Abstract
Hypoxia results in adaptive changes in the transcription of a range of genes including erythropoietin. An important mediator is hypoxia-inducible factor-1 (HIF-1), a DNA binding complex shown to contain at least two basic helix-loop-helix PAS-domain (bHLH-PAS) proteins, HIF-1α and aryl hydrocarbon nuclear receptor translocator (ARNT). In response to hypoxia, HIF-1α is activated and accumulates rapidly in the cell. Endothelial PAS domain protein 1 (EPAS-1) is a recently identified bHLH-PAS protein with 48% identity to HIF-1α, raising the question of its role in responses to hypoxia. We developed specific antibodies and studied expression and regulation of EPAS-1 mRNA and protein across a range of human cell lines. EPAS-1 was widely expressed, and strongly induced by hypoxia at the level of protein but not mRNA. Comparison of the effect of a range of activating and inhibitory stimuli showed striking similarities in the EPAS-1 and HIF-1α responses. Although major differences were observed in the abundance of EPAS-1 and HIF-1α in different cell types, differences in the inducible response were subtle with EPAS-1 protein being slightly more evident in normoxic and mildly hypoxic cells. Functional studies in a mutant cell line (Ka13) expressing neither HIF-1α nor EPAS-1 confirmed that both proteins interact with hypoxically responsive targets, but suggest target specificity with greater EPAS-1 transactivation (relative to HIF-1α transactivation) of the VEGF promoter than the LDH-A promoter.
OXYGEN AVAILABILITY is increasingly recognized as a major modulator of gene expression.1Studies of the hematopoietic growth factor erythropoietin have led to the definition of a widely operative system of gene regulation by oxygen2 that is dependent on activation of a transcriptional complex termed hypoxia inducible factor-1 (HIF-1).3 Affinity purification of HIF-1 from Hela cell nuclear extract led to the definition of the DNA binding complex as a heterodimer of two basic-helix-loop-helix PAS domain (bHLH-PAS) proteins, HIF-1α and a previously identified molecule termed the aryl hydrocarbon nuclear receptor translocator (ARNT).4 HIF-1 plays an important role not only in the regulation of erythropoietin but also in the hypoxia-inducible expression of many other genes with diverse functions in relation to the biology of oxygen. These include glucose transporters, glycolytic enzymes, vascular growth factors, and nitric oxide synthases.5 Studies of HIF-1 regulation have indicated that one of the major activation mechanisms involves rapid nuclear accumulation of the α subunit.4,6 Thus, when cells are exposed to an atmosphere of 1% hypoxia striking nuclear accumulation of HIF-1α occurs over a period of minutes to hours, whereas ARNT is present constitutively and increased only modestly (or not at all) with hypoxic stimulation.6 Current evidence indicates that in normoxic cells HIF-1α is targeted for rapid degradation by the proteasome through the operation of specific domains in the molecule,6-9 and that accumulation in hypoxia involves a reduction in this degradation.
Recent cloning experiments have greatly expanded the number of recognized bHLH-PAS proteins,10 raising an important question as to the possible involvement of other members of this family in the response to hypoxia. Among these a molecule first termed endothelial PAS protein-1 (EPAS-1) shows the closest sequence homology to HIF-1α (48% identity).11 This molecule was also independently identified and reported as HIF-like factor (HLF),12 member of PAS super-family 2 (MOP2),13and HIF-related factor (HRF).14 Although these reports have shown that EPAS-1, like HIF-1α, can dimerize with ARNT and activate transcription from similar DNA recognition sites, the regulatory characteristics of the EPAS-1 molecule are not yet clearly defined. In this report we have used specific antibodies to define and compare the distribution and regulation of EPAS-1 and HIF-1α in a wide range of tissue culture cells. We report that both molecules are expressed widely, although at greatly differing levels within this panel of cells. Both molecules show high-level induction by hypoxia at the level of protein but not mRNA. Their responses to a variety of pharmacological agents were also similar, strongly suggesting that they respond to a similar or identical sensing and signal transduction system.
MATERIALS AND METHODS
Cell culture.
Cell lines were cultured as recommended by the European Collection of Cell Cultures. The CHO-K1 derivative Ka13 and the endothelial line HMEC-1 have been described previously.15 16 Cells were plated onto 75-cm2 or 175-cm2 flasks 24 hours before experiments such that they were approaching confluence. Culture medium was replaced at the start of each experiment, and when short hypoxic exposures (<2 hours) were studied this medium was pre-equilibrated in the hypoxic incubator. Hypoxic exposure was in a NAPCO 7001 incubator (Precision Scientific, Chicago, IL) with 1% oxygen, 6% CO2, balance nitrogen. Exposure to experimental conditions was for 4 hours unless indicated otherwise. The effect of graded hypoxia was examined in cells cultured on Petriperm dishes (Heraeus, South Plainfield, NJ) in a set of five purpose-built gas-tight chambers, with continous monitoring of oxygen tension in each chamber. Culture media and chemicals were from Sigma (Poole, UK). Diphenylene iodonium chloride was from Calbiochem (Cambridge, MA).
RNA analysis.
Total RNA was extracted using RNAzol B (Biogenesis, Poole, UK). Ribonuclease protection assays were performed essentially as described previously,2 with parallel hybridization using 40 μg for HIF-1α, 40 μg for EPAS-1, and 1 μg for U6 small nuclear RNA. 32P-labeled riboprobes were generated using SP6 or T7 RNA polymerase. The templates used yielded protected fragments as follows: 221 bp for EPAS-1 (nucleotides 2542 to 2762, accession no. U81984), 255 bp for HIF-1α (nucleotides 764 to 1018, U22431), and 106 bp for U6 (nucleotides 1 to 107, X01366). After resolution on 8% polyacrylamide gels, quantification was performed using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Signals for HIF-1α mRNA and EPAS-1 mRNA were normalized to a value of 100 for EPAS-1 in Hep3B cells, allowing for the different number of labeled nucleotides in the two protected fragments.
Bacterial protein expression and immunization.
An Nco 1 restriction fragment encoding amino acids 535 to 631 of human EPAS-1 was cloned in frame into pGEX-4t-1 (Pharmacia Biotech, St Albans, UK) with a modified polylinker. Expression of glutathione-S-transferase (GST) fusion protein was induced by exposure of transformed Escherichia coli DH5α cells to 0.1 mmol/L isopropyl b-D-thiogalactopyranoside. After bacterial lysis, protein was affinity purified with glutathione Sepharose 4B (Pharmacia). To generate polyclonal antisera to EPAS-1, two rabbits were immunized with 200 μg protein in Complete Freund's Adjuvant (Difco, Detroit, MI), followed by six immunizations at 14-day intervals with 100 μg protein in Incomplete Freund's Adjuvant. To generate monoclonal antibodies (MoAbs), Balb/c mice were immunized with 50 μg protein in Complete Freund's Adjuvant followed by three further immunizations of 50 μg protein in phosphate-buffered saline (PBS) at 10-day intervals. Fusion of mouse splenocytes with myeloma cells was performed using standard techniques 4 days after the last immunization. Hybridoma supernatants were screened by enzyme-linked immunosorbent assay (ELISA) against the GST fusion protein and GST alone, and immunolabelling of COS-1 cells transfected with the human EPAS-1 expression plasmid, phEP1.11 Five MoAbs that reacted with EPAS-1 were obtained.
Protein extraction and immunoblot analysis.
For whole-cell extracts, adherent cells were washed with ice-cold PBS and removed by scraping. Cell pellets were homogenized in extraction buffer (7 mol/L urea/10% glycerol/10 mmol/L Tris-HCl pH 6.8/1% sodium dodecyl sulfate [SDS]/5 mmol/L dithiothreitol [DTT]/0.5 mmol/L phenylmethyl sulfonyl fluoride [PMSF] with 1 mg/L aprotinin, pepstatin, and leupeptin) using an IKA Ultra-Turrax T8 homogenizer (Janke & Kunkel, Staufen, Germany) for 5 seconds at full speed. Extracts were quantified using the BioRad DC protein assay (BioRad, Hemel Hempstead, UK). For differential nuclear and cytoplasmic extraction a modifed Dignam protocol was used.3 For immunoblotting, proteins were resolved in SDS/6% polyacrylamide gels and transferred to Immobilon P (Millipore, Bedford, MA) overnight in 10 mmol/L Tris/100 mmol/L glycine/10%methanol/0.05% SDS. Membranes were blocked with PBS/5% fat-free dried milk/0.1% Tween 20. For HIF-1α detection, MoAb 28b was used at 4 μg/mL. This antibody was from a fusion following immunization with a GST fusion protein including amino acids 329 to 530 of human HIF-1α. For EPAS-1, 190b supernatant was diluted 1:4. For detection of the Gal4 DNA binding domain in fusion proteins, RK5C1 (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 0.1 μg/mL. Detection was with horseradish peroxidase (HRP)-conjugated goat anti-mouse Igs (DAKO, Ely, UK) at 1:2,000 and enhanced chemiluminescence (SuperSignal Ultra; Pierce & Warriner, Chester, UK). After analysis, membranes were stained with Ponceau S to verify equal protein loading and transfer. For studies of antibody specificity and relative sensitivity, protein extracts containing a high level of EPAS-1 or HIF1α fused to a portion of the yeast transcription factor Gal4 were obtained by electroporating COS-1 cells with 10 μg pGN/EPAS19-870 or pGN/HIF1α28-826. These constructs were based on pcDNA3 (Invitrogen, Carlsbad, CA) and contained an SV40 origin of replication, the cytomegalovirus (CMV) promoter, and a bovine growth hormone poly A signal resulting in expression of in-frame fusions of sequence encoding amino acids 1-147 of Gal4 and polymerase chain reaction (PCR) products encoding the specified amino acids of EPAS-1 or HIF-1α.
Transient transfections and reporter gene assays.
For functional studies of EPAS-1 and HIF-1α, CHO Ka13 cells were cotransfected with 0.25 μg of expression plasmids for HIF-1α (pcDNA3/Neo/HIF-1α15) or EPAS-1 (phEP-111) or pcDNA3 without insert, 3 μg reporter plasmid, and 2 μg pCMVβGal15 (as a control for transfection efficiency).All reporter plasmids were based on pGL3 (Promega, Southampton, UK) but included different promoters as follows. p(24x6)TKLuc contained 6 copies of a 24-bp oligonucleotide (P24) from the mouse phosphoglycerate kinase-1 5′ enhancer,17placed 10 bp 5′ to the TATA box of the herpes simplex thymidine kinase promoter. pLDH-ALuc contained 233 bp from the mouse LDH-A promoter, extending from −186 to +47 relative to the transcriptional start site. pVEGFLuc contained a 1,786-bp BamH1 fragment from the human VEGF promoter, extending from −1288 to +480. Transfections were performed using DEAE/Dextran in 5-cm dishes with cells at 70% confluence. After transfection, dishes were incubated overnight at 37°C in complete medium, and the following day cells were trypsinized and divided into two 3.5-cm wells on separate plates for parallel 18-hour incubation in normoxia and hypoxia. Luciferase activities in cell lysates were determined using a commercially available luciferase assay system (Promega) and a TD-20e luminometer (Turner Designs, Sunnyvale, CA). Relative β-galactosidase activity in lysates was measured using o-nitrophenyl-β-D-galactopyranoside (0.67 mg/mL) as substrate in a 0.1 mol/L phosphate buffer (pH 7.0) containing 10 mmol/L KCl, 1 mmol/L MgSO4, and 30 mmol/L β-mercaptoethanol for 45 to 90 minutes. The OD420 was determined after stopping the reaction by the addition of 1 mol/L sodium carbonate.
Immunohistochemistry.
Cells were grown on lysine-coated glass slides (Polysine; BDH, Poole, UK). After washing in ice-cold PBS, cells were fixed in methanol at −20°C for 10 minutes and air dried. Slides were incubated with 28b or 190b hybridoma supernatant, followed by HRP-conjugated swine anti-rabbit Igs (DAKO). Detection was with 3′3′diaminobenzidine.
RESULTS
Distribution of EPAS-1 and HIF-1α mRNAs in tissue culture cells.
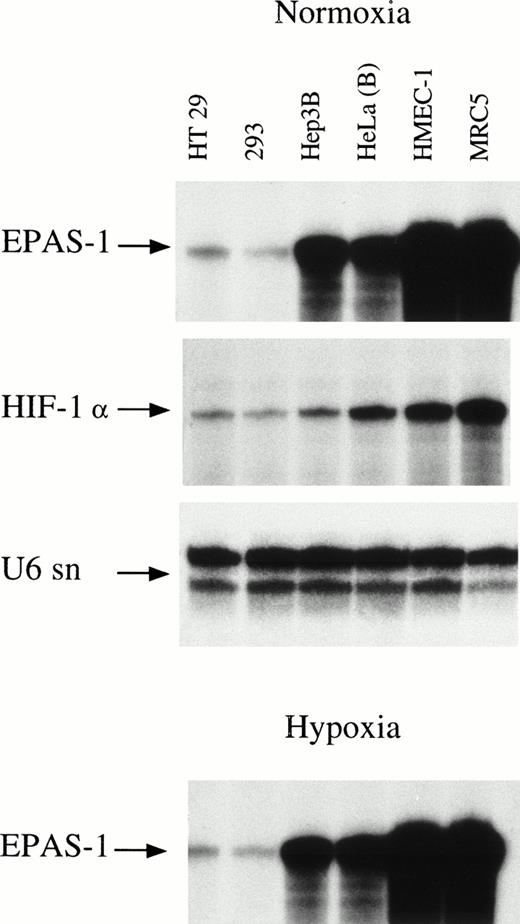
As a first step in this analysis, total RNA from cell lines derived from diverse human tissues was examined for EPAS-1 gene expression using RNAse protection (Fig 1 and Table 1). EPAS-1 mRNA was detected not only in endothelial cells, but also in fibroblast and epithelial cell lines. The abundance of EPAS-1 mRNA varied over a wide range (>100-fold) between cell lines, with cell lines with the highest levels of EPAS-1 mRNA being HMEC-1 (an endothelial capillary cell line) and MRC5 (fetal lung fibroblast). In contrast, a line of Epstein-Barr virus–transformed lymphocytes (RPMI 1788) showed the lowest level of expression among the cell lines examined, with signal near the limit of detection by RNAse protection assay. The level of EPAS-1 mRNA was determined both under normoxic conditions and after 4 hours of exposure to 1% oxygen, and was not altered by hypoxia in any cell line (Fig 1). Interestingly, two sublines of HeLa cells showed nearly a threefold difference in the level of EPAS-1 mRNA. HeLa(A) was a certified line obtained from the European Collection of Animal Cultures. PCR genotyping of both sublines using six highly polymorphic markers confirmed that the second subline, HeLa(B), were indeed HeLa cells.
Expression of EPAS-1 and HIF-1α in six human cell lines. Cells were cultured in parallel for 4 hours in normoxia (upper three panels) and 1% hypoxia (lower panel). Ribonuclease protection analysis of total RNA was performed for EPAS-1 and HIF-1α (40 μg each), and U6 small nuclear RNA (1 μg).
Expression of EPAS-1 and HIF-1α in six human cell lines. Cells were cultured in parallel for 4 hours in normoxia (upper three panels) and 1% hypoxia (lower panel). Ribonuclease protection analysis of total RNA was performed for EPAS-1 and HIF-1α (40 μg each), and U6 small nuclear RNA (1 μg).
Quantitative Data for EPAS-1 mRNA and HIF-1α mRNA Expression in a Range of Cell Lines
| Cell Line . | Tissue Origin . | EPAS-1 . | HIF-1α . | EPAS-1: HIF-1α . |
|---|---|---|---|---|
| RPMI 1788 | EBV lymphocyte | 4.4 | 13 | 0.34 |
| 293 | Embryonic kidney | 5.7 | 7.1 | 0.81 |
| HT29 | Colonic adenocarcinoma | 11 | 13 | 0.84 |
| HeLa (A) | Cervical carcinoma | 38 | 38 | 1.0 |
| HT1080 | Fibrosarcoma | 48 | 83 | 0.57 |
| HepG2 | Hepatoma | 90 | 39 | 2.3 |
| HeLa (B) | Cervical carcinoma | 99 | 25 | 3.9 |
| Hep3B | Hepatoma | 100 | 10 | 9.8 |
| BeWo | Choriocarcinoma | 242 | 26 | 9.4 |
| HMEC-1 | Microvascular endothelium | 952 | 46 | 21 |
| MRC5 | Foetal lung fibroblast | 1,284 | 133 | 9.6 |
| Cell Line . | Tissue Origin . | EPAS-1 . | HIF-1α . | EPAS-1: HIF-1α . |
|---|---|---|---|---|
| RPMI 1788 | EBV lymphocyte | 4.4 | 13 | 0.34 |
| 293 | Embryonic kidney | 5.7 | 7.1 | 0.81 |
| HT29 | Colonic adenocarcinoma | 11 | 13 | 0.84 |
| HeLa (A) | Cervical carcinoma | 38 | 38 | 1.0 |
| HT1080 | Fibrosarcoma | 48 | 83 | 0.57 |
| HepG2 | Hepatoma | 90 | 39 | 2.3 |
| HeLa (B) | Cervical carcinoma | 99 | 25 | 3.9 |
| Hep3B | Hepatoma | 100 | 10 | 9.8 |
| BeWo | Choriocarcinoma | 242 | 26 | 9.4 |
| HMEC-1 | Microvascular endothelium | 952 | 46 | 21 |
| MRC5 | Foetal lung fibroblast | 1,284 | 133 | 9.6 |
Expression is given relative to the level of EPAS-1 mRNA in Hep3B cells (100 arbitrary units).
To compare EPAS-1 and HIF-1α expression we also determined the level of HIF-1α mRNA in each cell line. The level of HIF-1α mRNA expression was less variable than that of EPAS-1 mRNA, ranging approximately 20-fold from the lowest expressing lines (293 cells and Hep3B cells) to the highest expressing lines (MRC5 and HT1080). HIF-1α mRNA was less abundant than EPAS-1 mRNA in 6 of the 11 cell lines examined (Table 1). We also examined the levels of HIF-1α mRNA after 4 hours of hypoxic exposure and again did not observe modulation in any cell line (data not shown).
Characterization of antibodies reactive against EPAS-1 and HIF-1α.
To permit analysis of EPAS-1 regulation at the protein level, and allow comparison with HIF-1α, antibodies were raised against recombinant immunogens (see Materials and Methods). To detect any cross-reactivity and determine the approximate sensitivity of these reagents in immunoblotting procedures, COS-1 cells were transfected with plasmids expressing chimeric genes in which near full-length cDNAs for EPAS-1 or HIF-1α were linked to an N-terminal Gal4 DNA binding domain. A total of five MoAbs and two polyclonal antisera raised against amino acids 535-631 of human EPAS-1 were tested. All seven gave a strong signal against the Gal4/EPAS-1 fusion protein, but not the Gal4/HIF-1α fusion (data not shown). One MoAb (MoAb 190b) was selected for the majority of further experiments described (Fig 2). For detection of HIF-1α, MoAb 28b, raised against an immunogen containing amino acids 329-530 of human HIF-1α, was tested similarly. This antibody reacted with the Gal4/HIF-1α fusion protein but not Gal4/EPAS-1 in the transfected COS-1 cells (Fig 2). Using an MoAb to Gal4 protein, the quantity of extract from each COS-1 cell transfection was adjusted so that equal amounts of HIF-1α and EPAS-1 fusion proteins were loaded for detection by either MoAb 28b or MoAb 190b. Under these conditions detection of HIF-1α with MoAb 28b gave approximately fourfold less signal than detection of EPAS-1 with MoAb 190b (Fig 2). Detection of the relevant Gal4 fusion proteins was substantially less sensitive with the MoAb to Gal4 than with either MoAb 28b or MoAb 190b.
Antibody specificity and relative sensitivity of MoAb 190b (EPAS-1) and MoAb 28b (HIF-1α). Immunoblots of whole cell extracts from COS-1 cells transfected with either pGN/EPAS19-870 or pGN/HIF-1α28-826, resulting in expression of fusion proteins with the N-terminal Gal4 DNA binding domain. Aliquots of the EPAS-1/GAL and HIF-1α/GAL extracts were analyzed in parallel using antibodies to Gal4 (RK5C1), EPAS-1 (190b), and HIF-1α (28b). After initial analysis with the Gal4 MoAb (not shown), the amount of each extract loaded was adjusted to give approximately equal signal with this antibody (left) indicating similar amounts of EPAS-1 and HIF-1α fusion proteins. The antibodies to EPAS-1 (middle) and HIF-1α (right) specifically recognized the appropriate fusion protein. Film exposure times are shown below each panel, indicating differences in the sensitivity of detection. The positions of MW markers are shown on the left.
Antibody specificity and relative sensitivity of MoAb 190b (EPAS-1) and MoAb 28b (HIF-1α). Immunoblots of whole cell extracts from COS-1 cells transfected with either pGN/EPAS19-870 or pGN/HIF-1α28-826, resulting in expression of fusion proteins with the N-terminal Gal4 DNA binding domain. Aliquots of the EPAS-1/GAL and HIF-1α/GAL extracts were analyzed in parallel using antibodies to Gal4 (RK5C1), EPAS-1 (190b), and HIF-1α (28b). After initial analysis with the Gal4 MoAb (not shown), the amount of each extract loaded was adjusted to give approximately equal signal with this antibody (left) indicating similar amounts of EPAS-1 and HIF-1α fusion proteins. The antibodies to EPAS-1 (middle) and HIF-1α (right) specifically recognized the appropriate fusion protein. Film exposure times are shown below each panel, indicating differences in the sensitivity of detection. The positions of MW markers are shown on the left.
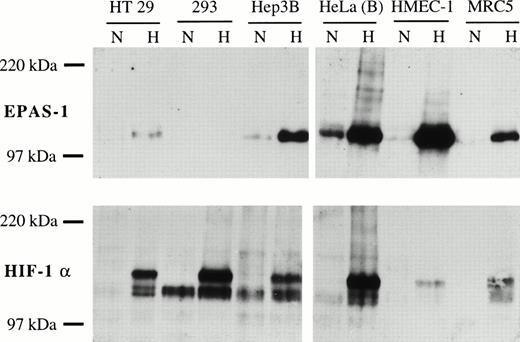
Expression of normoxic and hypoxic EPAS-1 and HIF-1α protein in six human cell lines. Cells were cultured in parallel in normoxia (N) or 1% oxygen (H) for 4 hours. Whole-cell extracts (50 μg) were analyzed for EPAS-1 (MoAb 190b, top) and HIF-1α (MoAb 28b, bottom). Exposure time was 30 seconds for EPAS-1 and 120 seconds for HIF-1α, so that sensitivity of detection for the two proteins is approximately equal. The HeLa(B) hypoxic extract was independently shown to contain approximately equal amounts of EPAS-1 and HIF-1α by direct comparison with transfected COS-1 cell extracts.
Expression of normoxic and hypoxic EPAS-1 and HIF-1α protein in six human cell lines. Cells were cultured in parallel in normoxia (N) or 1% oxygen (H) for 4 hours. Whole-cell extracts (50 μg) were analyzed for EPAS-1 (MoAb 190b, top) and HIF-1α (MoAb 28b, bottom). Exposure time was 30 seconds for EPAS-1 and 120 seconds for HIF-1α, so that sensitivity of detection for the two proteins is approximately equal. The HeLa(B) hypoxic extract was independently shown to contain approximately equal amounts of EPAS-1 and HIF-1α by direct comparison with transfected COS-1 cell extracts.
EPAS-1 and HIF-1α protein levels in normoxic and hypoxic tissue culture cells.
Previous studies of nuclear and whole cell extracts have shown striking increases in HIF-1α protein levels after hypoxic stimulation.4 6 To determine if EPAS-1 protein levels are induced by hypoxia and to compare the responses with those of HIF-1α across the range of cell types, whole-cell extracts were prepared following parallel normoxic and hypoxic incubation for 4 hours. EPAS-1 and HIF-1α were detected by immunoblotting using MoAbs 190b and 28b, respectively. EPAS-1 was detected as a single species with a somewhat lower mobility (apparent molecular weight [MW] 115 kD) than that predicted from the deduced amino acid sequence. In a number of cells, including endothelial, fibroblast-like, and epithelial lines, EPAS-1 protein was detectable in extracts of normoxic cells. In all these cell lines striking induction by hypoxia was observed (Fig. 3). Overall, there was a broad correlation between the level of mRNA expression and the hypoxic level of EPAS-1 protein. Thus, the highest levels of EPAS-1 protein in response to hypoxia were seen in HMEC-1, HepG2, and Hep3B cells. MRC5 cells, which showed the highest levels of EPAS-1 mRNA, showed an intermediate level of EPAS-1 protein, whereas EPAS-1 protein was not detected in RPMI 1788, consistent with the very low level of EPAS-1 mRNA in these cells. Essentially identical results were obtained using the other MoAbs and polyclonal antisera raised against EPAS-1 (data not shown).
The same extracts were analyzed in parallel for HIF-1α with MoAb 28b. As reported previously,4 18 HIF-1α was detected as several species which migrated more slowly than the predicted MW and was induced by hypoxia in all cell lines examined (Fig. 3). Overall, there was a poor correlation between immunodetectable HIF-1α in hypoxic cell extracts and the level of the mRNA. In particular, hypoxic HMEC-1 and MRC5 contained relatively low levels of HIF-1α protein, although these cells expressed the gene at a relatively high level as assessed by mRNA analysis. The relative amounts of EPAS-1 and HIF-1α were compared by reference to Gal4 fusion proteins. Hypoxic HMEC-1, Hep3B, and MRC5 cells were found to contain more EPAS-1 than HIF-1α. In hypoxic HeLa(B) and HepG2 cells the amount of HIF-1α and EPAS-1 is similar, whereas HIF-1α was more abundant than EPAS-1 in HT29, 293, and RPMI 1788 cells.
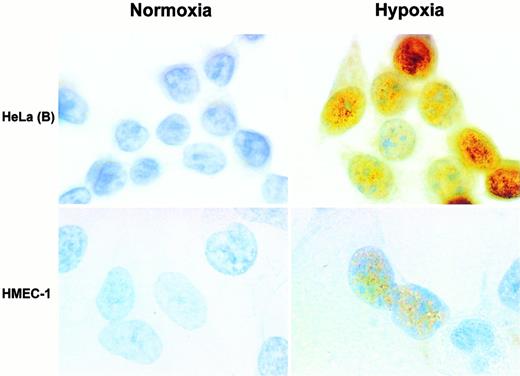
To characterize the regulation of EPAS-1 in more detail, we compared the induction of EPAS-1 and HIF-1α by hypoxia in HeLa(B) cells. These cells were selected because they showed a relatively high level of both proteins in response to 4 hours' exposure to 1% oxygen. It has previously been reported that accumulation of HIF-1α after hypoxic exposure is almost exclusively nuclear.4 Immunoblots of differential extracts of hypoxic HeLa(B) cells showed accumulation of both proteins in the nuclear fraction (data not shown), and immunolabeling of HeLa(B) and HMEC-1 cells grown on glass slides showed nuclear staining of EPAS-1 after hypoxic exposure, with little detectable cytoplasmic staining (Fig 4). Interestingly, as illustrated in hypoxic HMEC-1, the intensity of labeling for EPAS-1 varied from cell to cell; we have also observed this when labeling for HIF-1α.
Immunolabeling for EPAS-1 with MoAb 190b. Cells were exposed to normoxia (left panels) or 1% oxygen (right panels). Upper panels show HeLa(B) and lower panels HMEC-1. Nuclear labeling of variable intensity is seen in the hypoxic cells. Original magnification × 1,000.
Immunolabeling for EPAS-1 with MoAb 190b. Cells were exposed to normoxia (left panels) or 1% oxygen (right panels). Upper panels show HeLa(B) and lower panels HMEC-1. Nuclear labeling of variable intensity is seen in the hypoxic cells. Original magnification × 1,000.
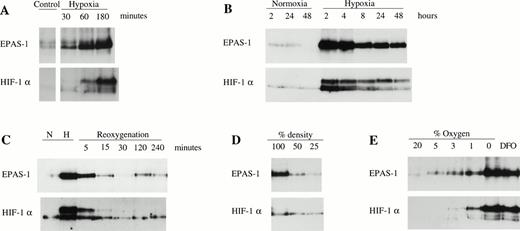
The time course of both HIF-1α and EPAS-1 accumulation was rapid, with a response being detected within 30 minutes of exposure to 1% oxygen (Fig 5A). Levels of both EPAS-1 and HIF-1α increased over the first 2 to 4 hours of exposure (Fig 5A and B), but were somewhat lower with prolonged hypoxia (Fig 5B). After return of hypoxic cultures to normoxic incubation (without changing the culture medium), the levels of both EPAS-1 and HIF-1α decreased very rapidly and were similar to the uninduced level after 30 minutes. Interestingly, following this, a transient reduction in the level of each protein below the normoxic level was consistently observed, suggesting a suppressive effect of reoxygenation (Fig 5C). Figure 5D and E shows the effect of cell density and graded hypoxia on expression of the two proteins. Responses were again similar. The normoxic expression levels were in each case reduced when cells were cultured at low density (25% of confluence) compared with standard density (at or approaching confluence). To test the responses to graded hypoxia, HeLa(B) cells were grown on Petriperm dishes to minimize oxygen gradients between the hypoxic atmosphere and the monolayer. Parallel exposure of near-confluent cells to a range of oxygen tensions induced both proteins, with significant increases in HIF-1α and EPAS-1 at 5% oxygen compared to normoxia, and further increases at 3% and 1% oxygen (Fig 5E). Interestingly, exposure to 5% oxygen appeared to result in a slightly greater increase in EPAS-1 compared to HIF-1α.
Characterization of induction of EPAS-1 and HIF-1α by hypoxia. Immunoblots of HeLa(B) cell whole-cell extracts (100 μg) with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) Cells were exposed to 1% oxygen for 30 to 180 minutes. (B) Cells were exposed to normoxia or 1% oxygen for 2 to 48 hours. (C) Cells were incubated in normoxia (N) or 1% oxygen (H) for 16 hours and harvested at different times following return to normoxia (Reoxygenation). (D) Cells were seeded at different densities and cultured under normoxic conditions. (E) Cells on gas-permeable dishes were exposed to different oxygen concentrations or 100 μmol/L DFO for 6 hours.
Characterization of induction of EPAS-1 and HIF-1α by hypoxia. Immunoblots of HeLa(B) cell whole-cell extracts (100 μg) with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) Cells were exposed to 1% oxygen for 30 to 180 minutes. (B) Cells were exposed to normoxia or 1% oxygen for 2 to 48 hours. (C) Cells were incubated in normoxia (N) or 1% oxygen (H) for 16 hours and harvested at different times following return to normoxia (Reoxygenation). (D) Cells were seeded at different densities and cultured under normoxic conditions. (E) Cells on gas-permeable dishes were exposed to different oxygen concentrations or 100 μmol/L DFO for 6 hours.
Pharmacological modulation of EPAS-1 and HIF-1α levels.
Important insights into the mechanism of oxygen sensing and/or signal transduction underlying HIF-1 activation have been gained from pharmacological interventions which perturb the response as measured either by HIF-1 or target gene activation. To compare further the regulation of EPAS-1 with HIF-1α, we measured the response of each protein to several of these interventions.
Cobaltous ions and iron chelators mimic hypoxia in activation of target genes such as erythropoietin and induce HIF-1α at the protein level.4,19,20 When Hela(B) cells were exposed to cobalt chloride (100 μmol/L) or desferrioxamine (100 μmol/L) a marked increase in EPAS-1 was observed, in parallel with a similar change in the level of HIF-1α (Fig 6A). Interestingly, in certain cell types there were differences betweeen EPAS-1 and HIF-1α in the level of induction achieved by 1% oxygen when compared with these stimuli. For instance, in Hep3B cells EPAS-1 was induced similarly by 1% hypoxia, cobalt chloride, or desferrioxamine (DFO), whereas HIF-1α was induced less effectively by hypoxia than the other stimuli (Fig 6A). In contrast to the effect of these stimuli, exposure to cyanide does not mimic the effect of hypoxia on HIF-1–responsive genes21 and was ineffective in stimulating either EPAS-1 or HIF-1α in the current experiments. In fact, a reduction in the level of both EPAS-1 and HIF-1α were observed when Hela(B) cells were exposed to 1 mmol/L potassium cyanide at either 21% or 1% oxygen (Fig 6B). When cells were exposed to cyanide for 4 hours, washed with fresh medium, and then exposed to hypoxia, the level of HIF-1α and EPAS-1 was similar to hypoxic cells that had not been exposed to cyanide (Fig 6B). This shows that the effect of this dose of cyanide is reversible, and is not simply caused by cell death.
Modulation of EPAS-1 and HIF-1α levels by different chemical agents. Immunoblots of whole-cell extracts (100 μg) with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) HeLa(B) and Hep3B cells were cultured in normoxia (N), 1% oxygen (H), 100 μmol/L DFO, or 100 μmol/L cobaltous chloride (CoCl2). (B) HeLa(B) cells were exposed to normoxia (N) or 1% oxygen (H) in the presence (+) or absence (−) of 1 mmol/L potassium cyanide (KCN). To ascertain that the effect of KCN was not caused by cell death, cells in the right-hand lane [+] were exposed to 1 mmol/L KCN for 4 hours, washed twice, and exposed to 1% oxygen for 4 hours in fresh medium. (C) HeLa(B) cells were exposed to the selective proteasomal inhibitor N-CBZ-Leu-Leu-Norvalinal (CBZ-LLL). (D) HeLa(B) cells were exposed to the antioxidant N-(2-mercaptopropionyl)-glycine (NMPG).
Modulation of EPAS-1 and HIF-1α levels by different chemical agents. Immunoblots of whole-cell extracts (100 μg) with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) HeLa(B) and Hep3B cells were cultured in normoxia (N), 1% oxygen (H), 100 μmol/L DFO, or 100 μmol/L cobaltous chloride (CoCl2). (B) HeLa(B) cells were exposed to normoxia (N) or 1% oxygen (H) in the presence (+) or absence (−) of 1 mmol/L potassium cyanide (KCN). To ascertain that the effect of KCN was not caused by cell death, cells in the right-hand lane [+] were exposed to 1 mmol/L KCN for 4 hours, washed twice, and exposed to 1% oxygen for 4 hours in fresh medium. (C) HeLa(B) cells were exposed to the selective proteasomal inhibitor N-CBZ-Leu-Leu-Norvalinal (CBZ-LLL). (D) HeLa(B) cells were exposed to the antioxidant N-(2-mercaptopropionyl)-glycine (NMPG).
Recently two other types of agent have been shown to stimulate HIF-1α expression. Enhanced HIF-1α immunoactivity and DNA binding activity has been observed in cells treated with peptide aldehyde inhibitors of the proteasome such as N-acetyl-leucinyl-leucinyl-norleucinal (Calpain inhibitor 1) and in cells treated with reducing agents such as N-(2-mercaptopropionyl)-glycine (NMPG).7 9 Although the effect of Calpain inhibitor 1 or the more selective proteasomal inhibitor N-carbobenzoxyl-L-leucinyl-L-leucinyl-L-norvalinal (CBZ-LLL) on HIF-1α abundance was rather variable, in each experiment the effect on HIF-1α and EPAS-1 was very similar (Fig 6C). Figure 6D shows that exposure to NMPG resulted in a similar increase in EPAS-1 and HIF-1α protein levels.
Characteristic reductions in HIF-1α or target gene expression in cells exposed to particular chemicals have also been used to explore the mechanism of oxygen sensing. Thus, hydrogen peroxide reduces the induction of HIF-1 target genes by hypoxia22 and destabilizes HIF-1α.6 In HeLa(B) cells we observed very similar responses of both HIF-1α and EPAS-1 to hydrogen peroxide. Although 100 μmol/L hydrogen peroxide had little effect (data not shown), cells exposed to 250 μmol/L and 1 mmol/L hydrogen peroxide showed marked reductions in both EPAS-1 and HIF-1α levels both in normoxia, and after exposure to hypoxia, cobalt chloride, and DFO (Fig 7A). Suppression of protein levels by exposure to hydrogen peroxide was somewhat greater in cells stimulated by cobalt or DFO when compared with cells stimulated by hypoxia, although in each case the response was again similar for both HIF-1α and EPAS-1.
Effect of hydrogen peroxide and diphenylene iodonium on levels of EPAS-1 and HIF-1α. Immunoblots of whole-cell extracts (100 μg) from HeLa(B) cells with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) Cells were incubated in normoxia (N), 1% oxygen (H), 100 μmol/L DFO or 100 μmol/L cobalt chloride (CoCl2). The presence (+) or absence (−) of 1 mmol/L hydrogen peroxide (H2O2) is indicated. (B) A similar experiment examining the effect of 5 μmol/L DPI.
Effect of hydrogen peroxide and diphenylene iodonium on levels of EPAS-1 and HIF-1α. Immunoblots of whole-cell extracts (100 μg) from HeLa(B) cells with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) Cells were incubated in normoxia (N), 1% oxygen (H), 100 μmol/L DFO or 100 μmol/L cobalt chloride (CoCl2). The presence (+) or absence (−) of 1 mmol/L hydrogen peroxide (H2O2) is indicated. (B) A similar experiment examining the effect of 5 μmol/L DPI.
In previous studies we have shown that diphenylene iodonium (DPI), an inhibitor of flavoprotein oxidodreductases, will abolish the induction of HIF-1 target genes by hypoxia.23 Interestingly, we found that doses of DPI which produced almost complete inhibition of the response to hypoxia had little effect on the response of the same genes to cobaltous ions or iron chelation. Therefore, we examined the effects of DPI on HIF-1α and EPAS-1 protein levels. DPI abolished the induction of both HIF-1α and EPAS-1 by hypoxia but, in contrast, had little effect on the induction of either molecule by cobaltous ions or DFO (Fig 7B).
Transactivation of hypoxia-responsive promoters by HIF-1α and EPAS-1.
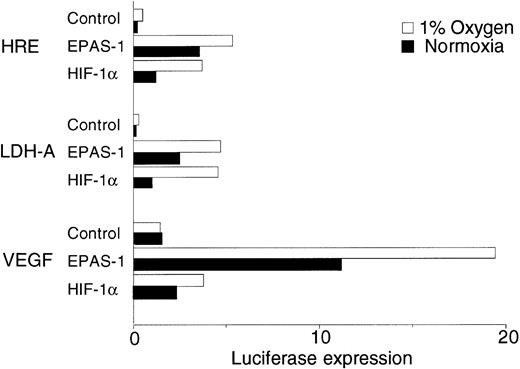
The above results show that despite wide variation in expression, HIF-1α and EPAS-1 show rather similar inducible responses. We next compared their ability to transactivate different hypoxia inducible promoters using a mutant CHO cell line (Ka13) which expresses neither HIF-1α nor EPAS-1 at detectable levels.15 In keeping with previous work, the activity of transiently transfected p(24x6)TKLuc, containing a multimerized hypoxia response element (HRE) adjacent to a minimal thymidine kinase promoter, was close to absent in the mutant Ka13 cells, and markedly enhanced by cotransfection of either EPAS-1 or HIF-1α expression plasmids (Fig 8). In contrast, two promoters without known hypoxia-inducible activity (the weak minimal TK promoter and the powerful CMV enhancer/promoter) showed preserved activity in mutant Ka13 cells and no response to cotransfection of EPAS-1 or HIF-1α (data not shown). We next tested activity on two hypoxia-inducible native promoters (from the LDH-A and VEGF genes). Interestingly, the LDH-A promoter had almost no activity in the mutant Ka13 cells, indicating that HRE function was critical. Cotransfection of either EPAS-1 or HIF-1α markedly stimulated activity. Responses were similar to those observed with the multimerized HRE, with EPAS-1 expression showing rather higher activity than HIF-1α, particularly in normoxic cells. In contrast, the VEGF promoter had substantial activity in mutant Ka13 cells. This activity was again considerably enhanced by cotransfection of either EPAS-1 or HIF-1α. However, the relative activities of the two molecules were quite different on this promoter. The ratio of EPAS-1 to HIF-1α activity with the VEGF promoter was considerably greater than that observed with either the LDH-A promoter or the simple multimerized HRE.
Transactivation by EPAS-1 and HIF-1α in Ka13 cells. Histogram showing the relative luciferase expression from transient cotransfections of CHO Ka13 cells with reporter plasmids containing a multiple HRE (p24x6TKLuc), the LDH-A promoter (pLDH-ALuc), or the VEGF promoter (pVEGFLuc) and an expression plasmid for either HIF-1α, EPAS-1, or an empty vector control. Values shown represent averaged data from seven experiments, with normalization to the normoxic value for LDH-A (1 arbitrary unit).
Transactivation by EPAS-1 and HIF-1α in Ka13 cells. Histogram showing the relative luciferase expression from transient cotransfections of CHO Ka13 cells with reporter plasmids containing a multiple HRE (p24x6TKLuc), the LDH-A promoter (pLDH-ALuc), or the VEGF promoter (pVEGFLuc) and an expression plasmid for either HIF-1α, EPAS-1, or an empty vector control. Values shown represent averaged data from seven experiments, with normalization to the normoxic value for LDH-A (1 arbitrary unit).
DISCUSSION
The identification of EPAS-1, a bHLH-PAS protein with a high degree of homology to HIF-1α, has raised the possibility that this might also be involved in adaptive responses to changes in oxygen tension. The initial reports showed that in the presence of ARNT the protein can form an HRE-binding complex, and that EPAS-1 can alter transcription driven by DNA sequences known to respond to hypoxia.11-13,15 To date, expression has only been studied at the mRNA level with several groups reporting analysis of whole-organ homogenates and in situ hybridization.11-14 These studies emphasized endothelial expression in developing rodent embryos, although other cell types including pulmonary epithelium and renal mesangial cells were also shown to express EPAS-1 mRNA.
Because the functional activation of the related bHLH-PAS family member HIF-1α involves major changes in the amount of protein,4which in most studies occur independently of any changes in mRNA levels,6 24 we developed MoAbs allowing specific detection of EPAS-1 protein. We examined expression in a range of cell types both at the RNA level by RNAse protection, and at the protein level by immunoblotting. EPAS-1 mRNA was detected in all 11 human cell lines examined, of which 10 were not endothelial. When cells were exposed to 1% oxygen, a major increase in EPAS-1 protein but not EPAS-1 mRNA was observed. The accumulation of EPAS-1 in hypoxia clearly supports a role in inducible responses to changes in oxygen tension, and raises the question as to the extent to which EPAS-1 and HIF-1α responses are similar and how they differ.
Therefore, we assayed these two proteins in parallel in extracts of HeLa(B) cells exposed to a range of experimental conditions known to activate HIF-1α. Overall, we observed very similar responses. Both proteins showed a similar time course of accumulation after hypoxic exposure, and a rapid decrease in protein level on return to 20% oxygen. For both molecules, accumulation also occurred following exposure of normoxic cells to cobaltous ions, desferrioxamine, the antioxidant NMPG, and peptide aldehyde inhibitors of the proteasome. Cyanide did not mimic these responses and decreased the levels of both proteins. Exposure of cells to hydrogen peroxide and to the flavoprotein inhibitor DPI reduced the levels of both proteins. Thus, across a range of experimental conditions previously shown to influence levels of HIF-1α, we observed concordance between the HIF-1α and EPAS-1 responses. Not only do both molecules respond to hypoxia, but it appears that the same, or strikingly similar, oxygen-sensing and signal transduction mechanisms (probably involving redox modulation of proteasomal destruction) regulate the abundance of both transcription factors.
In addition to providing comparative data on regulation of HIF-1α and EPAS-1, these experiments also offer some new insights into the underlying regulatory mechanisms. EPAS-1 protein (and to a lesser extent HIF-1α) could be readily detected in normoxic cells. Such levels could be suppressed by inhibitors such as hydrogen peroxide, and following re-oxygenation they were transiently depressed below the levels in cultures held in normoxia. Because re-oxygenation is frequently associated with enhanced oxygen radical production, this could be in keeping with the hypothesis that the oxygen sensitive signal is related to the cellular level of these species.22In studying the inhibitors of the response we also compared their action on hypoxia-induced protein accumulation with their action on responses to cobaltous ions and desferrioxamine. As we observed previously for the action of DPI on HIF-1 target gene expression,23 the action was stimulus specific, with induction by cobaltous ions and desferrioxamine being relatively resistant to DPI inhibition. Such an action might be explained if DPI were to enhance the generation of radicals whose effects on the signal pathway were blocked by iron chelation—for instance, by interference with Fenton chemistry. Interestingly, although such an argument has been advanced for interactions between inhibition of erythropoietin expression by hydrogen peroxide and stimulation by desferrioxamine,25 we did not find that activation of EPAS-1 or HIF-1α by desferrioxamine was resistant to hydrogen peroxide. Rather, we found the reverse, with hydrogen peroxide being more inhibitory to cells treated with desferrioxamine.
Also of note was the effect of cell density on both EPAS-1 and HIF-1α expression. Cell cultures at higher density consistently expressed higher levels of both proteins—an effect which might explain previous observations that certain HIF-1 target genes are expressed at higher levels as cells approach confluence, and that HRE activity (as assessed by transiently transfected reporter gene expression) is greater when transfected cells are grown at higher densities.2
Despite the similarities in HIF-1α and EPAS-1 responses, differences were observed. First, there were large differences in expression levels between cell lines. Although an endothelial cell line did express high levels of EPAS-1, no clear pattern was discernible, with related cell lines expressing quite different levels of one or other protein. Second, with the proviso that immunoblotting can give only approximate quantitation of protein levels, there appeared to be subtle differences in the inducible response. Overall, in cells that expressed both proteins the normoxic expression of EPAS-1 was somewhat more pronounced than that of HIF-1α (particularly the more hypoxically inducible high MW species). When comparing HIF-1α and EPAS-1 induction in HeLa(B) cells, it appeared that EPAS-1 may be induced by slightly less severe hypoxia (Fig 5E). Although the thresholds for target gene activation by HIF-1α and EPAS-1 might differ, these data would be consistent with EPAS-1 being a more important modulator under normoxic and less severely hypoxic conditions.
Other differences were observed in the transactivation studies. To compare the activity of each gene on different target sequences, we used a cell line deficient in both EPAS-1 and HIF-1α (Ka13), thus avoiding the potential complexity of interactions between the two molecules. Consistent with previous reports,11-13,15 both EPAS-1 and HIF-1α transactivated a multimerized minimal HRE, with EPAS-1 showing more activity in normoxic cells and less induction by hypoxia than HIF-1α. Comparison of the relative activities of the two molecules on the LDH-A and VEGF promoters indicated further differences in target gene specificity. Although both transcription factors activated both promoters, EPAS-1 was relatively more active on the VEGF promoter. Despite near-identical bHLH domains for EPAS-1 and HIF-1α, similar target gene selectivity has been reported by others with EPAS-1 but not HIF-1α activating the Tie-2 enhancer/promoter.11Consistent with the endothelium-restricted expression of Tie-2 we found these sequences to be inactive and unresponsive to either EPAS-1 or HIF-1α in Chinese hamster ovary (CHO) cells (data not shown), suggesting that cooperative interactions with other cell-specific proteins are necessary for target-specific transactivation. Interestingly, domain exchange experiments among DrosophilabHLH-PAS proteins have indicated a role for the PAS domain in such interactions.26
In conclusion, EPAS-1 is a second hypoxia-inducible transcription factor which shares extensive similarities in its mode of regulation with HIF-1α and the suggestion that this widely expressed protein be termed HIF-2α seems appropriate.27 Definition of regulatory domains in EPAS-1 and sequence comparison of these with HIF-1α should be useful in defining features that interact with the signal transduction mechanism. It is likely that differences in the cell-specific expression, induction, and target gene specificities of these two systems will be important in our understanding of the diversity of adaptive responses to oxygen availability.
ACKNOWLEDGMENT
The authors thank S. McKnight for pEP-1, E. Raybould for p(24x6)TKLuc, N. Bacon for the EPAS-1 riboprobe, and R. Bicknell for HMEC-1.
Supported by research grants from the Wellcome Trust, the Medical Research Council (UK), the Imperial Cancer Research Fund, the British Council/German Academic Exchange Service, and the Deutsche Forschungsgemeinschaft.
Address reprint requests to P.H. Maxwell, DPhil, Wellcome Trust Centre for Human Genetics, Windmill Rd, Oxford OX3 7BN, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 6. Modulation of EPAS-1 and HIF-1α levels by different chemical agents. Immunoblots of whole-cell extracts (100 μg) with MoAb 190b (upper panels) and MoAb 28b (lower panels). (A) HeLa(B) and Hep3B cells were cultured in normoxia (N), 1% oxygen (H), 100 μmol/L DFO, or 100 μmol/L cobaltous chloride (CoCl2). (B) HeLa(B) cells were exposed to normoxia (N) or 1% oxygen (H) in the presence (+) or absence (−) of 1 mmol/L potassium cyanide (KCN). To ascertain that the effect of KCN was not caused by cell death, cells in the right-hand lane [+] were exposed to 1 mmol/L KCN for 4 hours, washed twice, and exposed to 1% oxygen for 4 hours in fresh medium. (C) HeLa(B) cells were exposed to the selective proteasomal inhibitor N-CBZ-Leu-Leu-Norvalinal (CBZ-LLL). (D) HeLa(B) cells were exposed to the antioxidant N-(2-mercaptopropionyl)-glycine (NMPG).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/7/10.1182_blood.v92.7.2260/3/m_blod41944006w.jpeg?Expires=1763491704&Signature=ubuITVjbrHPUxLKy2K90l-hE49JDpwC8rBHoQ2trEwRSM4FYbTLzlfaQf3B9OZ~dApbjmAJif1kAxg~LvWF071YRLc9r1gL6g2jrURHWHHfXAbzgjXWL3DP~gOAfLmN7oS6Szx6QVyZp4mgunIs7WMQ2lDauwvkwhK1jsz1TPLYATEBDtu2IJXC-HOdMyhptkz8-4BUt4prjaZhkEKB29IY0QkhgqMoMjqnjG~-HiBS7eTPhfa2faXQ5JBTLx26GAdGcrCRd8SMH46Hi2xTqTk5vZvLZoZaRrflBYjIVD7COW~BVairvTtjL4Zj7Jqd9nu09nM1s4rMb7~GlD1mmkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal