Abstract
Microcytic anemia (mk) mice and Belgrade (b) rats have severe iron deficiency anemia due to defects in intestinal iron transport and erythroid iron utilization. Both animal mutants carry the same missense mutation in Nramp2, the first mammalian iron transporter to be identified. This mutation, in which glycine 185 is changed to arginine (G185R), occurs within predicted transmembrane domain 4 of the protein. We have performed site-directed mutagenesis of murine Nramp2, focusing on amino acids of transmembrane domain 4 that are highly conserved among Nramp-like proteins. We have expressed each mutant form in transfected cells and examined iron transport function, subcellular localization, and protein amounts. All tested forms of Nramp2 localize to the plasma membrane and to transferrin-containing endosomes. Most transmembrane domain 4 mutations affect the amount of protein detected and consequently show diminished iron transport. The G185R mutation, however, causes near total loss of Nramp2 function that cannot be fully explained by a decreased amount of protein, indicating that G185R disrupts iron transport through an alteration in the function of Nramp2, rather than degradation of the protein.
© 1998 by The American Society of Hematology.
ANIMAL MODELS HAVE served as important tools for understanding mammalian iron metabolism. The microcytic anemia (mk) mouse and the Belgrade (b) rat have inherited defects in iron transport that result in iron deficiency anemia.1-8 Their phenotypes are similar in that both red blood cell iron uptake and intestinal iron transport are affected. It is known that these two processes occur by distinct mechanisms – erythroid iron uptake involves receptor-mediated endocytosis of diferric transferrin, while intestinal iron absorption does not. However, these two processes are likely to share a common step, the actual transfer of iron across the plasma membrane. We have recently shown that mk and b animals both have missense mutations in the gene encoding Nramp2, an integral membrane protein whose biological function was previously unknown.9,10 The mutations have created the same amino acid substitution, converting a glycine residue to arginine (G185R). This provided compelling evidence that Nramp2 is important for iron transport. Additionally, biochemical studies of Nramp2 function in microinjected Xenopus oocytes indicate that Nramp2 acts as a proton-coupled transmembrane metal transporter.11 These findings strongly implicate Nramp2 as the apical iron transporter on intestinal epithelial cells and as the endosomal transporter in transferrin-cycle endosomes.
Nramp2 was initially described as a homolog of Nramp1, a protein involved in host defense.12 The Nramp proteins are the vertebrate representatives in a small family of related proteins conserved across species ranging from bacteria to man. Nramp1 is a determinant of resistance to intracellular pathogens.13Animals carrying the Nramp1-sensitive (bcg) allele are particularly susceptible to infection, owing to an inability of macrophages to attenuate pathogen replication within phagolysosomes.14 The bcg allele contains a missense mutation in which a glycine residue is converted to an aspartic acid (G169D). Until recently, the mechanism of action of Nramp1 was unknown. However, the fact that Nramp2 transports metals suggests that Nramp1 and other Nramp-related proteins may also be metal transporters. This assertion is further supported by data indicating that a yeast homolog, Smf1, transports manganese.15
Nramp proteins are predicted to have 12 transmembrane domains. Strikingly, the mk and b mutation in Nramp2and the bcg mutation in Nramp1 have occurred at adjacent, conserved residues within predicted transmembrane domain 4 (TMD4) of the proteins. When Nramp1 and Nramp2 are aligned based on homology, the bcg mutation (G169D) in Nramp1 corresponds to position 184 in Nramp2. This position is immediately upstream of themk and b mutation (G185R) in Nramp2.9 10This remarkably narrow spectrum of disease-causing mutations in rodent Nramp proteins suggests that TMD4 may serve a unique and important biologic function.
In the current study, we establish that the G185R mutation is functionally deleterious. While wild-type Nramp2 stimulates iron accumulation 75-fold, the mutant protein stimulates iron accumulation only twofold when assayed in transfected cells. A G184D mutation, analogous to the bcg mutation in Nramp1, but constructed in Nramp2, is also deleterious for iron transport. Other mutations within TMD4 have milder effects. We have considered possible mechanisms by which these TMD4 mutations may alter function of Nramp2. To address the possibility that these mutations affect subcellular localization, we first established the expression pattern of wild-type Nramp2 in transfected cells. Wild-type Nramp2 is expressed both on the plasma membrane and in endosomes, where it colocalizes with transferrin. This localization pattern is consistent with the hypothesis that Nramp2 functions both as the apical membrane iron transporter in intestinal cells and the endosomal iron transporter in erythroid cells. All TMD4 mutations in Nramp2 show an identical subcellular distribution, indicating that these mutations do not interfere with localization. The mutations do, however, appear to result in decreased Nramp2 protein on the cell surface. Decreased levels of mutant protein thus appear to contribute to loss of function. Loss of function of the G185R mutation, however, is too severe to be explained by diminished protein levels. Rather, this mutation appears to alter Nramp2 in a way that severely impairs its iron transport capability.
MATERIALS AND METHODS
Construction and Mutagenesis of Expression Constructs
The coding sequence of murine Nramp2 was cloned by reverse transcription-polymerase chain reaction (RT-PCR) from mouse spleen poly-A RNA and inserted as an EcoRI fragment into the eukaryotic expression vector pMT2. A FLAG epitope tag (Eastman-Kodak, Rochester, NY) was inserted in frame at the 5′ or 3′ end of the coding sequence to produce an Nramp2-FLAG fusion protein. Because initial iron transport and immunofluorescence results were identical with the two FLAG-tagged forms (data not shown), mutagenesis experiments were performed using a 3′ FLAGtagged version of the protein. Site-directed mutagenesis was performed using the QuikChange kit (Stratagene, La Jolla, CA), which uses reverse complement oligonucleotides to amplify circular plasmids. The sense strand oligonucleotides are: 5′GGGTCCCCCTGTGGGGCAGAGTCCTCA TCACCATC3′ for G185R; 5′GGGTCCCCCTGTGGGGCTCAGTCCTCATCACCATCG3′ for G185S; 5′CCTCATCACCATCGCAGGCACTTTTGTGTTTCTTT3′ for A192G; 5′CCTCATCACCATCGCAAACACTTTTGTGTTTCTTT3′ for A192N; 5′CCTCATCACCATCGCAGAAACTTTTGTGTTTCTTT3′ for A192E; 5′AAGGGTCCCCCTGTGGGACGGAGTCCTCATCACCA3′ for G184D (changes from wild-type are underlined). Constructs were confirmed by direct DNA sequence analysis.
Cell Culture and Transfection
Human embryonic kidney (HEK293T) cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO/BRL, Gaithersburg, MD) with 10% fetal calf serum (BioWhittaker, Walkersville, MD), 1% penicillin/streptomycin, and 1% L-glutamine and transfected by calcium phosphate precipitation, as previously described.10 All transfections were performed in 90 mm plates. All plates were seeded 18 hours before transfection with 5 × 106 cells/plate.
Iron Uptake Assay
Iron uptake in HEK293T cells was analyzed 48 hours after transfection, as previously described.10 Briefly, transfected cells were washed twice with 10 mL phosphate-buffered saline (PBS) prewarmed to 37°C and incubated in 10 mL incubation buffer (25 mmol/L Tris, 25 mmol/L 2-[N-morpholino] ethane sulfonic acid [MES], 140 mmol/L NaCl, 5.4 mmol/L KCl, 5 mmol/L glucose, 1.8 mmol/L CaCl2, 800 μmol/L MgSO4, pH 6.0) with 50 μmol/L ascorbic acid and 1 μmol/L 55Fe-nitrilotriacetic acid (55Fe-NTA) for 20 minutes at 37°C. At least 70% of the iron was shown to be in the ferrous state in these conditions (data not shown). Cells were removed from the plate by digestion with 1 mL of .25% trypsin-EDTA (GIBCO/BRL) and washed three times with 20 mL ice-cold PBS. 55Fe uptake was determined by resuspending the cell pellet in 1 mL of distilled water and measuring radioactivity by liquid scintillation. Iron uptake was identical for expressed Nramp2 lacking an epitope tag, and both 5′ and 3′ FLAG-tagged versions of the protein (data not shown). There was no difference in iron uptake between cells transfected with the antisense control construct used in these experiments and expression constructs carrying irrelevant cDNAs. This confirmed that the antisense murine cDNA did not inhibit expression of endogenous human Nramp2 in HEK293T cells (data not shown).
Immunofluorescence
Twenty-four hours posttransfection, HEK293T cells were split 1:10 and cultured on poly-D-lysine–coated coverslips. Forty-eight hours posttransfection, the coverslips were incubated with 50 μg/mL human transferrin conjugated to Texas Red (Molecular Probes, Eugene, OR) for 45 minutes at 37°C. After washing with PBS, coverslips were fixed in 3% paraformaldehyde, washed, and incubated in 50 mmol/L NH4Cl in PBS for 15 minutes. The cells were then permeabilized with 0.1% saponin, washed, and incubated with anti-FLAG antibody (Kodak; 1:5,000 dilution in 2 mmol/L sodium azide, 2% ovalbumin in PBS) for 30 minutes at 37°C. After washing, they were incubated with fluorescein isothiocyanate (FITC)-conjugated donkey antimouse antibody (Jackson Immunoresearch, West Grove, PA, 1:200 dilution in 2 mmol/L sodium azide, 2% ovalbumin in PBS) for 30 minutes at 37°C. Cells were washed, and coverslips were mounted in gelvatol (Monsanto, St Louis, MO).
Biotinylation and Protein Blotting
HEK293T cells were seeded on poly-D-lysine–coated 90 mm plates for transfection. Forty-eight hours posttransfection, cells were washed three times in cold PBS and then incubated for 30 minutes at 4°C with 5 mL of fresh biotin solution (0.5 mg/mL sulfo-NHS-LC biotin [Pierce Chemicals, Rockford, IL] in PBS with 0.1 mmol/L CaCl2, 1 mmol/L MgCl2). The sulfo-NHS-LC biotin reagent is membrane impermeable, so that only extracellular proteins are labelled. Unincorporated biotin was removed by three washes with cold PBS, and cells were lysed by the addition of 2 mL of RIPA buffer (50 mmol/L Tris-HCl pH 7.5, 1% Nonidet-P40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mmol/L NaCl, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF]) in the presence of 12.5 μg/mL leupeptin and 5 μg/mL pepstatin. Unsolubilized material was removed by microcentrifugation. A total of 400 μL of soluble lysate was precleared with 100 μL of 10% protein G-sepharose (Pharmacia, Uppsala, Sweden) in RIPA buffer, and then incubated with 1 μL anti-FLAG antiserum (Kodak) for 1 hour at 4°C. Immunoprecipitates were collected by incubation with 100 μL fresh 10% protein G-sepharose in RIPA buffer for 1 to 2 hours at 4°C, washed three times with RIPA buffer, boiled in sample buffer containing SDS and dithiothreitol, and fractionated on 10% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membranes and probed with streptavidin-horseradish peroxidase (HRP) to detect biotin. Proteins were visualized using enhanced chemiluminescence (ECL kit, Amersham, Arlington Heights, IL).
RESULTS
Mutations within TMD4 interfere with iron transport.
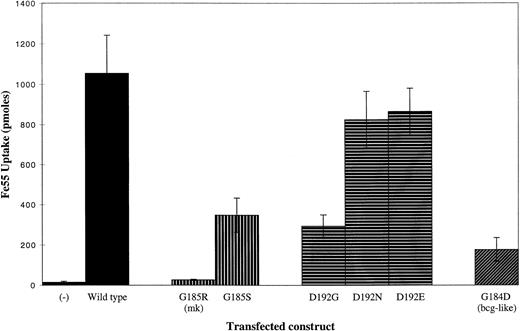
The G185R mutation found in mk and b animals results in the substitution of a large, basic residue for a small, nonpolar residue. To determine the functional consequences of the G185R mutation, we introduced wild-type and mutant forms of murine Nramp2 into HEK293T cells and studied their capacities for iron transport. Forty-eight hours after transfection, HEK293T cells were incubated for 20 minutes in pH 6.0 incubation media with 1 μmol/L55Fe-NTA in the presence of ascorbate. An antisenseNramp2 cDNA contained in the same expression vector was used as a negative control; it had no effect on basal HEK293T cell iron transport. Forced expression of wild-type Nramp2 stimulates iron uptake approximately 75-fold above basal levels (Fig 1). In contrast, expression of murine Nramp2 carrying the G185R mutation leads to a 35-fold decrease in iron uptake compared with wild-type. Substitution of a less bulky, polar residue, serine, for glycine 185 (G185S) inhibits iron transport to a moderate extent (Fig 1).
Iron transport activity of wild-type and mutant forms of Nramp2. HEK293T cells were transfected with the constructs indicated and uptake of 55Fe (in pmol) was measured. Cells were incubated for 20 minutes in pH 6.0 incubation buffer with 50 μmol/L ascorbate and 1 μmol/L 55Fe-NTA. (−) Indicates iron uptake by cells transfected with an antisense control, in which the murine Nramp2 cDNA was subcloned into pMT2 in a noncoding orientation.
Iron transport activity of wild-type and mutant forms of Nramp2. HEK293T cells were transfected with the constructs indicated and uptake of 55Fe (in pmol) was measured. Cells were incubated for 20 minutes in pH 6.0 incubation buffer with 50 μmol/L ascorbate and 1 μmol/L 55Fe-NTA. (−) Indicates iron uptake by cells transfected with an antisense control, in which the murine Nramp2 cDNA was subcloned into pMT2 in a noncoding orientation.
To begin to define the mechanism of loss of function in G185R Nramp2, we introduced a mutation at aspartate 192 (D192). This residue is invariant among Nramp-like proteins and is unusual in that it is a charged residue within a transmembrane domain. In a helical wheel projection of the predicted alpha helix formed by Nramp2 transmembrane domain 4, D192 lies in close proximity to G185 (Fig 2). We considered the possibility that the G185R mutation results in a novel ionic interaction between R185 and D192, preventing D192 from carrying out a function that is essential for iron transport. Our results indicate that this is unlikely, however, because mutations of D192 to glycine (D192G), asparagine (D192N), and glutamate (D192E) are less deleterious than the G185R mutation (Fig 1). To create an Nramp2 gene analogous to the bcg allele of Nramp1, we replaced glycine 184 with aspartate (G184D). This mutation reduces iron transport sixfold compared with wild-type Nramp2 (Fig 1).
Helical wheel representation of predicted TMD4 of Nramp1 and Nramp2. The structure of TMD4 of both Nramp1 and Nramp2 is represented as a helical wheel. The Nramp2 residue is given first when amino acid differences exist between Nramp1 and Nramp2. The positions of the G185R Nramp2 mutation in mk mice and the G169D Nramp1 mutation in bcg mice (corresponding to G184 in Nramp2) are shown. G185 is indicated with a gray circle and D192, which lies close to G185 in this projection, is indicated with a black circle.
Helical wheel representation of predicted TMD4 of Nramp1 and Nramp2. The structure of TMD4 of both Nramp1 and Nramp2 is represented as a helical wheel. The Nramp2 residue is given first when amino acid differences exist between Nramp1 and Nramp2. The positions of the G185R Nramp2 mutation in mk mice and the G169D Nramp1 mutation in bcg mice (corresponding to G184 in Nramp2) are shown. G185 is indicated with a gray circle and D192, which lies close to G185 in this projection, is indicated with a black circle.
To determine whether proteins carrying the G185R mutation can interfere with the function of wild-type Nramp2 protein in a dominant manner, cotransfection experiments were performed combining the two expression constructs. Cotransfection of equal amounts of wild-type Nramp2 and the G185R mutant construct resulted in iron transport at a level corresponding to the sum of the activity from each of the constructs alone (data not shown). This suggests that the G185R Nramp2 does not interact with wild-type Nramp2, as there is no evidence of a subunit poisoning effect from coexpression of mutant proteins. We conclude that it is unlikely that Nramp2 functions as a homomultimer.
Wild-type Nramp2 colocalizes with internalized transferrin and is expressed on the plasma membrane. The mechanisms by which the TMD4 mutants abrogate iron transport were further investigated by determining whether the mutations affect subcellular localization. We first established the subcellular localization of wild-type Nramp2. To do this, HEK293T cells were transfected with pMT2 constructs expressing epitope-tagged Nramp2, and Nramp2 was localized using an antiepitope antibody. We confirmed that addition of epitope tags had no effect on the iron transport activity of the expressed wild-type and mutant proteins (data not shown).
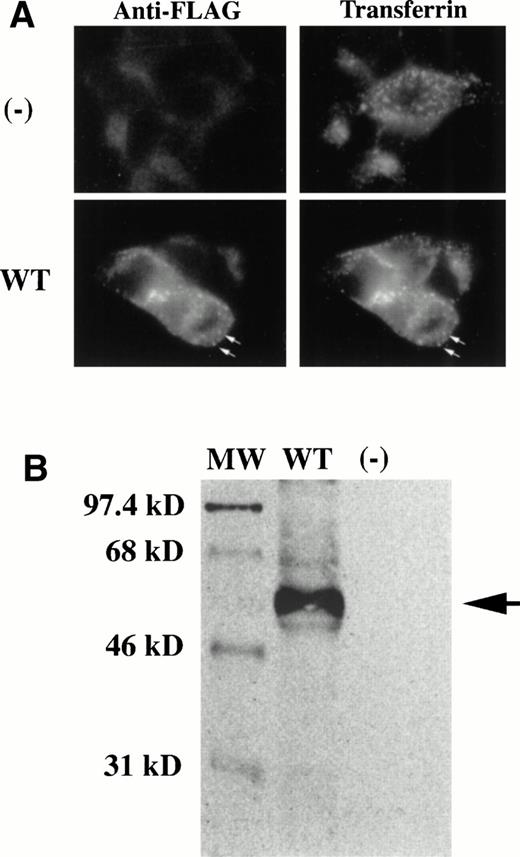
Because the b rat has been shown to have a defect in endosomal iron transport7,16 and is associated with the G185R mutation in Nramp2,10 we hypothesized that wild-type Nramp2 is present in transferrin cycle endosomes. Immunofluorescence was used to demonstrate that this is the case. The staining pattern for Nramp2, determined using a specific antiserum against the epitope tag on Nramp2, coincides with the fluorescence pattern of internalized Texas-red transferrin (Fig 3A). To confirm that the staining was specific for Nramp2, we demonstrated that cells transfected with Nramp2 in the antisense orientation are not stained. Nramp2 also colocalizes with the transferrin receptor as detected by immunofluorescence staining (data not shown). Furthermore, the punctate peripheral staining pattern of Nramp2 is consistent with the presence of Nramp2 in early transferrin cycle endosomes.
Wild-type Nramp2 colocalizes with cell-associated transferrin and is expressed on the cell surface. Expression constructs containing epitope-tagged Nramp2 cDNAs were transfected into HEK293T cells. (A) Two days posttransfection, cells were allowed to take up Texas-red transferrin, and then permeabilized for FITC immunostaining of the FLAG-Nramp2 proteins. Cells transfected withNramp2 in the antisense (-) orientation (upper left panel) did not stain for FLAG and served as a negative control. These cells did, however, take up Texas-red transferrin as expected (upper right panel). Cells transfected with FLAG-Nramp2 in the sense orientation show colocalization of Nramp2 protein with transferrin (bottom panels). Arrows highlight similarities in punctate staining of intracellular structures. (B) Two days posttransfection, cells were incubated with a membrane-impermeable form of biotin to label cell surface proteins. Cells were then solubilized in RIPA buffer, and FLAG-Nramp2 was immunoprecipitated using a specific antibody against the FLAG epitope. Proteins were resolved on a 10% SDS-polyacrylamide gel, transferred, and probed with streptavidin-HRP to detect biotin using enhanced chemiluminescence. Cells transfected with FLAG-Nramp2 in the sense direction resulted in abundant immunoprecipitation product (lane 2, arrow). Cells transfected with Nramp2 in the antisense direction did not produce any immunoprecipitation product (lane 3). Sizes of molecular weight standards (lane 1) are indicated on the left. In this system, we consistently find that the apparent molecular weight of Nramp2 is smaller than the predicted molecular weight of 63 kD.
Wild-type Nramp2 colocalizes with cell-associated transferrin and is expressed on the cell surface. Expression constructs containing epitope-tagged Nramp2 cDNAs were transfected into HEK293T cells. (A) Two days posttransfection, cells were allowed to take up Texas-red transferrin, and then permeabilized for FITC immunostaining of the FLAG-Nramp2 proteins. Cells transfected withNramp2 in the antisense (-) orientation (upper left panel) did not stain for FLAG and served as a negative control. These cells did, however, take up Texas-red transferrin as expected (upper right panel). Cells transfected with FLAG-Nramp2 in the sense orientation show colocalization of Nramp2 protein with transferrin (bottom panels). Arrows highlight similarities in punctate staining of intracellular structures. (B) Two days posttransfection, cells were incubated with a membrane-impermeable form of biotin to label cell surface proteins. Cells were then solubilized in RIPA buffer, and FLAG-Nramp2 was immunoprecipitated using a specific antibody against the FLAG epitope. Proteins were resolved on a 10% SDS-polyacrylamide gel, transferred, and probed with streptavidin-HRP to detect biotin using enhanced chemiluminescence. Cells transfected with FLAG-Nramp2 in the sense direction resulted in abundant immunoprecipitation product (lane 2, arrow). Cells transfected with Nramp2 in the antisense direction did not produce any immunoprecipitation product (lane 3). Sizes of molecular weight standards (lane 1) are indicated on the left. In this system, we consistently find that the apparent molecular weight of Nramp2 is smaller than the predicted molecular weight of 63 kD.
Before being recruited into endosomes, the transferrin receptor is first expressed on the cell surface. This expression pattern, along with the observation that the mk mouse has an apical intestinal iron absorption defect, led us to hypothesize that Nramp2 is also expressed on the plasma membrane. To test this, we treated intact, transfected cells with a membrane-impermeable form of biotin that labels only the extracellular portions of membrane proteins. The biotinylation reaction was stopped, cells were lysed, and proteins were solubilized with detergents. Specific antiserum recognizing the FLAG epitope on tagged Nramp2 was used for immunoprecipitation. Immunoprecipitated protein was fractionated on a denaturing gel, transferred to nitrocellulose, and probed with streptavidin linked to horseradish peroxidase to detect biotin. Nramp2 was easily detected, indicating that it was present on the plasma membrane at the time of biotinylation (Fig 3B). This assay is specific for Nramp2, as protein was not detected in cells transfected with Nramp2 in the antisense (-) orientation. To ensure that only cell-surface proteins are detected, an epitope-tagged version of a p45-MafG heterodimer that is expressed only in the nucleus was treated similarly, and no biotinylated protein was detected (data not shown).
TMD4 mutant forms of Nramp2 localize appropriately, but are decreased in amount.
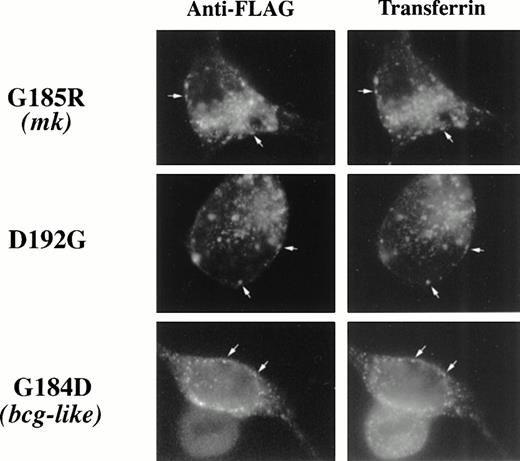
Having established that wild-type Nramp2 is expressed in transferrin cycle endosomes and on the plasma membrane, we sought to determine whether TMD4 mutations affect this pattern. Subcellular localization experiments were repeated using epitope-tagged G185R, D192G, and G184D mutant forms of Nramp2 in place of wild-type Nramp2. The staining patterns of mutant epitope-tagged Nramp2 proteins and Texas Red-transferrin were once again coincident (Fig 4), indicating that these mutations do not alter localization to the transferrin cycle endosome. Additionally, these TMD4 mutations do not appear to alter localization to the plasma membrane. Biotinylated Nramp2 could be detected on cells transfected with all three of these TMD4 mutants (Fig5), indicating that the mutant Nramp2 is still inserted into the plasma membrane. Because different techniques were used to detect endosomal and cell surface proteins, we cannot accurately determine the relative amounts of protein in each location. We would expect, however, that only cell surface protein would contribute to the nontransferrin bound iron uptake measured in our iron transport assay.
TMD4 mutations in Nramp2 do not alter localization to transferrin cycle endosomes. Epitope-tagged G185R, G184D, and D192GNramp2 cDNA mutant constructs were transfected into HEK293T cells and cells were treated as described in Fig 3A. All three mutant forms of Nramp2 show colocalization with Texas-red transferrin.
TMD4 mutations in Nramp2 do not alter localization to transferrin cycle endosomes. Epitope-tagged G185R, G184D, and D192GNramp2 cDNA mutant constructs were transfected into HEK293T cells and cells were treated as described in Fig 3A. All three mutant forms of Nramp2 show colocalization with Texas-red transferrin.
TMD4 mutations in Nramp2 do not alter localization to the plasma membrane, but result in decreased protein levels. Epitope-tagged wild-type, G185R, G184D, and D192G Nramp2 cDNA mutant constructs were transfected into HEK 293T cells, and cells were treated as described in Fig 3B. To quantitate the reduction in mutant protein levels, the cell lysate from cells transfected with wild-typeNramp2 was diluted 1:4, 1:10, and 1:40 in RIPA buffer. The amount of undiluted protein (lane 2) and diluted protein (lanes 3 to 5) was then compared with the amount of undiluted mutant protein detected (lanes 7 to 9). The antisense (-) control is shown in lane 6. Sizes of molecular weight standards (lane 1) are indicated on the left.
TMD4 mutations in Nramp2 do not alter localization to the plasma membrane, but result in decreased protein levels. Epitope-tagged wild-type, G185R, G184D, and D192G Nramp2 cDNA mutant constructs were transfected into HEK 293T cells, and cells were treated as described in Fig 3B. To quantitate the reduction in mutant protein levels, the cell lysate from cells transfected with wild-typeNramp2 was diluted 1:4, 1:10, and 1:40 in RIPA buffer. The amount of undiluted protein (lane 2) and diluted protein (lanes 3 to 5) was then compared with the amount of undiluted mutant protein detected (lanes 7 to 9). The antisense (-) control is shown in lane 6. Sizes of molecular weight standards (lane 1) are indicated on the left.
Although localization does not appear to be affected, the amount of Nramp2 protein expressed on the cell surface is decreased for all three of these TMD4 mutants (Fig 5). In four separate transfection experiments, reduced levels of mutant protein on the cell surface were seen when compared with wild-type protein. This reduction cannot be attributed to decreased mRNA levels, as equal amounts of mRNA expression are seen on Northern blot for mutant and wild-typeNramp2 (data not shown). Furthermore, reduced amounts of mutant protein were seen on Western blot (data not shown). These results are similar to earlier studies of the bcg allele ofNramp1, in which the G169D substitution led to rapid degradation of the mutant Nramp1 protein.17
To assess the degree to which mutant protein levels are decreased, we diluted the amount of cell lysate used for immunoprecipitation from cells expressing wild-type Nramp2 to determine a titration curve. Cell lysates were prepared undiluted (1:1) and diluted 1:4, 1:10, and 1:40 in RIPA buffer. The amount of undiluted (1:1) mutant Nramp2 was then compared against this titration curve. In a typical experiment, G185R, D192G, and G184D mutant forms of Nramp2 were present at levels between 1:4 and 1:10 dilutions of wild-type Nramp2 (Fig 5). Although not shown in this figure, the level of G185S protein was also reduced (data not shown). While this reduction in protein level can fully account for the reduced iron transport activity of D192G, G184D, and G185S Nramp2, it does not fully account for the reduced iron transport activity of G185R Nramp2. Because the G185R mutant results in a 35-fold decrease in iron transport compared with wild-type Nramp2, the decreased protein level can only account for a fraction of the reduction in iron transport. We therefore propose that the G185R mutation has a deleterious effect on the iron transport function of Nramp2.
DISCUSSION
The disease-causing mutations in the mk mouse and the brat have both been attributed to Nramp2.9,10Remarkably, all analyzed, spontaneous mutations in Nramp2 have been in the same codon. Three spontaneous allelic mk mutations have arisen independently1,18 (and Carol Linder, personal communication, March 1995). Although two of the three strains carrying mk alleles are now extinct, analyses of DNA from the surviving strain, carrying the mk 1J allele, and DNA from one of the extinct lines, carrying the mk 2J allele, have revealed identical mutations (G185R) in Nramp2.9Polymorphisms in closely linked markers confirm that mk 1J andmk 2J are distinct alleles and have not arisen by contamination of inbred stocks (our unpublished data). Additionally, the spontaneous mutation in b rats results in the same amino acid change (G185R).10 This grouping of Nramp2 mutations in codon 185 suggests that this codon may be predisposed to mutation or alternatively, its mutation may cause an easily-recognized phenotype.
Interestingly, the naturally-occurring bcg mutation inNramp1 occurs in the codon immediately upstream of codon 185, when Nramp1 and Nramp2 are aligned based on homology.13 This mutation is associated with increased susceptibility to a group of unrelated intracellular pathogens. The extensive homology between Nramp1 and Nramp2 raises the intriguing possibility that the bcg mutation may confer susceptibility by disrupting divalent cation transport out of phagolysosomes. The clustering of spontaneous mutations in Nramp proteins within two codons in TMD4 suggests that this region of the protein may have a DNA structure that is particularly prone to mutagenesis or result in a viable, but dramatic, phenotype when mutated.
To better understand the G185R mutation in Nramp2, we undertook characterization of wild-type Nramp2 and several mutant forms generated by site-directed mutagenesis. First, we showed that the G185R mutation is functionally deleterious and results in a 35-fold decrease in iron transport when compared with wild-type. To understand the mechanism of this decreased function, we compared protein localization and protein levels of wild-type and G185R Nramp2. Wild-type and G185R forms of Nramp2 are indistinguishable in their subcellular localization. Immunofluorescence shows colocalization of both forms with cell-associated transferrin, suggesting that they are present in transferrin cycle endosomes. Biotinylation of intact cells labels both proteins, indicating that they are expressed on the plasma membrane. Although the G185R mutation results in a decreased amount of protein when compared with wild-type, the fourfold to 10-fold decrease in protein level does not fully account for the 35-fold decrease in iron transport. We therefore propose that the G185R mutation results in a change in the protein that diminishes its actual iron transport capability. The fact that the G185S mutation appears to affect protein stability, rather than protein function, suggests that the deleterious effect of the G185R mutation is not due to loss of the glycine residue, but rather due to its replacement with a bulky, charged arginine residue. It remains unclear whether this results in a general conformational change in the protein or an alteration limited to a transmembrane segment through which metal ions pass.
We postulated that the G185R mutation might result in an ionic interaction between R185 and D192 that prevents the aspartic acid from performing some critical function (see helical wheel, Fig 2). To test this possibility, we performed site-directed mutagenesis of D192 and measured iron uptake in transfected cells. Surprisingly, the D192G, D192N, and D192E mutations conferred only moderate decreases in iron transport that could be explained by decreased protein levels. Although D192 is highly conserved among Nramp-like proteins, we conclude that it is not essential for iron transport function, and it probably is not affected by the G185R mutation.
The bcg-like G184D mutation was also shown to be deleterious in activity assays. Expression of the G184D form results in markedly reduced iron transport compared with wild-type. The sixfold reduction in iron transport can be fully attributed to the fourfold to 10-fold decrease in protein levels observed for this mutation. This result is consistent with the previously reported decrease in bcg Nramp1 protein levels when compared with wild-type Nramp1.17Whether wild-type Nramp1 confers resistance to infection by transporting iron out of the lysosome remains to be formally tested.
It is clear that TMD4 plays an important role in Nramp2 iron transport, but it is not clear why the G185R mutation has occurred at least three times in rodents, and why no other spontaneous mutations have been observed in Nramp2. It is possible that the severe nature of the G185R mutation has led to its detection, and yet the residual activity seen with this mutation has allowed the animals to remain viable. Other Nramp2 mutations may have occurred, but have not been identified, either because the phenotype was too weak to detect or because the mutation was lethal. It seems unlikely, however, that G185R would be the unique mutation resulting in near total loss of function. Alternatively, the G185R mutation may differentially affect ion transport; while it severely impairs iron transport, it may not impair transport of other essential metals. Gunshin et al11 have shown that Nramp2 transports manganese (Mn), cobalt (Co), nickel (Ni), zinc (Zn), copper (Cu), and cadmium (Cd) in addition to Fe. In this case, we would postulate that a mutation that interrupted transport of several metals would be lethal, but that loss of iron transport function alone would be compatible with survival. Although this has not been formally tested, it seems unlikely, as b rats have been shown to have a parallel defect in manganese transport,19 suggesting that the G185R mutation affects transport of at least one metal in addition to iron. A third possibility is that TMD4 of Nramp2 is involved in a protein-protein interaction and that the G185R mutation alters that interaction, leading to a recognizable phenotype. Perhaps the most likely reason for recurrence of the G185R mutation, however, is that TMD4 contains a hotspot for mutation by virtue of the structure of the DNA encoding it. This interpretation might also explain why the bcg mutation inNramp1 has occurred in an adjacent codon. However, if this is the case, the features of the DNA that make it prone to remutation are not obvious and will require further investigation.
ACKNOWLEDGMENT
We thank other members of the Andrews laboratory for useful discussions, Sam Lux for advice on membrane protein solubilization, Jim Cunningham and Rick Mitchell for suggestions for subcellular localization experiments, and Stuart Orkin for allowing us to use his fluorescence microscope.
M.A.S. is a medical student in the Harvard-M.I.T. Health Sciences and Technology Program. M.D.F. is the recipient of a National Institutes of Health K08 award. N.C.A. is an Assistant Investigator of the Howard Hughes Medical Institute.
Address reprint requests to Nancy C. Andrews MD, PhD, Howard Hughes Medical Institute, Enders 720, Children’s Hospital, 300 Longwood Ave, Boston, MA 02115; email: andrews_n@a1.tch.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal