Abstract
The IIb-IIIa glycoprotein complex is a favored target for allo-, auto-, and drug-dependent antibodies associated with immune thrombocytopenia. A soluble, recombinant form of the GPIIb-IIIa heterodimer that could be produced in large quantities and maintained in solution without detergent could provide a useful experimental tool for the study of platelet-reactive antibodies, but previous attempts to produce such a construct have yielded only small quantities of the end product. Using a baculovirus expression system and the dual-promoter transfer vector P2Bac, we were able to express soluble GPIIb-IIIa complex (srGPIIb-IIIa) lacking cytoplasmic and transmembrane domains in quantities of about 1,000 μg/L, about 40 times greater than reported previously. The high yield achieved may be related to inclusion of the entire extracellular region of the GPIIb light chain in the construct. srGPIIb-IIIa reacts spontaneously with fibrinogen, and this interaction is totally inhibited by the peptide RGDS. Reactions of 24 GPIIb-IIIa–specific antibodies evaluated (12 monoclonal, 3 allo-specific, 3 auto-specific, and 6 drug-dependent) with srGPIIb-IIIa were indistinguishable from reactions with platelet GPIIb-IIIa. Thus, srGPIIb-IIIa spontaneously assumes an active, ligand-binding conformation and contains epitopes for all monoclonal and human antibodies tested to date. srGPIIb-IIIa can be produced in large quantities, can readily be modified by site-directed mutagenesis, and should facilitate identification of epitopes recognized by GPIIb-IIIa–specific antibodies, study of the mechanism(s) by which certain drugs promote antibody binding to GPIIb-IIIa in drug-induced thrombocytopenia and structure-function relationships of GPIIb-IIIa.
© 1998 by The American Society of Hematology.
THE GLYCOPROTEIN complex IIb-IIIa (GPIIb-IIIa, integrin αIIbβ3) is a member of the integrin family of cell membrane receptors that play key roles in biologic processes ranging from embryogenesis to intercellular adhesion. Each integrin receptor complex consists of a heavy (α) and a light (β) chain associated as a calcium-dependent heterodimer.1 At least 17 α and 8 β subunits have been described to date.2 The human GPIIb-IIIa complex is restricted to megakaryocytes and platelets owing to the fact that GPIIb is expressed only in those tissues. When platelets are activated by any of a number of known agonists, a series of events are initiated that leads to conformational changes in GPIIb-IIIa, creating sites for its interaction with fibrinogen. One of these sites is recognized by ligands containing an RGD sequence and the other by a C-terminal sequence in the fibrinogen γ chain.3,4 This process is essential for platelet-platelet adhesion and for formation of an effective hemostatic plug. Activated GPIIb-IIIa can also react with von Willebrand factor, fibronectin, and vitronectin by way of RGD sequences in those molecules.5 Resting GPIIb-IIIa can be converted to an active configuration by small peptides containing the RGD sequence and by certain monoclonal antibodies (MoAbs), designated LIBS (ligand-induced binding site), that recognize conformational changes associated with activation.6-8 GPIIb-IIIa was the first human integrin to be sequenced and has been studied extensively because of its relevance in hemostasis. Studies at the molecular level have shown that GPIIb and GPIIIa must be cosynthesized to be expressed at the cell surface9,10 and have provided insights into the intracellular processing and trafficking and structural biology of the GPIIb-IIIa complex.3 4 However, the three-dimensional structure of the GPIIb-IIIa heterodimer has not yet been solved and understanding of the molecular changes responsible for its activation and interaction with fibrinogen is incomplete.
In addition to its important role in hemostasis, GPIIb-IIIa is a favored target for antibodies in immune thrombocytopenias. Ten years ago, we showed that a leucine to proline substitution in GPIIIa at position 33 from the N-terminus governs expression of the PlA1/PlA2 alloantigen system that provides the immunogenic stimulus in most cases of neonatal alloimmune thrombocytopenic purpura (NATP) and posttransfusion purpura (PTP).11 Subsequently, other amino acid substitutions on GPIIIa or GPIIb were found to correlate with alloantigens of the Bak (HPA-3),12 Pen (HPA-4),13 and Ca/Tu (HPA-6)14 systems and with several low frequency alloantigens.15 Antibodies in patients with immune thrombocytopenia induced by the drugs quinine, quinidine, sulfamethoxazole, and sulfisoxazole often react with the GPIIb-IIIa complex and many of these recognize combinatorial or conformational epitopes found only on the intact GPIIb-IIIa heterodimer.16,17 The same appears to be true of many, perhaps most, antibodies associated with autoimmune thrombocytopenic purpura (AITP).18 19
A soluble, recombinant form of GPIIb-IIIa (srGPIIb-IIIa) into which specific structural modifications can be experimentally introduced could be useful in studies to resolve the three-dimensional structure of the heterodimer, characterize structure-function relationships, and define the epitopes recognized by clinically significant allo-, auto-, and drug-dependent antibodies. A vexing problem concerning drug-induced antibodies is that most appear to recognize sites on a target glycoprotein created by a reversible (noncovalent) interaction between drug and protein, a process not yet understood at the molecular level.20,21 An srGPIIb-IIIa construct could enable studies to define the mechanism by which a sensitizing drug promotes tight binding of antibody to this glycoprotein complex. Finally, a soluble GPIIb-IIIa construct is likely to be free of some of the recognized shortcomings of intact platelets for antibody detection.22Because no detergent is required to maintain it in solution, it could also be superior to glycoprotein preparations isolated from solubilized platelets for detection and identification of allo-, auto-, and drug-dependent antibodies in patients with immune thrombocytopenia.
Previous attempts to produce soluble GPIIb-IIIa have yielded only small quantities of the end product.23-25 In this report, we describe construction and expression with high yield in a baculovirus system of cDNA constructs coding for the extracellular domains of GPIIb and GPIIIa and characterization of the functional and immunologic properties of the resulting soluble heterodimers (srGPIIb-IIIa).
MATERIALS AND METHODS
Antibodies
MoAbs and polyclonal antibodies used are characterized in Table 1. The human antibodies listed were obtained from the Platelet Antibody Reference Laboratory of The Blood Center of Southeastern Wisconsin.
Antibodies Used to Probe srGPIIb-IIIa
| Name . | Type-150 . | Specificity . | Source . |
|---|---|---|---|
| Tab | IgG2a MoAb | GPIIb | R. McEver |
| MBC132.1 | IgG1 MoAb | GPIIb | BCSEW |
| B1B5 | IgG1 MoAb | GPIIb light chain | J. Bennet |
| AP3 | IgG1 MoAb | GPIIIa (348-421) | P.J. Newman |
| AP5 | IgG1 MoAb | GPIIIa (1-6) (LIBS) | T.J. Kunicki |
| AP6 | IgM MoAb | GPIIIa (211-221) | T.J. Kunicki |
| D3 | IgG1 MoAb | GPIIIa (422-490) (LIBS) | L. Jennings |
| CRC54 | IgG1 MoAb | GPIIIa (1-100) LIBS | M.J. Berndt |
| Fire/Ice | Rabbit | GPIIIa | P.J. Newman |
| AP2 | IgG1 MoAb | GPIIb-IIIa complex | T.J. Kunicki |
| 10E5 | IgG2a MoAb | GPIIb-IIIa complex | B. Coller |
| 7E3 | IgG1 MoAb | GPIIb-IIIa complex | B. Coller |
| PAC-1 | IgM MoAb | GPIIb-IIIa ligand mimetic | S. Shattil |
| KR | Human allo-Ab | P1A1 (HPA-1a) | BCSEW |
| TR | Human allo-Ab | Pena (HPA-4b) | BCSEW |
| KE | Human allo-Ab | Baka (HPA-3a) | BCSEW |
| LB | Human AITP | GPIIIa | BCSEW |
| JN | Human AITP | GPIIIa | BCSEW |
| MD | Human AITP | GPIIb-IIIa complex | BCSEW |
| MA | Human quinine DDAb | GPIIIa | BCSEW |
| JW | Human quinine DDAb | GPIIIa | BCSEW |
| RB | Human quinine DDAb | GPIIIa | BCSEW |
| GW | Human quinidine DDAb | GPIIIa + GPIIb-IIIa complex | BCSEW |
| BC | Human SMX DDAb | GPIIb-IIIa complex | BCSEW |
| TB | Human SMX DDAb | GPIIb-IIIa complex | BCSEW |
| Name . | Type-150 . | Specificity . | Source . |
|---|---|---|---|
| Tab | IgG2a MoAb | GPIIb | R. McEver |
| MBC132.1 | IgG1 MoAb | GPIIb | BCSEW |
| B1B5 | IgG1 MoAb | GPIIb light chain | J. Bennet |
| AP3 | IgG1 MoAb | GPIIIa (348-421) | P.J. Newman |
| AP5 | IgG1 MoAb | GPIIIa (1-6) (LIBS) | T.J. Kunicki |
| AP6 | IgM MoAb | GPIIIa (211-221) | T.J. Kunicki |
| D3 | IgG1 MoAb | GPIIIa (422-490) (LIBS) | L. Jennings |
| CRC54 | IgG1 MoAb | GPIIIa (1-100) LIBS | M.J. Berndt |
| Fire/Ice | Rabbit | GPIIIa | P.J. Newman |
| AP2 | IgG1 MoAb | GPIIb-IIIa complex | T.J. Kunicki |
| 10E5 | IgG2a MoAb | GPIIb-IIIa complex | B. Coller |
| 7E3 | IgG1 MoAb | GPIIb-IIIa complex | B. Coller |
| PAC-1 | IgM MoAb | GPIIb-IIIa ligand mimetic | S. Shattil |
| KR | Human allo-Ab | P1A1 (HPA-1a) | BCSEW |
| TR | Human allo-Ab | Pena (HPA-4b) | BCSEW |
| KE | Human allo-Ab | Baka (HPA-3a) | BCSEW |
| LB | Human AITP | GPIIIa | BCSEW |
| JN | Human AITP | GPIIIa | BCSEW |
| MD | Human AITP | GPIIb-IIIa complex | BCSEW |
| MA | Human quinine DDAb | GPIIIa | BCSEW |
| JW | Human quinine DDAb | GPIIIa | BCSEW |
| RB | Human quinine DDAb | GPIIIa | BCSEW |
| GW | Human quinidine DDAb | GPIIIa + GPIIb-IIIa complex | BCSEW |
| BC | Human SMX DDAb | GPIIb-IIIa complex | BCSEW |
| TB | Human SMX DDAb | GPIIb-IIIa complex | BCSEW |
Abbreviations: LIBS, ligand-induced binding site; SMX, sulfamethoxazole; AITP, autoimmune thrombocytopenic purpura; DDAb, drug-dependent antibody; BCSEW, Blood Center of Southeastern Wisconsin.
Antibodies are murine monoclonals, unless otherwise stated.
Transfer Vector Construction
Throughout this report, Nucleotide 1 refers to the A of the translation start codon of either GPIIb (αIIb) or GPIIIa (β3) cDNA. Full-length αIIb cDNA cloned into pGEM7 as an EcoRI fragment (αIIb-pGEM7) was kindly provided by Dr Mortimer Poncz (Children’s Hospital of Philadelphia, Philadelphia, PA). A full-length β3 cDNA possessing a silent mutation at amino acid 726, thereby eliminating anEcoRI restriction endonuclease recognition site in pBluescript as an EcoRI fragment (β3 726-pBluescript), was a gift from Dr Gilbert White (University of North Carolina-Chapel Hill, Chapel Hill, NC).
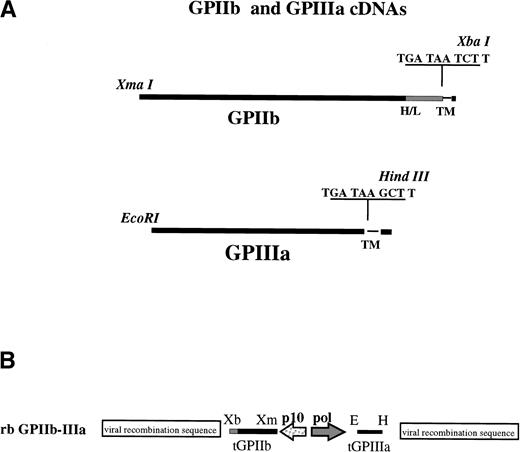
β3 was truncated at the junction (nt2154) between the extracellular and transmembrane domains after the generation of two stop codons and a unique HindIII recognition sequence adjacent to the transmembrane domain (nucleotide 2154) using site-directed mutagenesis (in vitro mutagenesis system; Amersham, Arlington Heights, IL) with mutagenic primer 5′ CCC AAG GGC CCD TGA TAA GCT TGG TCC TGC 3′ (Fig 1A). After mutagenesis, β3 was sequenced from the 5′EcoRI restriction site located at nucleotide −28 through the newly generated HindIII restriction site at nucleotide 2154 (AA1-718, including a 26 AA signal sequence) to ensure that no spontaneous mutations had occurred during the mutagenesis reaction. Truncated β3 comprising AA1-718 was shuttled as anEcoRI-HindIII fragment into the multiple cloning site of transfer vector p2Bac (Invitrogen, Madison, WI) located downstream of the baculovirus Poll-1 promoter, generating transfer plasmid p2Bac-β3 (Fig 1B).
Preparation of recombinant baculovirus transfer vectors for infection of SF9 cells. (A) Restriction sites and stop codons used to truncate IIb/β3 are shown. H/L denotes IIb heavy-light chain junction; TM indicates transmembrane domains. (B) Truncated IIb/β3 cDNAs were inserted into baculovirus transfer vector p2Bac under control of the baculovirus p10 and polyhedron (Pol) promoters, respectively, as shown. Indicated restriction enzyme sites are E (EcoRI), H (HindIII), Xm (Xma I), and Xb (Xba I).
Preparation of recombinant baculovirus transfer vectors for infection of SF9 cells. (A) Restriction sites and stop codons used to truncate IIb/β3 are shown. H/L denotes IIb heavy-light chain junction; TM indicates transmembrane domains. (B) Truncated IIb/β3 cDNAs were inserted into baculovirus transfer vector p2Bac under control of the baculovirus p10 and polyhedron (Pol) promoters, respectively, as shown. Indicated restriction enzyme sites are E (EcoRI), H (HindIII), Xm (Xma I), and Xb (Xba I).
Preparation of αIIb cDNA for insertion into transfer vector p2Bac-β3 involved generation of a uniqueXma I restriction sequence at nucleotide −1 to eliminate extraneous DNA upstream of the αIIb coding region. Mutageneic PCR primer 5′ACA CCC GGG ATG GCC AGA GCT TTG TGT CCA CTG3′ (nt 1-24) and polymerase chain reaction (PCR) primer 5′GGG AGC CTA CAT TTT CGG GTC TCA TCA CGG AGA GGT C3′, corresponding to nucleotides 305-339 were used to amplify nucleotides 1-339 of αIIb. The PCR fragment was digested with Xma I and Sac II (a unique Sac II restriction site is present in αIIb cDNA at nt 215) purified and ligated into the original αIIb-pBluescript plasmid digested with a unique Xma I (located in the multiple cloning site upstream of the αIIb insert) and SacII forming plasmid Xm-αIIb-pGEM7. The complete insert was sequenced to verify that no PCR mutations were generated.
αIIb was then truncated at the transmembrane junction (nt2980) by generating two stop codons and an Xba I restriction site in Xm-αIIb-pGEM7 with mutagenic PCR primer 5′ CAC CTC TAG ATT ATC A CC TCT CCT CCA AGG CCC GGA GCA GC corresponding to nucleotides 2955-2980 (noncoding) and PCR primer 5′GTC AAC CCT CTC AAG GTG GAC TGG3′ corresponding to nucleotides 2588-2611 (coding; see Fig 1A). The PCR fragment was digested with Xba I and Bgl II (a unique Bgl II restriction site is present in αIIb cDNA at nt 2673), purified, and ligated into Xm-αIIb-pGEM7 digested withXba I at a unique locus in the multiple cloning site downstream of the αIIb insertion and with Bgl II, forming plasmid XmXb-tαIIb-pGEM7. The complete PCR insert was sequenced to verify that no PCR mistakes were generated. Truncated αIIb comprising aa 1-993 was inserted as an XmaI-Xba I fragment into transfer vector p2Bac-β3downstream of the baculovirus P10 promoter, generating transfer plasmid p2Bac-αIIb-β3 (see Fig 1B). The αIIb cDNA insertion also possessed the native αIIb signal sequence at the 5′ end and two synthetic stop codons at the 3′ end to terminate translation, creating a construct lacking the transmembrane domain and cytoplasmic tail.
Generation of Recombinant Virus (rb αIIbβ3)
Generation of rb αIIbβ3 was accomplished by cotransfection of p2Bac-αIIbβ3 with wild-type Autographa californica nuclear polyhedrosis viral DNA (AcMNPV) into Sf9 insect cells (Spodoptera frugiperda; Invitrogen, Carlsbad, CA). Linear AcMNPV DNA (1 μg; Invitrogen) was incubated with transfer plasmid p2Bac-αIIbβ3 in the presence of a cationic liposome solution (Invitrogen) for 15 minutes with occasional vortexing. The DNA-liposome mixture (1 mL) was added to 1.5 × 106 Sf9 cells adherent to a T25 cm2flask previously washed with TNM-FH media without supplements. The transfection mixture was slowly rocked at room temperature for 4 hours. One milliliter of complete media was added to the cells, and they were incubated at 27°C for 48 hours. Virus stocks were harvested, and fresh media was added to the transfected cells, which were allowed to incubate another 4 days for visualization of infection. After incubation, occlusion bodies were visualized in 50% of the cells, confirming successful transfection. rb αIIbβ3 was enriched and purified using the end-point dilution method. Purity of the desired recombinant virus and verification of double homologous recombination events was achieved by PCR analysis using primers to amplify internal regions of the polyhedrin gene and internal primers specific for αIIband β3. Junctions of the recombinations were verified using PCR primers specific for the respective p10 or Pol promoters in combination with internal PCR primers specific for αIIband/or β3.
Production of Recombinant Protein
Recombinant protein expression was performed in 150-cm2tissue culture flasks seeded 24 hours previously with Sf9 or High five cells (Invitrogen) at a density of 2 × 106 (∼40% confluent) and infected with rb αIIbβ3. Optimal amounts of rb αIIbβ3 required to infect Sf9 or High five cells at this density and thereby produce the highest amounts of soluble, recombinant αIIbβ3 (srGPIIb-IIIa) were determined in titration experiments to be five infectious units per cell. Optimal duration of infection was determined to be 4 days postinfection for Sf9 cells and 3 days postinfection for High five cells in initial experiments. After infection, cell supernatants were harvested and used without further purification in the experiments described.
Quantitation of srGPIIb-IIIa
The amounts of srGPIIb-IIIa in cell supernatants were quantified by a sandwich enzyme-linked immunosorbent assay (ELISA). Microtiter wells were coated overnight at 4°C with MoAb AP2 (10 μg/mL in 15 mmol/L sodium carbonate, 35 mmol/L sodium bicarbonate, pH 9.6). Wells were washed three times with 20 mmol/L Tris, 145 mmol/L NaCl, 1 mmol/L CaCl2, and 0.05% Tween 20, pH 7.6 (wash buffer), and were saturated with 2% bovine serum albumin (BSA) in wash buffer for 1 hour at room temperature. Cell culture supernatants were added as well as serial dilutions of purified platelet GPIIb-IIIa26 from 0 to 1 mg/mL in increments of 0.1 mg/mL to generate a standard curve. The quality and purity of platelet GPIIb-IIIa was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Coomassie staining demonstrated the presence of only two bands corresponding in size to GPIIb and GPIIIa (data not shown). After 1 hour of incubation, wells were washed as described above and biotinylated MoAb AP3 (5 μg/mL) was added after incubation for 1 hour at room temperature. The wells were washed and alkaline-phosphatase–labeled streptavidin was added for 45 minutes at room temperature. After washing, bound alkaline phosphatase was detected with p-nitrophenyl phosphate (PNPP) substrate.
Electrophoresis and Immunoblotting
Cell supernatants were concentrated about fivefold with a Centricon 30 concentrator (Amicon, Beverly, MA) and were treated with an equal volume of sample buffer consisting of 0.04 mol/L Tris, 2% glycerol, 1.6% SDS, and 0.2% bromophenol blue, pH 6.8 (nonreducing), and 2% β-mercaptoethanol (reducing). Samples were boiled and electrophoresed through 8% SDS polyacrylamide gels according to Laemmli.27 Gels were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA) using CAPs buffer (0.01 mol/L CAPs, 10% methanol, pH 11). After an overnight incubation in 3% BSA, 20 mmol/L Tris, 145 mmol/L NaCl, and 0.05% Tween 20, various antibodies (3 μg/mL) were added, and the membrane was incubated for 4 hours. Bound Ig was detected with peroxidase-conjugated goat antimouse IgG antibody (Jackson Immunoresearch, West Grove, PA) using enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Immunoprecipitation
Immunoprecipitations involving mouse MoAb were performed using protein A Sepharose preloaded with rabbit antimouse Fc-specific antibodies (Jackson Immunoresearch) and then loaded with a mouse MoAb specific for GPIIb and/or GPIIIa. Beads were added to 7 mL of cell culture supernatant that had been precleared with fresh, unloaded protein A sepharose. After incubation, the beads were washed several times in wash buffer and absorbed proteins were dissociated by boiling in 25 mmol/L Tris, 1.0% SDS, pH 7.0, and subjected to SDS-PAGE electrophoresis. For studies involving human drug-dependent antibodies, culture supernatant was incubated with human serum in the presence or absence of 0.4 mmol/L quinine or quinidine for 60 minutes at 4°C. The resulting immune complexes were then absorbed at 4°C for 4 hours with protein A sepharose beads incubated previously with 3% BSA. Glycoproteins were eluted from the beads and characterized as described above.
Biotin-Labeled Fibrinogen
Two milligrams of purified fibrinogen (American Bioproducts, Parsippany, NJ) was biotinylated with 2.5 μmol/L NHS-LC-Biotin (Pierce, Rockford, IL) in phosphate-buffered saline (PBS) 2 hours at 25°C. Excess biotin was removed by dialysis in PBS. The function of the biotinylated fibrinogen was then examined in flow cytometry and aggregation studies. Preliminary studies established that, at a concentration of 170 μg/mL, the biotinylated fibrinogen bound readily to platelets treated with the LIBS MoAb D3, but not to untreated platelets and supported aggregation of gel-filtered platelets (Sepharose CL-2B; Pharmacia, Piscataway, NJ) activated with thrombin receptor activating peptide (TRAP)28 (not shown). TRAP-activated platelets, added in excess, absorbed all detectable amounts of biotin-labeled fibrinogen present in solution as determined by ELISA. Briefly, polyclonal goat antifibrinogen was used to capture serial dilutions of known amounts of biotinylated fibrinogen to generate a standard curve as well as biotinylated fibrinogen present in the unbound fraction of the platelet reaction. Captured fibrinogen was then detected with alkaline-phosphatase–labeled streptavidin. Although unbound fibrinogen was readily detectable in reactions with unsaturating amounts of platelets, no unbound fibrinogen was detected in reactions with saturating amounts of platelets indicating that the labeled protein was fully bindable.
Fibrinogen Binding
MoAb Tab (anti-GPIIb) was immobilized in microtiter wells at a concentration of 10 μg/mL in 0.05 mol/L sodium carbonate buffer, pH 9.0, overnight at 4°C. Wells were washed in 20 mmol/L TRIS, 0.145 mol/L NaCl, 1 mmol/L CaCl2, 0.05% Tween-20, pH 7.6, and were blocked with 3.5% BSA in wash buffer for 1 hour. SrGPIIb-IIIa (0.4 μg) from cell culture supernatant or platelet GPIIb-IIIa was then added and allowed to bind to the immobilized antibody for 4 hours at room temperature. Unbound material was removed by washing three times and the wells were blocked again with 3.5% BSA in wash buffer for 1 hour at room temperature. After washing, the immobilized GPIIb-IIIa was incubated with buffer or LIBS MoAbs AP5, D3, or CRC54 for 1 hour at room temperature. Increasing concentrations of biotinylated fibrinogen were then added. After incubation for 14 hours at room temperature, unbound fibrinogen was removed by washing three times with wash buffer, and bound fibrinogen was detected with alkaline-phosphatase–labeled streptavidin and subsequent addition of PNPP. Optical densities were read 45 minutes after PNPP addition. RGDS peptide used to inhibit specific binding of fibrinogen to its receptor was obtained from the Protein Chemistry Core Laboratory of the Blood Research Institute.
Deglycosylation of srGPIIb-IIIa
Enzymes used were endoglycosidase-H (Genzyme, Cambridge, MA), which removes N-linked high-mannose carbohydrate residues, and N-Glycanase (Genzyme), which removes all N-linked oligosaccharides. Fifty micrograms of srGPIIb-IIIa was partially purified by immunoprecipitation with MoAb AP2 bound to protein A sepharose. The immunoprecipitated material was eluted by boiling. The eluted protein was digested with endoglycosidase-H according to Newman et al29 using 150 mU/mL endo-H per 50 μg protein in 1.4 mmol/L phenylmethylsulfonyl fluoride (PMSF) and 4 mmol/L leupeptin for 15 hours at 37°C. Digestion with N-glycanase was performed according to Plummer et al30 by resuspending 50 μg of srGPIIb-IIIa in 0.5 mmol/L NaPO4, pH 8.6, 0.5% SDS, 50 mmol/L β-mercaptoethanol. The material was then denatured by boiling for 5 minutes. NP-40 detergent (1% final concentration) and 0.3 U of N-glycanase were then added, followed by digestion for 15 hours at 37°C.
RESULTS
Preliminary Observations
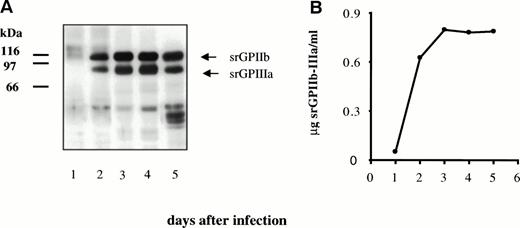
Production of recombinant protein was monitored daily in aliquots of cell culture supernatant. As shown in Fig2A, two bands of apparent molecular weights 116 kD and 85 kD were recognized in Western blots by GPIIb and GPIIIa-specific antibodies. The soluble recombinant protein was captured in microtiter wells using immobilized MoAb AP2, specific for the GPIIb-IIIa complex, and captured protein was detected with biotin-labeled AP3, specific for GPIIIa. Known amounts of platelet GPIIb-IIIa were used to generate a standard curve for the purpose of quantitation. Levels of recombinant protein were maximum at 3 to 4 days (Fig 2B) and averaged about 0.8 mg of protein complex per liter.
Secretion of srGPIIb-IIIa by transfected SF9 insect cells. (A) Western blot analysis of secreted proteins on days 1 through 5 using a mixture of MBC 132.1 (anti-GPIIb) and rabbit anti-GPIIIa for detection. (B) Levels of srGPIIb-IIIa recovered in culture supernatants on days 1 through 5 after transfection.
Secretion of srGPIIb-IIIa by transfected SF9 insect cells. (A) Western blot analysis of secreted proteins on days 1 through 5 using a mixture of MBC 132.1 (anti-GPIIb) and rabbit anti-GPIIIa for detection. (B) Levels of srGPIIb-IIIa recovered in culture supernatants on days 1 through 5 after transfection.
Molecular Characterization of srGPIIb-IIIa
On the basis of protein content alone, molecular weights of srGPIIb and srGPIIIa were expected to be 105 and 75 kD, respectively. The higher values observed (116 and 85 kD) were assumed to be a consequence of glycosylation. Treatment of srGPIIb with N-glycanase and srGPIIIa with endo-H led to shifts in apparent molecular weight (MW) in SDS gels to 108 kD and 75 kD, indicating that srGPIIb and srGPIIIa contain approximately 7% and 12% N-linked oligosaccharides, respectively (data not shown).
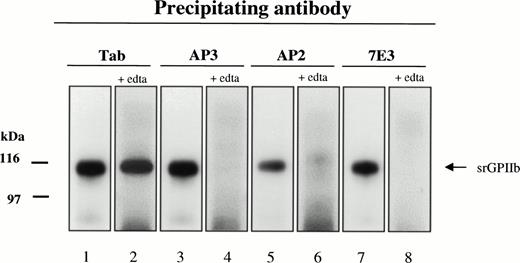
The platelet GPIIb-IIIa complex is a cation-dependent, noncovalently associated heterodimer.31-33 The nature of srGPIIb-GPIIIa association was examined by immunoprecipitating the recombinant protein with MoAbs specific for either the intact GPIIb-IIIa complex or its individual subunits with and without prior treatment with EDTA. In the absence of EDTA, srGPIIb was precipitated by MoAb AP3, specific for GPIIIa, and Tab, specific for GPIIb as well as by the complex-specific MoAbs AP2 and 7E3 (Fig 3). After treatment with 10 mmol/L EDTA, srGPIIb was precipitated only by the GPIIb-specific antibody Tab. In a reciprocal experiment in which GPIIIa was identified with the GPIIIa-specific antibody, Fire/Ice, srGPIIIa was precipitated by AP3, but not by Tab in the presence of EDTA, and no precipitate was obtained with AP2 or 7E3 (data not shown). These findings indicate that srGPIIb and srGPIIIa are noncovalently associated as a cation-dependent heterodimer.
Immunoblot of srGPIIb precipitated from day-4 culture supernatant by MoAbs Tab (anti-GPIIb H), AP3 (anti-GPIIIa), and AP2 and 7E3 (anti-GPIIb-IIIa complex) in the absence (lanes 1, 3, 5, and 7) and presence (lanes 2, 4, 6, and 8) of 10 mmol/L EDTA. All four MoAbs precipitated srGPIIb from untreated culture medium. After the addition of EDTA to dissociate srGPIIb-IIIa, precipitation was achieved only with Tab.
Immunoblot of srGPIIb precipitated from day-4 culture supernatant by MoAbs Tab (anti-GPIIb H), AP3 (anti-GPIIIa), and AP2 and 7E3 (anti-GPIIb-IIIa complex) in the absence (lanes 1, 3, 5, and 7) and presence (lanes 2, 4, 6, and 8) of 10 mmol/L EDTA. All four MoAbs precipitated srGPIIb from untreated culture medium. After the addition of EDTA to dissociate srGPIIb-IIIa, precipitation was achieved only with Tab.
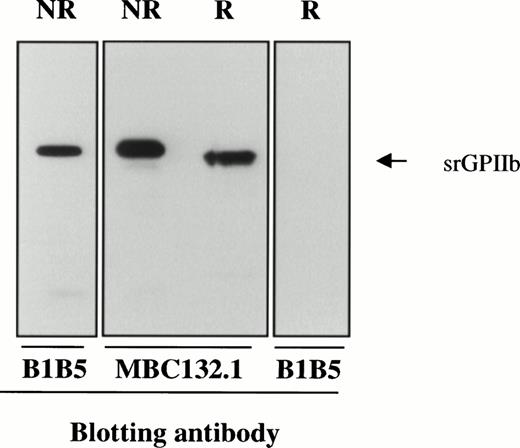
After its synthesis, pro-GPIIb is cleaved immediately C-terminal to dibasic residues at positions 856-857 and/or 860-861 to produce an N-terminal heavy chain and a C-terminal light chain that are disulfide linked.34 As shown in Fig 4, srGPIIb is recognized by MoAbs MBC132.1 and B1B5 specific for the heavy and light chains, respectively. After reduction, the band recognized by MBC132.1 has an apparent MW of about 104 and is no longer recognized by the light chain-specific antibody B1B5. The decrease in apparent MW of GPIIb of about 6 kD after reduction is approximately the amount predicted after dissociation of the disulfide-linked light chain fragment (srGPIIbβ).
Immunoblots of srGPIIb with B1B5 (anti-GPIIb L chain) and MBC 132.1 (anti-GPIIb H chain). After reduction (R), the apparent MW of srGPIIb decreased by about 6 kD and binding of B1B5 was lost because of dissociation of GPIIb L chain.
Immunoblots of srGPIIb with B1B5 (anti-GPIIb L chain) and MBC 132.1 (anti-GPIIb H chain). After reduction (R), the apparent MW of srGPIIb decreased by about 6 kD and binding of B1B5 was lost because of dissociation of GPIIb L chain.
Recognition of srGPIIb-IIIa by Other MoAbs
Recognition of srGPIIb-IIIa by Antibodies From Patients With Immune Thrombocytopenia
Alloantibodies.
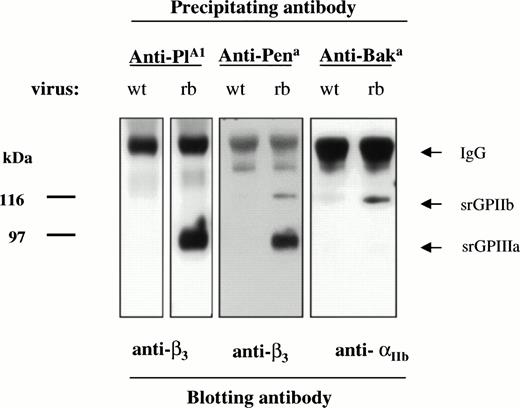
On the basis of their known amino acid sequences, srGPIIb was expected to carry the alloantigen Baka/HPA-3a (Iso 843) and srGPIIIa was expected to carry PlA1/HPA-1a (Leu 33) and Pena/HPA-4b (Arg 143). As shown in Fig 5, srGPIIb-IIIa was immunoprecipitated by alloantibodies specific for each of these alloantigens. No bands were precipitated by normal plasma (data not shown).
Immunoprecipitation of srGPIIb and srGPIIIa by human antibodies specific for the alloantigens PlA1 (HPA-1a), Pena (HPA-4b), and Baka (HPA-3a). As predicted from the known amino acid sequences of srGPIIb and srGPIIIa, anti-PlA1 and anti-Pena recognized srGPIIIa and anti-Baka recognized srGPIIb. No bands were obtained with normal serum (not shown).
Immunoprecipitation of srGPIIb and srGPIIIa by human antibodies specific for the alloantigens PlA1 (HPA-1a), Pena (HPA-4b), and Baka (HPA-3a). As predicted from the known amino acid sequences of srGPIIb and srGPIIIa, anti-PlA1 and anti-Pena recognized srGPIIIa and anti-Baka recognized srGPIIb. No bands were obtained with normal serum (not shown).
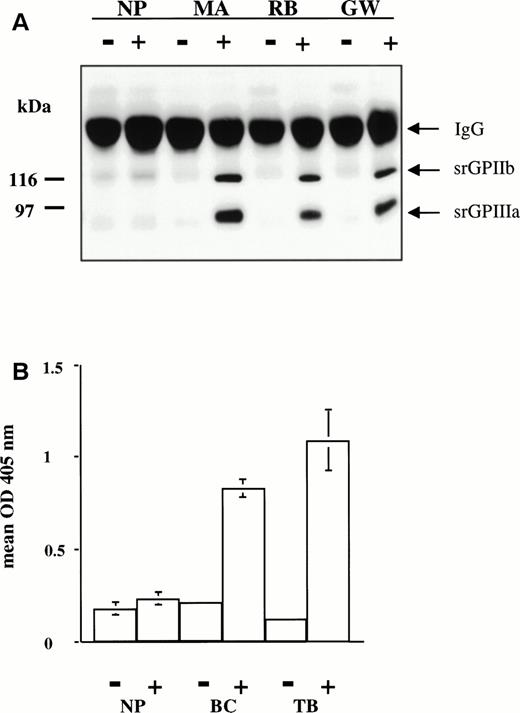
Drug-dependent antibodies.
Studies were performed with serum from 6 patients. MA, RB, and JW developed acute, severe thrombocytopenia while taking quinine; GW developed similar symptoms while taking quinidine; and BC and TB experienced thrombocytopenia while taking sulfamethoxazole. In previous studies, it was shown that MA, RB, and JW are specific for GPIIIa; that GW contains two antibodies, one specific for GPIIIa and the other specific for the GPIIb-IIIa complex16; and that BC and TB recognize only the intact GPIIb-IIIa complex.17 As shown in Fig 6A, MA, RB, and GW reacted with srGPIIb-IIIa in the presence, but not in the absence of the drug that provoked thrombocytopenia. Similar reactions were obtained with the quinine-dependent antibody JW (data not shown). The same antibodies were tested for their ability to recognize srGPIIb-IIIa and platelet GPIIb-IIIa captured in microtiter wells by immobilized Tab (anti-GPIIb). In each case, positive reactions were obtained in the presence of the sensitizing drug, but not in its absence (data not shown). The sulfamethoxazole-dependent antibodies BC and TB gave weak and inconsistent immunoprecipitation reactions with both srGPIIb-IIIa and platelet GPIIb-IIIa. Therefore, these antibodies were studied by a modified antigen capture ELISA35 in which antibody, drug, and target GP complex were first incubated in suspension for 30 minutes at room temperature, and the resulting immune complexes were captured in microtiter wells by immobilized MoAb Tab (anti-GPIIb). After washing, immobilized IgG was detected by ELISA. As shown in Fig 6B, BC and TB reacted with srGPIIb-IIIa in the presence, but not in the absence, of drug. Similar reactions were obtained when the antibodies were allowed to react with platelet GPIIb-IIIa rather than srGPIIb-IIIa (data not shown).
(A) Reactions of drug-dependent antibodies MA, RB, and GW with srGPIIb-IIIa in the presence (+) and absence (−) of 0.4 mmol/L quinine (MA and RB) and quinidine (GW). All three drug-dependent antibodies precipitated bands corresponding to srGPIIb and srGPIIIa in the presence of drug only. No precipitation was seen with normal plasma (NP). (B) Reactions of sulfamethoxazole-dependent antibodies BC and TB with srGPIIb-IIIa in the presence (+) and absence (−) of 0.4 mmol/L sulfamethoxazole. IgG bound to srGPIIb-IIIa was detected by modified antigen capture ELISA. No reaction was obtained with normal plasma (NP).
(A) Reactions of drug-dependent antibodies MA, RB, and GW with srGPIIb-IIIa in the presence (+) and absence (−) of 0.4 mmol/L quinine (MA and RB) and quinidine (GW). All three drug-dependent antibodies precipitated bands corresponding to srGPIIb and srGPIIIa in the presence of drug only. No precipitation was seen with normal plasma (NP). (B) Reactions of sulfamethoxazole-dependent antibodies BC and TB with srGPIIb-IIIa in the presence (+) and absence (−) of 0.4 mmol/L sulfamethoxazole. IgG bound to srGPIIb-IIIa was detected by modified antigen capture ELISA. No reaction was obtained with normal plasma (NP).
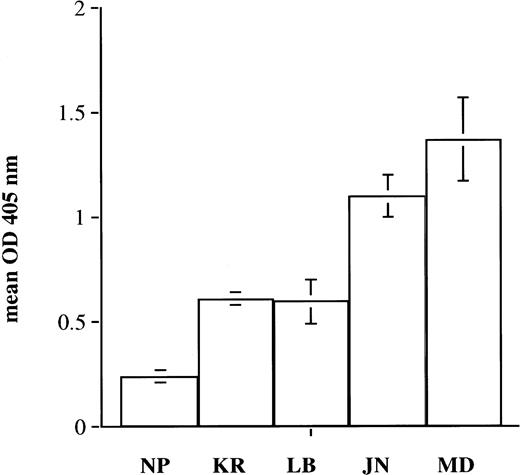
Autoantibodies.
Three sera from patients with autoimmune thrombocytopenia (LB, JN, and MD) were previously shown to be specific for GPIIIa (JN and LB) or for the GPIIb-IIIa complex (MD) in immunoprecipitation studies using biotin-labeled normal target platelets and by their failure to react with platelets from a patient with type I Glanzmann’s thrombasthenia. Autospecificity was confirmed by showing that each antibody reacted as strongly with autologous platelets obtained after splenectomy-induced remission as with platelets from normal donors (data not shown). Each of the three autoantibodies recognized srGPIIb-IIIa immobilized in microtiter wells (Fig 7). In parallel studies, similar reactions were obtained with platelet GPIIb-IIIa (data not shown). Reactions of antibody MD but not JN or LB with srGPIIb-IIIa were abolished by EDTA, confirming that MD is specific for an epitope or epitopes found on intact but not on dissociated GPIIb-IIIa complex.
Reactions of autoantibodies LB and JN (anti-GPIIIa) and MD (anti-GPIIb-IIIa complex) with srGPIIb-IIIa. srGPIIb-IIIa was immobilized in microtiter wells and was used as a target for normal plasma (NP), an anti-PlAl antibody (KR), and autoantibodies LB, JN, and MD. Bound IgG was detected by ELISA. Brackets indicate mean ± 2 SD.
Reactions of autoantibodies LB and JN (anti-GPIIIa) and MD (anti-GPIIb-IIIa complex) with srGPIIb-IIIa. srGPIIb-IIIa was immobilized in microtiter wells and was used as a target for normal plasma (NP), an anti-PlAl antibody (KR), and autoantibodies LB, JN, and MD. Bound IgG was detected by ELISA. Brackets indicate mean ± 2 SD.
Functional Characterization of srGPIIb-IIIa
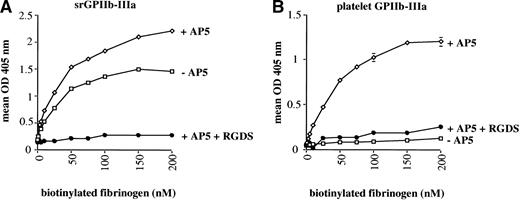
For these studies, srGPIIb-IIIa and platelet GPIIb-IIIa were immobilized in microtiter wells precoated with the GPIIb-specific MoAb, Tab, known not to interfere with the reaction between fibrinogen and the GPIIb-IIIa heterodimer.36 Binding of biotinylated fibrinogen to the immobilized soluble recombinant complex and to platelet-derived GPIIb-IIIa was then measured by ELISA. Under these conditions, fibrinogen bound readily to srGPIIb-IIIa (Fig 8A). This binding was enhanced slightly by the LIBS MoAb AP5 and was totally inhibited by RGD peptide at 2.0 mmol/L. Similar results were obtained when the ligand mimetic MoAb PAC-1 was added instead of biotinylated fibrinogen (data not shown). In contrast, fibrinogen reacted with platelet GPIIb-IIIa only when AP5 was present. This binding was also blocked by RGD peptide (Fig8B). Likewise, platelet GPIIb-IIIa did not spontaneously bind PAC-1 in similar studies but did so upon addition of AP5 (data not shown). In similar experiments, LIBS MoAbs D3 and CRC54 influenced fibrinogen binding to srGPIIb-IIIa and platelet GPIIb-IIIa in the same way as AP5 (data not shown). Half saturation of srGPIIb-IIIa by fibrinogen, with and without AP5 occurred at a concentration of about 30 nmol/L. Half saturation of platelet GPIIb-IIIa in the presence of AP5 occurred at about 40 nmol/L.
Binding of biotinylated fibrinogen to srGPIIb-IIIa (A) and platelet GPIIb-IIIa (B). Fibrinogen reacted spontaneously with srGPIIb-IIIa and this binding was enhanced slightly by the LIBS MoAb AP5. Binding to platelet GPIIb-IIIa occurred only when AP5 was added. In both cases, fibrinogen binding was completely inhibited by RGDS peptide. Data points are the average of triplicate determinations, which varied from each other by no more than ±5%.
Binding of biotinylated fibrinogen to srGPIIb-IIIa (A) and platelet GPIIb-IIIa (B). Fibrinogen reacted spontaneously with srGPIIb-IIIa and this binding was enhanced slightly by the LIBS MoAb AP5. Binding to platelet GPIIb-IIIa occurred only when AP5 was added. In both cases, fibrinogen binding was completely inhibited by RGDS peptide. Data points are the average of triplicate determinations, which varied from each other by no more than ±5%.
DISCUSSION
The purpose of the present study was to achieve synthesis of soluble, recombinant glycoprotein IIb-IIIa complex srGPIIb-IIIa in quantities sufficient to permit studies of its structure-function relationships and immunologic properties.
Bennett et al37 first established the feasibility of producing soluble, recombinant forms of GPIIb-IIIa by demonstrating normal processing and secretion of the truncated heterodimer in COS cells using cDNA constructs coding for the extracellular domains of both molecules. Truncated GPIIIa, transfected alone, was also synthesized and secreted, but truncated GPIIb was processed normally only in cells cotransfected with GPIIIa. Several groups have prepared recombinant, soluble, GPIIb-IIIa lacking transmembrane and cytoplasmic domains and various parts of their extracellular domains for functional and structural studies of the heterodimeric complex. Wippler et al23 constructed a tandem vector containing cDNA coding for the GPIIb heavy chain and for residues 1-469 of GPIIIa, encompassing the region N-terminal to the cysteine-rich, protease-resistant extracellular domain. The product, designated αIIbH; t-β3, was expressed in a baculovirus system at a level of about 0.0114 μg/mL (7.8 × 10−11 mol/L). The synthesized heterodimer reacted spontaneously with fibrinogen with an affinity comparable to that of platelet GPIIb-IIIa, indicating that the cysteine-rich region of GPIIIa is not required for assembly and function of the heterodimer. Gulino et al24 expressed the entire extracellular domains of GPIIb and GPIIIa in COS cells using a twofold vector that contained both cDNAs and achieved production of soluble, truncated GPIIb-IIIa at a level of about 0.048 μg/mL (2.7 × 10−10 mol/L). Their soluble heterodimer also reacted spontaneously with fibrinogen with an affinity slightly greater than that of platelet GPIIb-IIIa and was recognized by MoAbs specific for GPIIb, GPIIIa, and the GPIIb-IIIa complex. McKay et al,25 using baculovirus, produced a heterodimer containing only the first 223 amino acids of GPIIb and residues 111-318 of GPIIIa for the purpose of defining sequences necessary for association of the two chains, but did not quantify the amount of soluble heterodimer synthesized.
Our expression system achieved soluble GPIIb-IIIa production of about 0.85 μg/mL (4.7 × 10−9 mol/L), about 17 and 60 times greater than the levels obtained by Gulino et al24in COS cells and Wippler et al23 in baculovirus, respectively. Several previous reports provide a possible explanation for the relatively high yield of srGPIIb-IIIa achieved in the present study. As already noted, Bennett et al37 showed that GPIIb and GPIIIa lacking transmembrane and cytoplasmic domains form a complex that is secreted by COS cells. In contrast, Frachet et al38observed that cDNA coding for the GPIIb heavy chain alone is not surface-expressed when cotransfected with cDNA coding for the extracellular domain of GPIIIa. However, satisfactory expression of the heterodimer was achieved when cDNA coding for full-length GPIIb (H plus L chain) was used.38 Together, these observations suggest that at least the extracellular part of the GPIIb light chain is needed for efficient processing and secretion of truncated GPIIb-IIIa. Thus, efficient synthesis and secretion of the soluble heterodimer achieved in the present study may be due to the inclusion of cDNA coding for the extracellular region of GPIIbH in our construct. Milligram quantities of srGPIIb-IIIa can readily be produced in a single 4-day culture in this system. Isolation of 1 mg of GPIIb-IIIa from platelets requires the equivalent of several units of donated blood as starting material, is labor intensive, and yields an end product that can be maintained in solution only with detergents.
The two protein chains synthesized in the present study exhibited the mobility predicted for srGPIIb and srGPIIIa in SDS gels. The reaction of the larger product (srGPIIb) with MoAb BlB5 (anti-GPIIbL) and loss of B1B5 binding associated with increased mobility of the major band after reduction (Fig 4) indicates that cleavage of pro-GPIIb into H and L chains and formation of a disulfide link between the cleavage products occurred normally in the baculovirus expression system. The oligosaccharide content of srGPIIb and srGPIIIa was about 7% and 12%, respectively, as judged by the change in their mobilities after enzymatic deglycosylation. The oligosaccharide content of platelet GPIIb and GPIIIa is about 15% to 18%.39,40 GPIIb oligosaccharides, in contrast to those of GPIIIa, are mainly complex and fully processed.40,41 The lower saccharide content of srGPIIb relative to platelet GPIIb may reflect the relative ineffectiveness of insect cells in synthesizing complex sugars.42
Reactions of srGPIIb-IIIa with MoAbs specific for GPIIIa (AP3, AP5, AP6, D3, and CRC54), GPIIb (Tab, B1B5, and MBC 132.1), and the GPIIb-IIIa a complex (AP2, 10E5, 7E3, and PAC-1) indicate that the construct is similar to wild-type GPIIb-IIIa in expressing the epitopes recognized by each of these antibodies.
A principal reason for producing srGPIIb-IIIa was to develop a tool that could be used to detect and study clinically significant antibodies that react with the GPIIb-IIIa complex. As shown in Figs 5-7, srGPIIb-IIIa reacted with alloantibodies specific for alloantigens carried on GPIIb (Baka) and GPIIIa (PlA1 and Pena), with six drug-dependent antibodies induced by quinine, quinidine, and sulfamethoxazole and with three autoantibodies from patients with chronic AITP. The two sulfamethoxazole-dependent antibodies and one of the three autoantibodies (MD) recognize only the intact GPIIb-IIIa complex and are thus specific for epitopes containing amino acids from both chains or for conformational epitopes on one chain that are dependent on its interaction with the other. Recent studies indicate that many autoantibodies reactive with GPIIb-IIIa are of this type.18,19 Reactions of the autoantibody from patient MD are particularly noteworthy. This antibody, which was described in a recently published abstract,43 binds to GPIIb-IIIa on platelets suspended at room temperature in PBS, pH 7.4, containing citrate. However, its reactions are abolished when sodium EDTA, but not calcium EDTA is added. When sodium EDTA is added to intact platelets at room temperature and physiologic pH, the GPIIb-IIIa complex remains intact, but its ability to bind fibrinogen is impaired,44apparently as the result of a structural rearrangement, not yet fully characterized, that is reported by certain MoAbs.45 46Binding of antibody MD to srGPIIb-IIIa indicates that the recombinant heterodimer possesses the EDTA-sensitive epitope or epitopes for which this antibody is specific.
An interesting aspect of the present study is that srGPIIb-IIIa reacted spontaneously with fibrinogen with an average Kd on the order of 30 nmol/L, as judged by the concentration of fibrinogen at which half saturation of immobilized srGPIIb-IIIa was achieved (Fig 8). Half saturation of platelet GPIIb-IIIa studied in parallel was achieved at a fibrinogen concentration of 40 nmol/L. After the addition of LIBS MoAb AP5 to srGPIIb-IIIa, there was a slight increase in fibrinogen bound, but no change in average Kd. The effect of two other LIBS MoAbs, D3 and CRC54, on binding of fibrinogen to srGPIIb-IIIa was similar to that of AP5. In the same experimental system, fibrinogen reacted with platelet GPIIb-IIIa only after activation by LIBS antibody, as expected because platelet GPIIb-IIIa was purified in a nonactivating manner without RGDS-affinity chromatography.24,26 These findings indicate that the recombinant heterodimer is predominantly in an active configuration, like a similar construct prepared by Gulino et al24 and a smaller one produced by Wippler et al.23 The experimental system we used (binding of biotinylated fibrinogen) did not lend itself to Scatchard analysis, but our estimated Kd for fibrinogen binding to immobilized srGPIIb-IIIa is in the range (12 to 70 nmol/L) obtained by others in studies using isolated platelet GPIIb-IIIa.47-49
In summary, we have shown that soluble, recombinant GPIIb-IIIa can be produced free of detergent in large quantities in a baculovirus expression system. Soluble, recombinant GPIIb-IIIa differs from platelet GPIIb-IIIa in having no requirement for activation to bind fibrinogen. However, its reactions with all MoAbs specific for GPIIb, GPIIIa, and the GPIIb-IIIa complex and with allo-, drug-dependent, and autoantibodies studied to date are indistinguishable from those of platelet GPIIb-IIIa. Desired structural modifications can be introduced into srGPIIb-IIIa using standard tools of molecular biology. These properties should make srGPIIb-IIIa useful for detection of clinically significant antibodies specific for the GPIIb-IIIa heterodimer, for characterization of epitopes recognized by such antibodies, and for elucidation of the mechanism(s) by which drugs promote tight binding of antibodies to membrane glycoproteins and cause acute thrombocytopenia in sensitive patients.
ACKNOWLEDGMENT
The authors are indebted to Drs Rodger McEver (Oklahoma City, OK), Joel Bennet (Philadelphia, PA), Thomas Kunicki (LaJolla, CA), Lisa Jennings (Memphis, TN), Michael Berndt (Melbourne, Australia), Barry Coller (New York, NY), and Sanford Shattil (LaJolla, CA) for their gifts of valuable reagents and MoAbs used in these studies.
Supported by Grants No. HL-13629, HL-44612, and HL-03464 and Training Grant No. HL-07209 from the National Heart, Lung, and Blood Institute.
Address reprint requests to Julie A. Peterson, PhD, Blood Research Institute, 8727 Watertown Plank Rd, Milwaukee, WI 53226-3584.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal