critical role in clot retraction for any one of the hypothesized platelet binding sites on fibrin. However, our results do indicate that the three putative binding sites on fibrinogen are involved in the initial recruitment of platelets to the fibrin clot. Because Aα DE2/γ407 fibrinogen does support clot retraction, we conclude that at least one of the RGE sites and/or an unidentified site on fibrin interacts with platelets during the second, clot tension phase of retraction. Likely candidates for the novel fibrin site include other regions on fibrinogen found to impair platelet aggregation, another αIIbβ3-driven process. These include two regions on the γ chain identified in the dysfibrinogens Vlissingen and Frankfurt VII, which have a deletion of γ 319-320 and a substitution of Thr for Met at γ 310, respectively. These two variants from heterozygous patients supported platelet aggregation less than 50% of that observed with normal fibrinogen, indicating these residues can directly or indirectly influence binding to αIIbβ3.39 Moreover, as stated previously, the second phase may involve a second receptor to mediate the development of clot tension. Therefore, the presumptive novel fibrin site may be one of several sites on fibrinogen or fibrin that interact with other integrin and nonintegrin receptors. These include γ 190-201, which was found to interact with MAC-1 on leukocytes, γ 117-133, which is found to inhibit binding of fibrinogen to endothelial cells or B lymphoblastoid Daudi cells expressing intracellular adhesion molecule 1 (ICAM-I), and β 15-42, which is hypothesized to interact with a 130-kD glycoprotein during endothelial cell spreading on fibrin.40-43 Finally, the possibility exists that the site on fibrin required for clot retraction is buried in fibrinogen and only becomes available after conformational changes as a result of the thrombin-catalyzed transition from fibrinogen to fibrin. For example, neoantigenic sequences on fibrin, such as α 148-160, may only be available for interaction with αIIbβ3 after conversion of fibrinogen to fibrin or after subsequent polymerization of fibrin.44 This and other fibrin-exposed sequences are leading candidates for a novel binding site important in clot retraction.
ACKNOWLEDGMENT
We thank Li Fang Ping for her tissue culture work and Dr Leslie V. Parise for critical review of this manuscript.
Supported by the National Institutes of Health Grants No. HL 31048 and HL 45100 (S.T.L.) and a predoctoral fellowship from the American Heart Association, NC Affiliate (M.M.R.).
Address reprint requests to Susan T. Lord, PhD, University of North Carolina, Department of Pathology and Laboratory Medicine, CB# 7525, 605 Brinkhous-Bullitt Bldg, Chapel Hill, NC 27599.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal