Abstract
Childhood lymphoblastic leukemia (ALL) is usually assumed to have been permanently eradicated in patients in long-term remission, but occasionally can recur after many years. To learn more about the problem, we studied a group of children whose leukemia had been in remission for 10 or more years before relapse and tried to determine whether they had true recurrences or second malignancies. We studied children treated on Medical Research Council ALL protocols between 1970 and 1984 and followed up by the Clinical Trial Service Unit in Oxford. Detailed clinical and laboratory data was collected from the centers concerned on all who were reported to have had a recurrence of their leukemia after 10 or more years from the time of achieving first complete remission (CR1). To prove that the relapse was a true recurrence rather than a second or secondary leukemia, DNA extracted from archived marrow smears was subjected to polymerase chain reaction (PCR) analysis for the presence of an identical Ig heavy chain (IgH) or T-cell receptor (TCR) gene rearrangement at initial diagnosis and subsequent relapse. A total of 1,134 of 2,746 children had survived 10 years or more (range, 10 to 24 years) in CR1 and of those, 12 (approximately 1%) had subsequently relapsed. Relapse blast cells were shown to express the common ALL antigen (CD 10) in all cases and an identical clonal IgH or TCR gene rearrangement was found on PCR analysis of DNA from diagnosis and relapse in all eight cases where DNA extraction was successful. A further program of therapy was successful in inducing a second CR in all patients, four of whom have succumbed to a second relapse after 12 to 27 months. The remaining eight are in continuing CR2 at a follow-up of 12 to 108 months (median, 52) from relapse. Although the risk of relapse of childhood ALL after 10 years in remission appears to be small (around 1%), it persists. This raises questions about how blasts can survive quiescent for so long and when we can truly be confident of cure, if ever.
EVEN AFTER TREATMENT with the best current therapy, approximately one in four children with lymphoblastic leukemia (ALL) relapse.1 The risk of relapse is mainly during the period immediately after stopping treatment and diminishes with time so that patients in complete remission (CR) for 10 years are considered to be effectively cured of their disease.2Relapse after that time period has been reported,3 but until a few years ago, it was difficult to be certain whether these patients had a true recurrence of the original clone or a second or secondary leukemia.
Confirmation of a true relapse can now be obtained by documenting the presence of an identical clone-specific molecular signature in lymphoblasts at diagnosis and recurrence. Immunoglobulin (IgH) and T-cell receptor (TCR) gene loci consist of dispersed gene segments, which undergo somatic recombination early in B- and T-cell ontogeny to produce functional Ig or TCR molecules. Clonal IgH or TCR rearrangements are detectable in almost every case of ALL. Each rearranged gene is unique and therefore provides a good clonal marker to monitor minimal residual disease in patients in apparent remission.
Such molecular techniques have been used by several investigators, including ourselves, to confirm relapse in cases of late recurrence.4-6 These cases not only raise fundamental questions about the biology of childhood ALL, they also generate anxiety about the resilience of cure in patients whose leukemia has been in long-term remission. To answer the latter question in particular, we studied long-term follow-up data on a large cohort of children treated on the MRC national ALL trials. We tried to estimate the frequency of very late relapse, what type of leukemia gives rise to it, and what type of patient is at risk.
MATERIALS AND METHODS
Patient Population and Data Collection
Since the late 1960s, an increasing proportion, now over 90%, of children diagnosed with ALL in the United Kingdom have been treated on MRC childhood leukemia studies. Long-term follow-up of these patients is undertaken by individual treatment centers and reported every 2 years to the Clinical Trial Service Unit (CTSU) in Oxford. Data are available for nearly all patients treated between 1970 and 1984 with less than 1% lost to follow-up at 10 years. Median follow-up of surviving patients is 18 years. Information on the number of patients surviving for 10 years in first remission and those who subsequently relapsed was extracted from the CTSU database. Where detailed clinical treatment or outcome information on patients with late relapse was unavailable in the database, individual centers were contacted to obtain this information. The actuarial risk of relapse after 10 years in first complete remission (CR1) was calculated using Kaplan-Meier life table analysis.
Molecular Analysis
Central morphology review and archiving of marrow smears is undertaken on all cases entered into MRC childhood leukemia studies. Diagnosis and relapse marrow smears were retrieved from the central archive or were kindly provided by individual centers from their local archives where these were unavailable in the central archives.
DNA was extracted from marrow smears archived at diagnosis and relapse using a previously described technique.7 Briefly, material was scraped from the slide using a sterile blade and DNA was extracted by proteinase K digestion, phenol/chlorofom extraction, and ethanol precipitation. Polymerase chain reaction (PCR) amplification of the IgH gene (Frameworks 1, 2, and 3) or TCR gene (Vδ2-Dδ3) were performed on 1 μg of patient DNA. The three IgH PCR reactions used a 3′ consensus primer to the joining region of the IgH gene and a consensus 5′ primer to the appropriate framework region of the variable gene.8,9 The TCR δ region PCR used a 5′ Vδ2 primer and a 3′ Dδ3 primer.10 PCR products were visualized using polyacrylamide gel electrophoresis and ethidium bromide staining. Clonal products were sequenced with forward and reverse primers using an ABI 373 automated sequencer (Applied Biosystems, Foster City, CA).
RESULTS
Risk of Late Relapse
A total of 2,746 children were treated on Medical Research Council (MRC) ALL trials between 1970 and 1984. Details of study treatment protocols and patient outcome have been published elsewhere.11-15 A total of 1,134 children survived for 10 years in CR1, of whom 12 subsequently relapsed giving an actuarial risk of 1% over the next 10 years.
Clinical Characteristics of Relapse Cases
Diagnosis.
There were equal numbers of males and females within the 12 relapse cases (Table 1). Based on their age and white blood cell count at presentation, all had standard risk disease according to current risk stratification criteria for childhood ALL.16 Although the diagnosis of ALL was based primarily on cytomorphology and cytochemistry, it was further confirmed in six patients by documenting CD10 expression (common ALL antigen) by the lymphoblasts. Treatment varied according to the then current study protocol, but most patients did not receive any postinduction intensive consolidation therapy,17 and all could be regarded as having received inadequate treatment by current standards.1
Patient Characteristics and Treatment at Initial Presentation
| Patient . | Age (yr; diagnosis/ relapse) . | Sex . | WCC (109/L) . | Immunophenotype . | Karyotype . | Treatment . | CR1 Duration (yr) . | Clonal Marker . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10/34 | M | 14 | NK | NK | UKALL I | 24 | IgH FR2 (260 bp) |
| 2 | 11/21 | F | 2 | NK | NK | UKALL III | 10 | Failed* |
| 3 | 3/13 | F | 18 | NK | NK | UKALL V | 10 | IgH FR3 (125 bp) |
| 4 | 11/31 | M | 12 | CD10+ | NK | UKALL V | 20 | IgH FR3 (120 bp) |
| 5 | 4/15 | F | 10 | NK | NK | UKALL V | 11 | Failed* |
| 6 | 3/13 | M | 14 | CD10+ | Normal | UKALL V | 14 | IgH FR3 (130 bp) |
| 7 | 8/22 | F | 2 | NK | NK | UKALL V | 14 | IgH FR2 (250 bp) |
| 8 | 7/19 | F | 8 | CD10+ | NK | UKALL V | 11 | IgH FR3 |
| 9 | 7/18 | M | 60 | NK | NK | UKALL VI | 11 | TCRδ (vδ2-Dδ-Dδ3 240 bp) |
| 10 | 5/18 | F | 21 | CD10+ | NK | UKALL VIII | 12 | IgH FR3 (120 bp) |
| 11 | 7/19 | M | 8 | CD10+ | NK | UKALL VIII | 12 | Failed* |
| 12 | 4/14 | M | 2 | CD10+ | 46 XY Gq ?Ph | UKALL VIII | 10 | Failed* |
| Median (range) | 7 (3-11) | 11 (2-60) | 12 (10-24) |
| Patient . | Age (yr; diagnosis/ relapse) . | Sex . | WCC (109/L) . | Immunophenotype . | Karyotype . | Treatment . | CR1 Duration (yr) . | Clonal Marker . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10/34 | M | 14 | NK | NK | UKALL I | 24 | IgH FR2 (260 bp) |
| 2 | 11/21 | F | 2 | NK | NK | UKALL III | 10 | Failed* |
| 3 | 3/13 | F | 18 | NK | NK | UKALL V | 10 | IgH FR3 (125 bp) |
| 4 | 11/31 | M | 12 | CD10+ | NK | UKALL V | 20 | IgH FR3 (120 bp) |
| 5 | 4/15 | F | 10 | NK | NK | UKALL V | 11 | Failed* |
| 6 | 3/13 | M | 14 | CD10+ | Normal | UKALL V | 14 | IgH FR3 (130 bp) |
| 7 | 8/22 | F | 2 | NK | NK | UKALL V | 14 | IgH FR2 (250 bp) |
| 8 | 7/19 | F | 8 | CD10+ | NK | UKALL V | 11 | IgH FR3 |
| 9 | 7/18 | M | 60 | NK | NK | UKALL VI | 11 | TCRδ (vδ2-Dδ-Dδ3 240 bp) |
| 10 | 5/18 | F | 21 | CD10+ | NK | UKALL VIII | 12 | IgH FR3 (120 bp) |
| 11 | 7/19 | M | 8 | CD10+ | NK | UKALL VIII | 12 | Failed* |
| 12 | 4/14 | M | 2 | CD10+ | 46 XY Gq ?Ph | UKALL VIII | 10 | Failed* |
| Median (range) | 7 (3-11) | 11 (2-60) | 12 (10-24) |
*Failed DNA extraction.
Relapse.
Although relapse was diagnosed after 10 to 15 years in CR1 in 10 children, it did not occur until after 20 years in two patients (Table 2). All relapses were in the marrow except one, a patient who had a pelvic lymphoma with no morphological evidence of marrow disease. Blast cells were of precursor B lymphoid origin in all cases. Four of seven patients in whom a blast cell karyotype analysis was successful had a clonal abnormality. One of these was a Philadelphia translocation, which had, in retrospect, probably been present at diagnosis.
Patient Characteristics, Treatment, and Outcome After Relapse
| Patient . | Site . | Immunotype . | Karyotype . | Treatment . | Response . | CR2 Duration (mo) . | Current Status . | Clonal Marker . |
|---|---|---|---|---|---|---|---|---|
| 1 | Marrow + testes | Common | Hypodiploid 45XY | Chemo + XRT | CR2 | 27 | CCR2 | IgH FR2 (260 bp) |
| 2 | Marrow | Common | NK | Chemo | CR2 | 26 | Relapse death | Failed* |
| 3 | Marrow | Pre-B | NK | Chemo | CR2 | 134 | CCR2 | IgH FR3 (125 bp) |
| 4 | Marrow | Common | 46XY,del 14q,t(12;17) | Chemo | CR2 | 18 | CCR2 | IgH FR3 (120 bp) |
| 5 | Pelvic | Common (APAAP) | NK | Chemo + autograft | CR2 | 106 | CCR2 | Failed* |
| 6 | Marrow | Common | Normal | Chemo | CR2 | 113 | CCR2 | IgH FR3 (130 bp) |
| 7 | Marrow | Pre-B | Tetraploid 92-94, del 4q, t(9;12)del10q | Chemo + autograft | CR2 | 26 | Relapse death | IgH FR2 (250 bp) |
| 8 | Marrow | Common | Normal | Chemo | CR2 | 94 | CCR2 | IgH FR3 |
| 9 | Marrow | Common | Normal | Chemo | CR2 | 96 | CCR2 | TCRδ (vδ2-Dδ-Dδ3 240 bp) |
| 10 | Marrow | Common | NK | Chemo + allograft | CR2 | 36 | CCR2 | IgH FR3 (120 bp) |
| 11 | Marrow | Common | NK | Chemo + allograft | CR2 | 12 | Relapse death | IgH FR2 (260 bp) |
| 12 | Marrow | Common | 46XY t(9:22)(q34;q11) | Chemo | CR2 | 27 | Relapse death | IgH FR3 (120 bp) |
| Median (range) | 30 (12-134) | |||||||
| Patient . | Site . | Immunotype . | Karyotype . | Treatment . | Response . | CR2 Duration (mo) . | Current Status . | Clonal Marker . |
|---|---|---|---|---|---|---|---|---|
| 1 | Marrow + testes | Common | Hypodiploid 45XY | Chemo + XRT | CR2 | 27 | CCR2 | IgH FR2 (260 bp) |
| 2 | Marrow | Common | NK | Chemo | CR2 | 26 | Relapse death | Failed* |
| 3 | Marrow | Pre-B | NK | Chemo | CR2 | 134 | CCR2 | IgH FR3 (125 bp) |
| 4 | Marrow | Common | 46XY,del 14q,t(12;17) | Chemo | CR2 | 18 | CCR2 | IgH FR3 (120 bp) |
| 5 | Pelvic | Common (APAAP) | NK | Chemo + autograft | CR2 | 106 | CCR2 | Failed* |
| 6 | Marrow | Common | Normal | Chemo | CR2 | 113 | CCR2 | IgH FR3 (130 bp) |
| 7 | Marrow | Pre-B | Tetraploid 92-94, del 4q, t(9;12)del10q | Chemo + autograft | CR2 | 26 | Relapse death | IgH FR2 (250 bp) |
| 8 | Marrow | Common | Normal | Chemo | CR2 | 94 | CCR2 | IgH FR3 |
| 9 | Marrow | Common | Normal | Chemo | CR2 | 96 | CCR2 | TCRδ (vδ2-Dδ-Dδ3 240 bp) |
| 10 | Marrow | Common | NK | Chemo + allograft | CR2 | 36 | CCR2 | IgH FR3 (120 bp) |
| 11 | Marrow | Common | NK | Chemo + allograft | CR2 | 12 | Relapse death | IgH FR2 (260 bp) |
| 12 | Marrow | Common | 46XY t(9:22)(q34;q11) | Chemo | CR2 | 27 | Relapse death | IgH FR3 (120 bp) |
| Median (range) | 30 (12-134) | |||||||
Abbreviations: NK, not known (not performed or failed); CR, complete remission; IgH, immunoglobulin heavy chain gene rearrangement; FR2 and 3, framework 2 and 3; TCR, T-cell receptor rearrangement; CCR, continuing complete remission.
Failed DNA extraction.
All patients were successfully reinduced into a second complete remission and the majority received intensive and continuing chemotherapy to consolidate and maintain the remission without a marrow transplant. Of the 12 patients, eight remain in a second complete remission at a median follow-up of 52 months and the actuarial disease-free survival 5 years after relapse is 56%. Of the four patients who relapsed a second time, two had received an autologous transplant and two chemotherapy alone.
Molecular analysis.
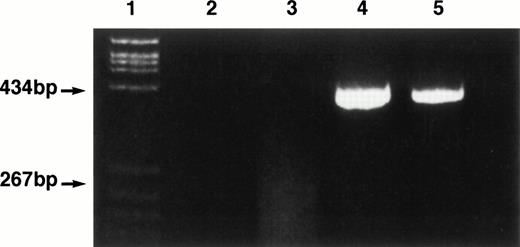
Amplifiable DNA could be extracted from both diagnosis and relapse marrow smears in 8 of 12 cases studied. Polyacrylamide gel electrophoresis of the amplified DNA showed an identical size clonal TCR (1 case) or IgH (7 cases) gene rearrangement at diagnosis and relapse in these 8 cases (Tables 1 and 2, Fig 1, patient 1). Automated sequence analysis confirmed complete sequency homology of rearrangement at diagnosis and relapse in seven patients. The remaining patient has been reported previously5 and was shown to have an identical FR3 IgH rearrangement by dot hybridization using a clone-specific oligonucleotide probe at diagnosis, end of treatment, and relapse. In two cases, a clonal IgH rearrangement could be detected at relapse (Table 2, patients 11 and 12), but we failed to extract DNA from the diagnostic smear.
Ethidium bromide-stained 4% polyacrylamide gel of IgH framework 1 PCR products obtained at diagnosis and relapse in patient 1. Lane 1, PBR 322/HaeIII size marker. Lane 2, water blank. Lane 3, normal bone marrow sample showing a typical polyclonal smear. Lane 4, patient 1 at diagnosis. Lane 5, patient 1 at relapse.
Ethidium bromide-stained 4% polyacrylamide gel of IgH framework 1 PCR products obtained at diagnosis and relapse in patient 1. Lane 1, PBR 322/HaeIII size marker. Lane 2, water blank. Lane 3, normal bone marrow sample showing a typical polyclonal smear. Lane 4, patient 1 at diagnosis. Lane 5, patient 1 at relapse.
DISCUSSION
With the large number of children treated on MRC ALL studies between 1970 and 1984, almost all of whom were rigorously followed for more than 18 years, we can be confident that the 1% risk of late relapse uncovered in this study is representative. Therefore, physicians treating children with ALL can be reassured that the vast majority of long-term remitters are truly cured of their disease. Whether even this small risk is faced by patients treated on later protocols is uncertain, as all of the patients reported received less intensive treatment than is used in current protocols.1 17
The evidence provided in this study that relapse can occur after 10 years in remission raises some fundamental questions about the biology of childhood ALL. Like the requirement for a prolonged “maintenance” treatment program, late relapse seems to be unique to this cancer. Together with recent evidence that PCR techniques, similar to those used in this study, can detect residual leukemic cells in a substantial proportion of patients in long-term remission,18 our findings suggest that cells from the original clone can remain dormant for long periods of time. Although the mechanisms responsible for this dormancy are not known, they are likely to involve either an ability of the clonal cells to remain out of cell cycle (in G0) for long periods, or the ability of host immune surveillance to maintain minimal residual leukemia in limbo, or a combination of the two. Clinical recurrence would only arise when there was either a breach in immune surveillance or a return to cell cycle triggered by the acquisition of new mutations within the dormant cell genome allowing clonal escape. The absence of subclones with mutated IgH or TCR genes at late relapse (there was complete sequency homology between the clonal rearrangements found at diagnosis and relapse) in the patients reported suggests the former to be a more likely explanation. However, we do not have sufficient information to exclude immunophenotypic or karyotypic clonal evolution at relapse, so this hypothesis remains unsubstantiated.
The relative ease with which the leukemia could be reinduced into an easily maintained second remission suggests that the lymphoblasts retain chemosensitivity. If so, there is every reason to suppose that such patients can be salvaged simply with more intensive conventional chemotherapy than that which they received on the first occasion and without recourse to progenitor cell transplantation.
ACKNOWLEDGMENT
We are grateful to all previous and current members of the MRC Childhood Leukaemia Working Party and the following physicians, data and laboratory managers who contributed material for this study: Birmingham Children's Hospital, Professor J.R. Mann and P. Short; Queen Elisabeth Hospital, Birmingham, D. Boffey; Bristol Royal Hospital for Sick Children, Professor A. Oakhill; Addenbrooke's Hospital, Cambridge, Dr V. Broadbent, Dr R. Marcus, and D. Bloxham; Walsgrave Hospital, Coventry, Dr M.J. Strevens; Royal Hospital for Sick Children, Edinburgh, Dr A. Thomas and Dr P Shaw; Milton Keynes General Hospital. Dr S.S. Jolloh and Dr D.J. Moir; and North Staffordshire Hospital, Stoke-on-Trent, Dr R.N. Ibbotson.
L.F. is supported by a grant from Parents Association of Children with Tumours (P.A.C.T.), a Sheffield-based children's cancer parent support group.
Address reprint requests to Ajay Vora, MD, Department of Paediatric Haematology, The Children's Hospital, Sheffield S10 2TH, UK; e-mail:A.J.Vora@Sheffield.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal