Abstract
We report the results of a phase III open-label, randomized, multicenter trial comparing tacrolimus/methotrexate to cyclosporine/methotrexate for graft-versus-host disease (GVHD) prophylaxis after HLA-identical sibling marrow transplantation in patients with hematologic malignancy. The primary objective of this study was to compare the incidence of moderate to severe (grade II-IV) acute GVHD. Secondary objectives were to compare the relapse rate, disease-free survival, overall survival, and the incidence of chronic GVHD. Patients were stratified according to age (<40 v ≥40) and for male recipients of a marrow graft from an alloimmunized female. There was a significantly greater proportion of patients with advanced disease randomized to tacrolimus arm (P = .02). The incidence of grade II-IV acute GVHD was significantly lower in patients who received tacrolimus than patients in the cyclosporine group (31.9% and 44.4%, respectively; P = .01). The incidence of grade III-IV acute GVHD was similar, 17.1% in cyclosporine group and 13.3% in the tacrolimus group. There was no difference in the incidence of chronic GVHD between the tacrolimus and the cyclosporine group (55.9% and 49.4%, respectively; P = .8). However, there was a significantly higher proportion of patients in the cyclosporine group who had clinical extensive chronic GVHD (P = .03). The relapse rates of the two groups were similar. The patients in the cyclosporine arm had a significantly better 2-year disease-free survival and overall survival than patients in the tacrolimus arm, 50.4% versus 40.5% (P = .01) and 57.2% versus 46.9% (P = .02), respectively. The significant difference in the overall and disease-free survival was largely the result of the patients with advanced disease, 24.8% with tacrolimus versus 41.7% with cyclosporine (P = .006) and 20.4% with tacrolimus versus 28% with cyclosporine (P = .007), respectively. There was a higher frequency of deaths from regimen-related toxicity in patients with advanced disease who received tacrolimus. There was no difference in the disease-free and overall survival in patients with nonadvanced disease. These results show the superiority of tacrolimus/methotrexate over cyclosporine/methotrexate in the prevention of grade II-IV acute GVHD with no difference in disease-free or overall survival in patients with nonadvanced disease. The survival disadvantage in advanced disease patients receiving tacrolimus warrants further investigation.
ACUTE GRAFT-VERSUS-HOST disease (GVHD) is a common immunologic complication which occurs in 40% to 50% of the recipients of allogeneic bone marrow transplantation (BMT). The immunologic event leading to injury of the target organs—skin, liver, and gut—involves activation and clonal expansion of the donor's effector T cells in response to the recipients' disparate major or minor histocompatibility antigens.1 The morbidity and mortality of this complication correlates with the severity of the organ involvement.2-4 The main treatment strategy for acute GVHD routinely entails the intensification of immunosuppression which often leads to serious infectious complications.5-7 Death in patients with acute GVHD is usually due to organ failure or overwhelming infections. Thus, major research efforts in allogeneic BMT over the past 2 decades have focused on the prevention of GVHD, mainly with pharmacologic immunosuppressive agents. Since 1985, the combination of cyclosporine and short-course methotrexate (cyclosporine/methotrexate) has been studied extensively for the prevention of acute GVHD against single agents such as cyclosporine or methotrexate in randomized trials.8-13 Subsequently, this combination has been adopted as standard care in most centers.
In the late 1980s, tacrolimus or FK506, a potent macrolide lactone immunosuppressant, was introduced into clinical trials for the prevention of rejection in recipients of solid-organ transplants.14 In addition to its efficacy in the prevention of organ rejection, tacrolimus has been shown to be effective in the prevention of acute GVHD in murine and canine models.15-17 Canine studies had shown a combination of methotrexate and tacrolimus to be better than either drug alone.18 Moreover, several pilot studies in recipients of allogeneic marrow transplantation using tacrolimus for the treatment19,20 and prevention of acute GVHD21-24 suggested the efficacy of this agent, thus providing the rationale for this randomized clinical trial comparing the combination of methotrexate and tacrolimus to methotrexate and cyclosporine for the prevention of acute GVHD in HLA-identical sibling marrow transplantation in patients with hematologic malignancy.
We now report the results of a phase III open-label, randomized, multicenter trial. The primary objective of this study was to compare the incidence of moderate to severe (grade II-IV) acute GVHD. The secondary objectives were to compare the relapse rate, overall survival, disease-free survival (DFS), and the incidence of chronic GVHD.
Patient Demographics and Baseline Characteristics
| Characteristics . | Tacrolimus N = 165 (%) . | Cyclosporine N = 164 (%) . | P Value . |
|---|---|---|---|
| Age | |||
| Median (range) | 40.0 (17-61) | 40.0 (16-63) | 1.0* |
| 12-19 | 3 (2) | 10 (6) | 0.26-151 |
| 20-29 | 34 (20) | 25 (15) | |
| 30-39 | 44 (27) | 46 (28) | |
| 40-49 | 56 (34) | 57 (35) | |
| ≥50 | 28 (17) | 26 (16) | |
| Sex | 0.39-151 | ||
| Male | 93 (56) | 100 (61) | |
| Female | 72 (44) | 64 (39) | |
| Race | 0.25-151 | ||
| White | 146 (89) | 135 (82) | |
| African-American | 7 (4) | 13 (8) | |
| Others | 12 (7) | 16 (10) | |
| Karnofsky score | |||
| Median (range) | 80 (20-100) | 80 (20-100) | 0.89* |
| CMV serology (donor/ recipient) | 0.18-151 | ||
| Negative/negative | 38 (23) | 30 (19) | |
| Negative/positive | 35 (21) | 25 (15) | |
| Positive/negative | 22 (13) | 20 (12) | |
| Positive/positive | 69 (42) | 89 (54) | |
| Unknown | 1 (1) | 0 (0) | |
| Stage of malignancy | 0.02-151 | ||
| Advanced | 68 (41) | 48 (29) | |
| Nonadvanced | 97 (59) | 116 (71) | |
| Diagnosis | 0.70-152 | ||
| Myelodysplasia | 7 (4) | 13 (8) | |
| Acute lymphoid leukemia | 18 (11) | 14 (9) | |
| Acute myeloid leukemia | 45 (27) | 44 (27) | |
| Chronic myeloid leukemia | 51 (31) | 57 (35) | |
| Non-Hodgkin's lymphoma | 13 (8) | 16 (10) | |
| Hodgkin's disease | 2 (1) | 2 (1) | |
| Myeloma | 14 (9) | 9 (5) | |
| Chronic lymphoid leukemia | 6 (4) | 4 (2) | |
| Others | 9 (5) | 5 (3) |
| Characteristics . | Tacrolimus N = 165 (%) . | Cyclosporine N = 164 (%) . | P Value . |
|---|---|---|---|
| Age | |||
| Median (range) | 40.0 (17-61) | 40.0 (16-63) | 1.0* |
| 12-19 | 3 (2) | 10 (6) | 0.26-151 |
| 20-29 | 34 (20) | 25 (15) | |
| 30-39 | 44 (27) | 46 (28) | |
| 40-49 | 56 (34) | 57 (35) | |
| ≥50 | 28 (17) | 26 (16) | |
| Sex | 0.39-151 | ||
| Male | 93 (56) | 100 (61) | |
| Female | 72 (44) | 64 (39) | |
| Race | 0.25-151 | ||
| White | 146 (89) | 135 (82) | |
| African-American | 7 (4) | 13 (8) | |
| Others | 12 (7) | 16 (10) | |
| Karnofsky score | |||
| Median (range) | 80 (20-100) | 80 (20-100) | 0.89* |
| CMV serology (donor/ recipient) | 0.18-151 | ||
| Negative/negative | 38 (23) | 30 (19) | |
| Negative/positive | 35 (21) | 25 (15) | |
| Positive/negative | 22 (13) | 20 (12) | |
| Positive/positive | 69 (42) | 89 (54) | |
| Unknown | 1 (1) | 0 (0) | |
| Stage of malignancy | 0.02-151 | ||
| Advanced | 68 (41) | 48 (29) | |
| Nonadvanced | 97 (59) | 116 (71) | |
| Diagnosis | 0.70-152 | ||
| Myelodysplasia | 7 (4) | 13 (8) | |
| Acute lymphoid leukemia | 18 (11) | 14 (9) | |
| Acute myeloid leukemia | 45 (27) | 44 (27) | |
| Chronic myeloid leukemia | 51 (31) | 57 (35) | |
| Non-Hodgkin's lymphoma | 13 (8) | 16 (10) | |
| Hodgkin's disease | 2 (1) | 2 (1) | |
| Myeloma | 14 (9) | 9 (5) | |
| Chronic lymphoid leukemia | 6 (4) | 4 (2) | |
| Others | 9 (5) | 5 (3) |
*Student's t-test.
By Pearson's chi-squared test.
By Fisher's exact test.
Preparative Regimens Used in Patients With Advanced and Nonadvanced Disease
| Preparative Regimens . | Tacrolimus n (%) . | Cyclosporine n (%) . | P Values* . |
|---|---|---|---|
| Nonadvanced disease | 97 (100) | 116 (100) | .90 |
| Cyclophosphamide + TBI | 20 (21) | 19 (16) | |
| Cyclophosphamide + TBI + ara-C | 7 (7) | 6 (5) | |
| Cyclophosphamide + TBI + VP-16 | 7 (7) | 12 (10) | |
| TBI + VP-16 | 8 (8) | 9 (8) | |
| Busulfan + cyclophosphamide | 29 (30) | 33 (28) | |
| Busulfan + cyclophosphamide + ara-C | 20 (21) | 26 (22) | |
| Busulfan + cyclophosphamide + VP-16 | 2 (2) | 5 (4) | |
| Busulfan + cyclophosphamide + Thio-TEPA | 2 (2) | 5 (4) | |
| Other regimens | 2 (2) | 1 (1) | |
| Advanced disease | 68 (100) | 48 (100) | 1.0 |
| Cyclophosphamide + TBI | 8 (12) | 7 (15) | |
| Cyclophosphamide + TBI + ara-C | 7 (10) | 6 (12) | |
| Cyclophosphamide + TBI + VP-16 | 10 (15) | 7 (15) | |
| TBI + VP-16 | 3 (4) | 1 (2) | |
| Busulfan + cyclophosphamide | 9 (13) | 6 (12) | |
| Busulfan + cyclophosphamide + ara-C | 3 (4) | 2 (4) | |
| Busulfan + cyclophosphamide + VP-16 | 5 (7) | 5 (10) | |
| Busulfan + cyclophosphamide + Thio-TEPA | 11 (16) | 8 (17) | |
| Other regimens | 12 (18) | 6 (12) |
| Preparative Regimens . | Tacrolimus n (%) . | Cyclosporine n (%) . | P Values* . |
|---|---|---|---|
| Nonadvanced disease | 97 (100) | 116 (100) | .90 |
| Cyclophosphamide + TBI | 20 (21) | 19 (16) | |
| Cyclophosphamide + TBI + ara-C | 7 (7) | 6 (5) | |
| Cyclophosphamide + TBI + VP-16 | 7 (7) | 12 (10) | |
| TBI + VP-16 | 8 (8) | 9 (8) | |
| Busulfan + cyclophosphamide | 29 (30) | 33 (28) | |
| Busulfan + cyclophosphamide + ara-C | 20 (21) | 26 (22) | |
| Busulfan + cyclophosphamide + VP-16 | 2 (2) | 5 (4) | |
| Busulfan + cyclophosphamide + Thio-TEPA | 2 (2) | 5 (4) | |
| Other regimens | 2 (2) | 1 (1) | |
| Advanced disease | 68 (100) | 48 (100) | 1.0 |
| Cyclophosphamide + TBI | 8 (12) | 7 (15) | |
| Cyclophosphamide + TBI + ara-C | 7 (10) | 6 (12) | |
| Cyclophosphamide + TBI + VP-16 | 10 (15) | 7 (15) | |
| TBI + VP-16 | 3 (4) | 1 (2) | |
| Busulfan + cyclophosphamide | 9 (13) | 6 (12) | |
| Busulfan + cyclophosphamide + ara-C | 3 (4) | 2 (4) | |
| Busulfan + cyclophosphamide + VP-16 | 5 (7) | 5 (10) | |
| Busulfan + cyclophosphamide + Thio-TEPA | 11 (16) | 8 (17) | |
| Other regimens | 12 (18) | 6 (12) |
Abbreviations: TBI, total body irradiation; ara-C,cytosine arabinoside.
By Fisher's exact test.
Distribution of Doses of Methotrexate Administered to Patients in Two Treatment Arms
| No. of Doses . | Tacrolimus (N = 165) . | Cyclosporine (N = 164) . |
|---|---|---|
| 0 | 3 | 2 |
| 1 | 0 | 1 |
| 2 | 13 | 8 |
| 3 | 46 | 42 |
| 4 | 103 | 111 |
| No. of Doses . | Tacrolimus (N = 165) . | Cyclosporine (N = 164) . |
|---|---|---|
| 0 | 3 | 2 |
| 1 | 0 | 1 |
| 2 | 13 | 8 |
| 3 | 46 | 42 |
| 4 | 103 | 111 |
P = .61 by Fisher's exact test.
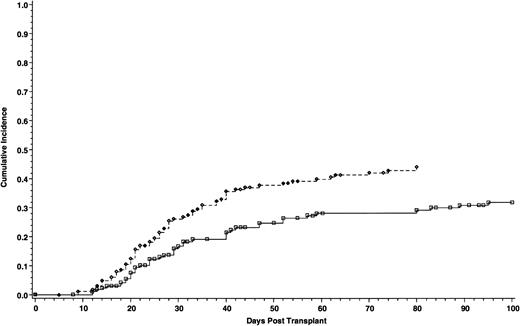
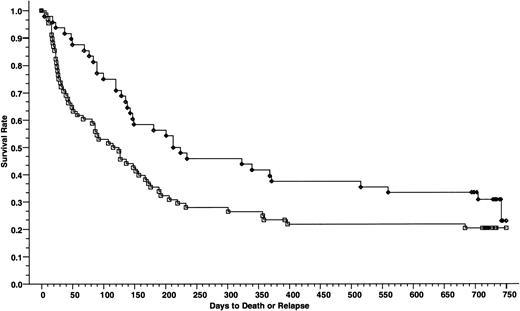
Cumulative incidence of grade II-IV acute GVHD of 165 patients who received cyclosporine/methotrexate, 44.4% (◊) and 164 patients who received tacrolimus/methotrexate, 31.9% (□); absolute difference = 12.5%, 95% CI = −23.9 to −1.2 (P = .01, Wilcoxon).
Cumulative incidence of grade II-IV acute GVHD of 165 patients who received cyclosporine/methotrexate, 44.4% (◊) and 164 patients who received tacrolimus/methotrexate, 31.9% (□); absolute difference = 12.5%, 95% CI = −23.9 to −1.2 (P = .01, Wilcoxon).
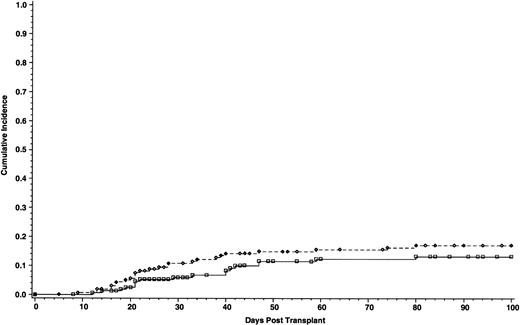
Cumulative incidence of grade III-IV acute GVHD of 165 patients who received cyclosporine/methotrexate, 17.1% (◊) and 164 patients who received tacrolimus/methotrexate 13.3% (□); absolute difference = 3.8%, 95% CI interval = −12.1 to 4.5 (P = .2, Wilcoxon).
Cumulative incidence of grade III-IV acute GVHD of 165 patients who received cyclosporine/methotrexate, 17.1% (◊) and 164 patients who received tacrolimus/methotrexate 13.3% (□); absolute difference = 3.8%, 95% CI interval = −12.1 to 4.5 (P = .2, Wilcoxon).
Organ Involvement in Patients With Grade II-IV Acute GVHD
| Stage of Malignancy . | Tacrolimus . | Cyclosporine . | P Value3-150 . |
|---|---|---|---|
| Nonadvanced | N = 27 (%) | N = 44 (%) | .07 |
| Skin only | 2 (7) | 9 (20) | |
| Skin + GI | 4 (15) | 12 (27) | |
| Skin + liver | 2 (7) | 4 (9) | |
| Skin + GI + liver | 3 (11) | 9 (20) | |
| GI only | 9 (34) | 6 (14) | |
| GI + liver | 6 (22) | 2 (5) | |
| Liver only | 1 (4) | 2 (5) | |
| Advanced disease | n = 16 (%) | n = 23 (%) | .52 |
| Skin only | 2 (13) | 4 (18) | |
| Skin + GI | 4 (25) | 6 (26) | |
| Skin + liver | 1 (6) | 1 (4) | |
| Skin + GI + liver | 8 (50) | 6 (26) | |
| GI only | 0 (0) | 4 (18) | |
| GI + liver | 1 (6) | 1 (4) | |
| Liver only | 0 (0) | 1 (4) |
| Stage of Malignancy . | Tacrolimus . | Cyclosporine . | P Value3-150 . |
|---|---|---|---|
| Nonadvanced | N = 27 (%) | N = 44 (%) | .07 |
| Skin only | 2 (7) | 9 (20) | |
| Skin + GI | 4 (15) | 12 (27) | |
| Skin + liver | 2 (7) | 4 (9) | |
| Skin + GI + liver | 3 (11) | 9 (20) | |
| GI only | 9 (34) | 6 (14) | |
| GI + liver | 6 (22) | 2 (5) | |
| Liver only | 1 (4) | 2 (5) | |
| Advanced disease | n = 16 (%) | n = 23 (%) | .52 |
| Skin only | 2 (13) | 4 (18) | |
| Skin + GI | 4 (25) | 6 (26) | |
| Skin + liver | 1 (6) | 1 (4) | |
| Skin + GI + liver | 8 (50) | 6 (26) | |
| GI only | 0 (0) | 4 (18) | |
| GI + liver | 1 (6) | 1 (4) | |
| Liver only | 0 (0) | 1 (4) |
Abbreviation: GI, gastrointestinal.
By Fisher's exact test.
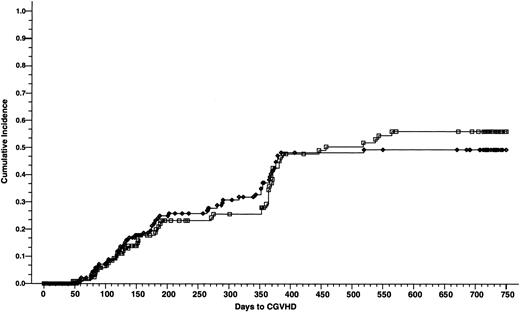
Cumulative incidence of chronic GVHD of 165 patients who received cyclosporine/methotrexate, 49.4% (◊) and 164 patients who received tacrolimus/methotrexate, 55.9% (□); absolute difference = 6.5%, 95% CI = −8.0 to 21.1 (P = .8, Wilcoxon).
Cumulative incidence of chronic GVHD of 165 patients who received cyclosporine/methotrexate, 49.4% (◊) and 164 patients who received tacrolimus/methotrexate, 55.9% (□); absolute difference = 6.5%, 95% CI = −8.0 to 21.1 (P = .8, Wilcoxon).
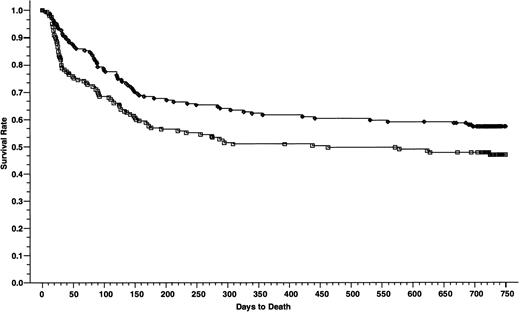
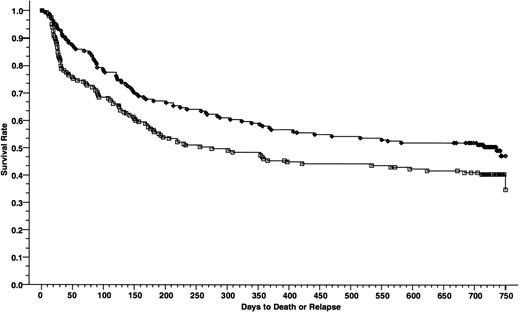
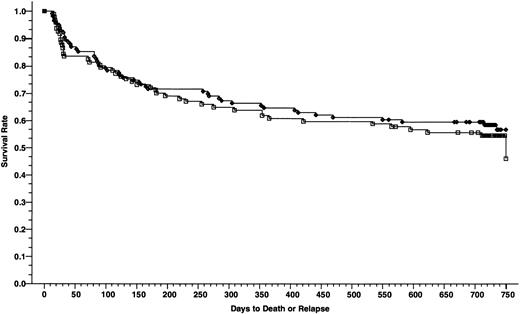
Overall survivals at 2 years of 165 patients who received cyclosporine/methotrexate, 57.2% (◊) and 164 patients who received tacrolimus/methotrexate 46.9% (□); absolute difference = 10.3%, 95% CI interval = −21.1 to 0.5 (P = .02, Wilcoxon).
Overall survivals at 2 years of 165 patients who received cyclosporine/methotrexate, 57.2% (◊) and 164 patients who received tacrolimus/methotrexate 46.9% (□); absolute difference = 10.3%, 95% CI interval = −21.1 to 0.5 (P = .02, Wilcoxon).
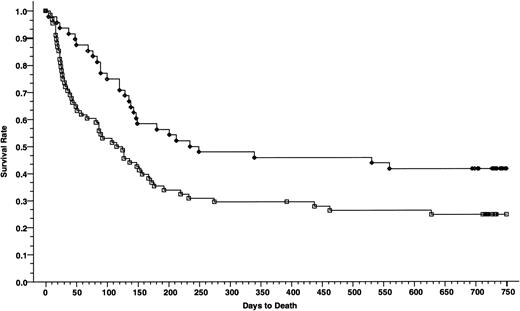
Overall survivals at 2 years of patients with advanced disease; 48 patients received cyclosporine/methotrexate, 41.7% (◊) and 68 patients received tacrolimus/methotrexate, 24.8% (□); absolute difference = 16.9%, 95% CI = −34.3 to 0.4 (P = .006, Wilcoxon).
Overall survivals at 2 years of patients with advanced disease; 48 patients received cyclosporine/methotrexate, 41.7% (◊) and 68 patients received tacrolimus/methotrexate, 24.8% (□); absolute difference = 16.9%, 95% CI = −34.3 to 0.4 (P = .006, Wilcoxon).
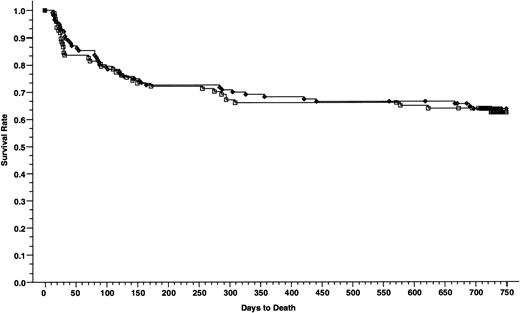
Overall survivals at 2 years of patients with nonadvanced disease; 116 patients received cyclosporine/methotrexate 63.6% (◊) and 97 patients received tacrolimus/methotrexate, 62.4% (□); absolute difference = 1.2%, 95% CI = −14.4 to 11.9 (P = .79, Wilcoxon).
Overall survivals at 2 years of patients with nonadvanced disease; 116 patients received cyclosporine/methotrexate 63.6% (◊) and 97 patients received tacrolimus/methotrexate, 62.4% (□); absolute difference = 1.2%, 95% CI = −14.4 to 11.9 (P = .79, Wilcoxon).
Causes of Death by Disease Stage
| Disease Stage and Causes . | Tacrolimus . | Cyclosporine . | P Value* . |
|---|---|---|---|
| Nonadvanced disease | N = 36 (%) | N = 43 (%) | .99 |
| GVHD (%) | 9 (25) | 13 (30) | |
| Infection (%) | 6 (17) | 7 (16) | |
| Regimen-related toxicity (%)4-151 | 11 (30) | 11 (26) | |
| Relapse (%) | 8 (22) | 9 (21) | |
| Others (%) | 2 (6) | 3 (7) | |
| Advanced disease | N = 53 (%) | N = 28 (%) | .05 |
| GVHD (%) | 3 (6) | 6 (21) | |
| Infection (%) | 10 (19) | 4 (14) | |
| Regimen-related toxicity (%)4-151 | 15 (28) | 2 (7) | |
| Relapse (%) | 22 (41) | 15 (54) | |
| Others (%) | 3 (6) | 1 (4) |
| Disease Stage and Causes . | Tacrolimus . | Cyclosporine . | P Value* . |
|---|---|---|---|
| Nonadvanced disease | N = 36 (%) | N = 43 (%) | .99 |
| GVHD (%) | 9 (25) | 13 (30) | |
| Infection (%) | 6 (17) | 7 (16) | |
| Regimen-related toxicity (%)4-151 | 11 (30) | 11 (26) | |
| Relapse (%) | 8 (22) | 9 (21) | |
| Others (%) | 2 (6) | 3 (7) | |
| Advanced disease | N = 53 (%) | N = 28 (%) | .05 |
| GVHD (%) | 3 (6) | 6 (21) | |
| Infection (%) | 10 (19) | 4 (14) | |
| Regimen-related toxicity (%)4-151 | 15 (28) | 2 (7) | |
| Relapse (%) | 22 (41) | 15 (54) | |
| Others (%) | 3 (6) | 1 (4) |
*By Fisher's exact test.
Patients with venocclusive disease of the liver were included.
DFSs at 2 years of 165 patients who received cyclosporine/methotrexate, 50.4% (◊) and 164 patients who received tacrolimus/methotrexate 40.5% (□); absolute difference = 9.9%, 95% CI = −20.7 to 0.8 (P = .01, Wilcoxon).
DFSs at 2 years of 165 patients who received cyclosporine/methotrexate, 50.4% (◊) and 164 patients who received tacrolimus/methotrexate 40.5% (□); absolute difference = 9.9%, 95% CI = −20.7 to 0.8 (P = .01, Wilcoxon).
DFSs of patients with advanced disease; 48 patients received cyclosporine/methotrexate, 30.8% (◊) and 68 patients received tacrolimus/methotrexate, 20.4% (□); absolute difference = 10.4%, 95% CI = −26.7 to 6.0 (P = .007, Wilcoxon).
DFSs of patients with advanced disease; 48 patients received cyclosporine/methotrexate, 30.8% (◊) and 68 patients received tacrolimus/methotrexate, 20.4% (□); absolute difference = 10.4%, 95% CI = −26.7 to 6.0 (P = .007, Wilcoxon).
DFSs of patients with nonadvanced disease; 116 patients received cyclosporine/methotrexate, 58.4% (◊) and 97 patients received tacrolimus/methotrexate, 54.5% (□); absolute difference = 3.9%, 95% CI = −17.3 to 9.5 (P = .55, Wilcoxon).
DFSs of patients with nonadvanced disease; 116 patients received cyclosporine/methotrexate, 58.4% (◊) and 97 patients received tacrolimus/methotrexate, 54.5% (□); absolute difference = 3.9%, 95% CI = −17.3 to 9.5 (P = .55, Wilcoxon).
Adverse Events in Tacrolimus and Cyclosporine Groups
| Adverse Events . | Tacrolimus N = 165 (%) . | Cyclosporine N = 164 (%) . | P Value . |
|---|---|---|---|
| Incidence of serum creatinine >2 mg/dL | |||
| Within 8 wk posttransplant | 99 (60) | 79 (48) | .03 |
| Within 26 wk posttransplant | 111 (67) | 98 (60) | .16 |
| Incidence of serum creatinine 2× baseline | |||
| Within 8 wk posttransplant | 132 (80) | 119 (73) | .11 |
| Within 26 wk posttransplant | 139 (84) | 131 (80) | .30 |
| Incidence of hemodialysis | |||
| Within 8 wk posttransplant | 31 (19) | 13 (8) | .004 |
| Within 26 wk posttransplant | 32 (19) | 16 (10) | .01 |
| Nonadvanced disease | 13/97 (13) | 13/116 (11) | .63 |
| Advanced disease | 19/68 (28) | 3/48 (6) | .003 |
| Incidence of veno-occlusive disease | |||
| Within 20 d posttransplant | 44 (27) | 37 (23) | .39 |
| Hyperglycemia requiring insulin5-150 | |||
| At 8 wk | 17 (10) | 7 (4) | .06 |
| At 26 wk | 1 (0.6) | 0 (0) | 1.0 |
| Hypertension requiring treatment while on study drug at 26 wk | 34 (21) | 66 (40) | .001 |
| Adverse Events . | Tacrolimus N = 165 (%) . | Cyclosporine N = 164 (%) . | P Value . |
|---|---|---|---|
| Incidence of serum creatinine >2 mg/dL | |||
| Within 8 wk posttransplant | 99 (60) | 79 (48) | .03 |
| Within 26 wk posttransplant | 111 (67) | 98 (60) | .16 |
| Incidence of serum creatinine 2× baseline | |||
| Within 8 wk posttransplant | 132 (80) | 119 (73) | .11 |
| Within 26 wk posttransplant | 139 (84) | 131 (80) | .30 |
| Incidence of hemodialysis | |||
| Within 8 wk posttransplant | 31 (19) | 13 (8) | .004 |
| Within 26 wk posttransplant | 32 (19) | 16 (10) | .01 |
| Nonadvanced disease | 13/97 (13) | 13/116 (11) | .63 |
| Advanced disease | 19/68 (28) | 3/48 (6) | .003 |
| Incidence of veno-occlusive disease | |||
| Within 20 d posttransplant | 44 (27) | 37 (23) | .39 |
| Hyperglycemia requiring insulin5-150 | |||
| At 8 wk | 17 (10) | 7 (4) | .06 |
| At 26 wk | 1 (0.6) | 0 (0) | 1.0 |
| Hypertension requiring treatment while on study drug at 26 wk | 34 (21) | 66 (40) | .001 |
Patient population: all randomized patients who received a marrow transplant and at least one dose of study drug (tacrolimus group = 165 patients; cyclosporine group = 164 patients).
Patients without history of diabetes mellitus (tacrolimus group = 163 patients; cyclosporine group = 160 patients).
MATERIALS AND METHODS
Subjects.
Patients must have fulfilled all the following eligibility criteria to be included in the study: (1) a documented hematologic malignancy; (2) recipient of a genotypically HLA-identical marrow transplantation; (3) age of 12 years of age or older; and (4) patients or legal guardians must sign the informed consent form approved by the Institutional Review Board. Patients were ineligible if they had one of the following exclusion criteria: (1) pregnant or unwilling to maintain an effective contraception during the study; (2) a previous marrow transplant; (3) serum creatinine ≥ 3.0 mg/dL; (4) carriers of any of the human immunodeficiency viruses; and (5) use of a T-depleted marrow graft. In addition, patients must have met the criteria of patient selection according to the clinical protocols for marrow transplantation at the study sites.
Study design.
The primary endpoint of this study was the development of grade II-IV acute GVHD. The secondary end points were DFS and the incidence of chronic GVHD. We used the method of Makuch and Simon25 to calculate the sample size based on the primary end point—the development of moderate to severe (grade II-IV) acute GVHD, assuming that there was no difference in treatment efficacy between the two groups. An estimated sample size of 150 patients in each treatment arm was derived to ensure a probability of 80% that the upper 95% confidence limit for the true difference in efficacy did not exceed 0.15.
Patients with hematologic malignancies who were candidates for BMT in each center were randomized in a 1:1 allocation ratio to receive tacrolimus or cyclosporine for GVHD prophylaxis. Patients were stratified according to age (<40 v ≥40 years) and whether they were a male recipient of a marrow graft from an alloimmunized female. The stage of underlying malignancy was categorized as ‘nonadvanced disease’ if patients had chronic myeloid leukemia in chronic or accelerated phase, myelodysplasia, or if the disease was in remission; otherwise they were categorized as having ‘advanced disease.’ This study was monitored by an independent Data Safety Monitoring Board (DSMB). Results of interim analyses performed by the DSMB were blinded to the investigators and sponsor. The analysis for this report was based on the intent-to-treat principle.
Treatment protocol.
Preparative regimens were assigned according to the institutional protocols at the clinical sites. Supportive care such as protective isolation techniques, antimicrobial policy, and the use of intravenous (IV) immune globulin was standardized within each clinical site and was uniformly applied to the patients in both groups. All blood components were irradiated before administration.
The day of transplantation was designated as day 0. Either tacrolimus or cyclosporine was initiated on day −1 of transplantation. The starting dose of tacrolimus (0.03 mg/kg/d) or cyclosporine (3 mg/kg/d) was administered by continuous IV infusion. The dose of cyclosporine or tacrolimus was calculated according to patient's lean body weight. The route of administration was converted from IV to oral at the ratio of 1:4 when patients were able to tolerate oral intake. Dose modifications of tacrolimus and cyclosporine were dependent primarily on serum creatinine and the blood levels of tacrolimus or cyclosporine. A dose reduction of at least 25% was mandatory for patients with a creatinine elevated to greater than 2.0 times baseline and at least a 50% reduction for patients with a creatinine greater than 3 times baseline. Whole blood levels of tacrolimus26 and cyclosporine27 were obtained three times a week during the first 28 days posttransplantation, weekly between day 29 to day 56, and every 4 weeks from day 57 to day 180. During the first 56 days of transplantation, the whole blood level of tacrolimus by IMx assay (Abbott, Abbott Park, IL) and cyclosporine by monoclonal or high-performance liquid chromatography (HPLC) assay were maintained between 10 and 40 ng/mL and 150 and 450 ng/mL, respectively. Approximately halfway through the course of this clinical trial, the upper therapeutic level of tacrolimus was reduced to 30 ng/mL. This modification was recommended by the DSMB when excessive renal toxicity associated with higher levels of tacrolimus was recognized during the analysis of the solid-organ transplantation trials.28 The dose of tacrolimus and cyclosporine were tapered by 20% per month beginning on day 57 posttransplant and discontinued at the end of 6 months.
All patients also received a short course of methotrexate, 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11. The doses of methotrexate were adjusted according to the severity of mucosal toxicity, weight gain, fluid retention, renal impairment, and hepatic dysfunction. Elevation of serum creatinine to greater than twice of the baseline, transaminases greater than 200 U/L, or total bilirubin greater than 5.0 mg/dL required at least a 50% dose reduction. More severe renal or hepatic dysfunction, fluid retention, and severe fibrinous mucositis with threatening airway obstruction resulted in withholding the methotrexate. Monitoring of methotrexate blood levels and the decision to rescue patients with leucovorin were left to the discretion of the investigators at the clinical sites. However, leucovorin rescue was not permitted earlier than 24 hours after the administration of methotrexate.
The development of acute and chronic GVHD were monitored throughout the study. The severity of acute GVHD was graded by the investigators at each clinical site using the consensus criteria.29 The clinical and laboratory parameters collected to assess the grading of acute GVHD included percent of body-surface area with skin rash, volume of diarrhea, total bilirubin, and Karnofsky performance status. Tissue biopsy samples were obtained to confirm the diagnosis of acute GVHD whenever clinically feasible. The diagnosis and grading of chronic GVHD were established according to clinical and pathologic criteria proposed by Sullivan et al.30
Study end points and statistical analysis.
The difference in the distribution of categorical data was compared using chi-square test or Fisher's exact test when appropriate. StatXact software (Cytel Software Corp, Cambridge, MA) for microcomputer was used to perform the analysis of categorical data.31 Student's t-test was used to compare the means of continuous variables between two groups. The time to the development of acute GVHD was calculated from the date of transplantation to the date of onset of acute GVHD. Patients who did not develop acute GVHD were censored on the date of their last contact, relapse, or death, whichever occurred first. DFS was calculated from the date of transplantation to the date of disease recurrence or death, whichever occurred first. The Kaplan-Meier method was used to calculate the estimates of the distributions of the time to events—probabilities of relapse, acute and chronic GVHD, overall survival, and DFS.32 The Wilcoxon test was used to compare the Kaplan-Meier estimates between groups; and the 95% confidence interval (CI) around the difference in Kaplan-Meier estimates between the treatment groups was calculated using the variance derived from Greenwood's formula. We used the Cox proportional hazards models to estimate the relative risks of an event adjusted for the stage of malignancy.33 All P values were two-sided and a value of < .05 was considered statistically significant.
RESULTS
Patient demographics and characteristics.
From May 1993 to November 1994, 16 marrow transplantation centers in North America enrolled 332 patients. Three of the 332 patients (1 in the cyclosporine group and 2 in the tacrolimus group) randomized in the study did not undergo marrow transplantation because of death or life-threatening complications just before the procedure and were considered ineligible. Of the remaining 329 patients, 165 patients were randomized to receive tacrolimus and 164 patients were randomized to receive cyclosporine. With the exception of the stage of malignancy (advanced v nonadvanced disease), demographic characteristics including the age of the patients, sex, Karnofsky performance status, diagnosis, race, positive serology for cytomegalovirus (CMV), and male recipients of an alloimmunized female donor were distributed equally between the two treatment groups. By chance, there was a significantly larger number of patients with advanced disease assigned to the tacrolimus group (P = .02) (Table 1). Within the subgroups of patients with nonadvanced and advanced disease, the distributions of preparative regimens between two treatment groups were not different (Table 2). The distribution for the number of doses of methotrexate given to the patients between two treatment arms were also not significantly different (Table 3).
Acute GVHD.
The incidence of grade II-IV acute GVHD was significantly lower in patients who received tacrolimus than patients in the cyclosporine group—31.9% and 44.4%, respectively. The absolute difference between the two groups was 12.5% (95% CI for the difference, −23.9 to −1.2; P = .01) (Fig 1). However, the incidence of grade III-IV acute GVHD was similar, 17.1% with cyclosporine and 13.3% with tacrolimus. The absolute difference between the two groups was 3.8% (95% CI for the difference, −12.1 to 4.5; P = .2) (Fig 2). Using the Cox regression model, the relative risk of grade II-IV acute GVHD in the cyclosporine group compared with the tacrolimus group was 1.61 when adjusted for disease stage (P = .01). The distributions of the organ involvement by acute GVHD were not significantly different between patients who received tacrolimus or cyclosporine regardless of disease stage (Table 4).
Chronic GVHD.
At 2 years of follow up, there was no difference in the incidence of chronic GVHD between the tacrolimus and the cyclosporine groups—55.9% and 49.4%, respectively (absolute difference 6.5%; 95% CI, −8.0 to 21.1) (P = .8) (Fig 3). Chronic GVHD developed in 50 patients in the tacrolimus group (27 patients had extensive disease and 23 patients had limited disease) and 54 patients in the cyclosporine group (41 patients had extensive disease and 13 patients had limited disease). There was a significantly higher proportion of patients in the cyclosporine group with clinical extensive disease (P = .03).
Survival and relapse.
The patients who received cyclosporine had a significantly better survival than patients who received tacrolimus—the 2-year survival rates were 57.2% and 46.9%, respectively (absolute difference 10.3%; 95% CI, −21.1 to 0.5) (P = .02) (Fig 4). When the overall survival was adjusted for the disease stage using Cox regression model, the relative risk of death was 0.75 in favor of cyclosporine group (P = .07). However, the advantage in the overall survival was limited to the patients with advanced disease, 24.8% in the tacrolimus group and 41.7% in the cyclosporine group, respectively (absolute difference 16.9%; 95% CI, −34.3 to 0.4) (P = .006) (Fig 5). The survival rates in patients with nonadvanced disease were not different, 62.4% in the tacrolimus group and 63.6% in the cyclosporine group, respectively (absolute difference 1.2%; 95% CI, −14.4 to 11.9) (P = .79) (Fig 6). Collectively, the distributions for the causes of death between the two treatment groups in patients with nonadvanced disease were not different (Table5). However, in the patients with advanced disease, there was a higher frequency of deaths from GVHD in patients who received cyclosporine (P = .06, Fisher's) and from regimen-related toxicity in patients who received tacrolimus (P = .04, Fisher's). Cox regression models were used to evaluate the interactions of tacrolimus with other nephrotoxic agents (aminoglycosides, furosemide, vancomycin, and amphotericin-B) and its association with early posttransplant mortality (deaths within day 56 after transplantation). None of these agents were found to have significant association with early posttransplant mortality when administered concomitantly with tacrolimus (data not shown).
Forty-one patients in the tacrolimus group and 36 patients in the cyclosporine group relapsed. There was no difference in relapse rates between patients who received tacrolimus (24.8%; 95% CI, 18.2 to 31.4) and patients who received cyclosporine (22.0%; 95% CI, 15.7 to 28.3) (P = .54). The relapse rates were similar regardless of the stage of malignancy (data not shown).
The DFS at 2 years for patients receiving tacrolimus and cyclosporine was 40.5% and 50.4%, respectively (absolute difference 9.9%; 95% CI, −20.7 to 0.8) (P = .01) (Fig 7). The significant difference in DFS was largely the result of a difference among patients with advanced disease, 20.4% in the tacrolimus group and 30.8% in the cyclosporine group, respectively (absolute difference 10.4%; 95% CI, −26.7 to 6.0, P = .007) (Fig8). The DFSs among patients with nonadvanced disease were similar between the two groups, 54.5% in the tacrolimus group and 58.4% in the cyclosporine group (absolute difference 3.9%; 95% CI, −17.3 to 9.5) (P = .55) (Fig 9).
Engraftment.
The median times to the recovery of absolute neutrophil counts ≥0.5 × 109/L did not differ between the tacrolimus and cyclosporine groups (19 and 20 days, respectively) (P = .78). Neutrophil recovery was also not different in the subgroups of patients who had advanced or nonadvanced disease (advanced disease: 18 days in the tacrolimus group and 19 days in the cyclosporine group; nonadvanced disease: 21 days in the tacrolimus group and 21 days in the cyclosporine group). The frequencies and durations of platelet and red blood cell transfusions did not differ significantly between the tacrolimus and cyclosporine groups, or between subgroups of advanced or nonadvanced disease (data not shown).
Toxicities.
The incidences of renal toxicity, veno-occlusive disease of the liver, and hyperglycemic events during 26 weeks of scheduled immunosuppressive therapy are shown in Table 6. The incidence of serum creatinine increasing above 2 mg/dL within 8 weeks of transplantation was significantly higher in the tacrolimus group (P = .03) but was not different from the cyclosporine arm at 26 weeks (P = .16). The incidence of renal failure requiring hemodialysis was significantly higher in the tacrolimus group, of which the patients with advanced disease accounted for most of the events and the difference between treatment groups. The incidence of hyperglycemia requiring insulin within 8 weeks of transplantation (in patients with no prior history of diabetes) was significantly higher in the tacrolimus group but was not different at 26 weeks. The incidence of hypertension requiring antihypertensive medications was significantly higher in the cyclosporine group (P = .001). The incidence of veno-occlusive disease was similar. Neurologic side effects were also similar in both groups: approximately 25% of the patients had tremor or headache and 10% or less had confusion, nervousness, or paresthesia.
DISCUSSION
Since the late 1960s, the pharmacologic prophylaxis of acute GVHD after allogeneic marrow transplantation has evolved from the introduction of monotherapy using methotrexate or cyclosporine to the development of combination therapy—cyclosporine/methotrexate.11,34-40Similar to cyclosporine in its development as an immunosuppressant, the success of tacrolimus in kidney,41-43 liver,44and heart45 transplantation served as a leading indicator for its possible application in the prevention of acute GVHD in allogeneic marrow transplantation. Indeed, experimental animal studies showed synergism between tacrolimus and methotrexate for prevention of acute GVHD and showed significant improvement of survival over monotherapy.18 Preceding the initiation of this present study, there were three pilot studies of tacrolimus conducted to study the pharmacokinetics of tacrolimus and to estimate its efficacy and safety of tacrolimus for the prevention of acute GVHD in HLA-matched sibling and unrelated donor transplantation.21-23 Two of these studies were conducted in the recipients of histocompatible sibling donor marrow transplantation.21,22 In a study on 27 patients who received tacrolimus monotherapy, the rate of grade II-IV acute GVHD was 41%.21 In the other study on 18 patients who received either tacrolimus alone or in combination with methotrexate or steroid, the rate of grade II-IV acute GVHD was 44%.22 The remaining study, conducted on the recipients of an unrelated marrow transplantation, reported an incidence of grade II-IV acute GVHD of 42%.23 Taken together, these data strongly suggested substantial efficacy of tacrolimus for the prevention of acute GVHD in allogeneic marrow transplantation.
The results of this study confirm the efficacy of tacrolimus and also suggest the superiority of tacrolimus/methotrexate over that of cyclosporine/methotrexate for the prevention of acute GVHD in recipients of an allogeneic marrow transplantation. The difference in the efficacy of these two regimens could not be attributed to the cumulative dose of methotrexate given or pharmacologic interaction between tacrolimus and methotrexate.46 However, the survival of patients who had advanced disease was significantly poorer in the tacrolimus/methotrexate group. It is intriguing that the survival of patients with advanced disease who received cyclosporine in this study was much better than in other studies reported in the literature.36,47,48 Moreover, in the cyclosporine arm, we observed an unexpectedly higher incidence of fatal regimen-related toxicity in patients with nonadvanced disease compared to patients with advanced disease (26% v 7%, respectively). The reasons for these contradictory findings are unclear. On the other hand, the patients with advanced disease in the tacrolimus group had a survival rate comparable to similar patients in other studies using cyclosporine. Several subsequent studies of tacrolimus which included patients with advanced disease found no increase in treatment-related toxicity or mortality.24 49-51
By our definition of ‘advanced disease,’ this group of patients comprised a heterogeneous population with diverse diagnoses and perhaps receiving a higher intensity of preparative regimen, which might have heightened the organ toxicity in patients who received tacrolimus/methotrexate. Consequently, patients with advanced disease were a less than ideal population for a study of this nature in which many of these adverse factors may confound the analysis. This contention has been emphasized by other investigators in several studies, in which only good-risk (nonadvanced disease) patients were included in their study designs.9-13,52 The survival outcomes of nonadvanced disease patients in our study were consistent with previous randomized trials in patients with good-risk disease.9,10,13 In this subset of patients, the overall survival and DFS of patients who received tacrolimus/methotrexate and cyclosporine/methotrexate were similar. Recently two other randomized studies using the same study design have compared tacrolimus/methotrexate and cyclosporine/methotrexate. One study was conducted in patients with nonadvanced disease receiving a matched unrelated donor transplantation,53 and the other study included both sibling matched donor and unrelated donor transplantation.54 Both of these studies reported superiority of tacrolimus/methotrexate over that of cyclosporine/methotrexate in the prevention of acute GVHD, with no difference in survival or relapse.
Based on the superior results of tacrolimus for the prevention of acute GVHD shown in this and other recently completed randomized trials, this agent is likely to have a major role in the prevention of acute GVHD in allogeneic transplantation. The lower incidence of extensive chronic GVHD in patients treated with tacrolimus in this study is unique and needs confirmation. The long-term impact of tacrolimus on the clinical course of extensive chronic GVHD remains a subject of further investigation. Regarding the nephrotoxicity of tacrolimus in our current patient population, a retrospective analysis to correlate whole blood tacrolimus levels with toxicity and efficacy was undertaken. There was no correlation between whole blood concentration of tacrolimus and acute GVHD. On the other hand, the incidence of nephrotoxicity was significantly increased when the whole blood concentration of tacrolimus exceeded 20 ng/mL.55 It is possible that the upper limit of the targeted blood level of 30 to 40 ng/mL used in this study might have adversely influenced the survival. Further studies to define the drug levels to minimize toxicity while preserving a high level of immunosuppressive effect of this agent are crucial to optimize its therapeutic index.
ACKNOWLEDGMENT
We thank the members of the Data Safety Monitoring Board (Thomas Fleming, PhD [Chair], Donald Steinmuller, MD, and Elena Bloom, MD), the statistical staff at Fujisawa USA who provided the technical supports (Jin Zhu, PhD, Ronald Kershner, PhD, Sarah Young, PhD, Revathy Rangarajan, MS, and Jay Erdman, MS), the data managers, clinical research associates, and the nursing and medical staffs at each study site, all of whom had substantial contribution to the success of this study.
Supported by Fujisawa, USA, Deerfield, IL.
Address reprint requests to Voravit Ratanatharathorn, MD, Associate Professor of Internal Medicine, B1-207 Cancer Center, University of Michigan Medical Center, 1500 E Medical Center Dr, Ann Arbor, MI 48109-0914; e-mail: vratanat@umich.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal