Abstract
In contrast to childhood acute lymphoblastic leukemia (ALL), the cell-biological features, clinical characteristics, and treatment outcome of CD10− pro-B ALL have not yet been determined in larger series of adult patients. Therefore, we studied 57 adult patients with newly diagnosed pro-B ALL (median age, 30 years) enrolled in two consecutive German multicenter ALL studies (03/87 and 04/89). Extensive immunophenotypic characterization of leukemic blasts could be performed on all patients, whereas adequate cytogenetic data were available in 33 cases and molecular studies in 18 cases, using reverse transcription-polymerase chain reaction to detect MLL-AF-4 transcripts. Twenty-two patients demonstrated a t(4;11)(q21;q23) and/or MLL-AF-4 rearrangements, and 6 patients had other structural abnormalities, including a t(9;22)(q34;q11) (N = 2). Nine patients had a normal karyotype. Patients with 11q23 abnormalities tended to be younger (median age, 29 years) and were characterized by male predominance (64%), hyperleukocytosis (median leukocyte count, 168 × 109/L), and a frequent coexpression of CD65s (64%) as compared with patients with other cytogenetic abnormalities or a normal karyotype. Twelve of 16 (75%) pro-B ALL patients in study 03/87 and 30 of 41 (73%) in study 04/89 achieved a complete remission (CR). Sixteen of 30 patients in study 04/89 remain in continuous CR (CCR) in contrast to only 2 of 12 patients in study 03/87. Interestingly, all 7 patients treated with high-dose cytarabine and mitoxantrone as consolidation in study 04/89 remain alive and leukemia-free. One patient in study 03/87 and 8 in study 04/89 underwent autologous (N = 2) or allogeneic (N = 7) bone marrow transplantation (BMT). The median remission duration was 420 days for patients in study 03/87 and has not yet been reached in study 04/89. The median survival time of all pro-B ALL patients was 571 days in study 03/87 and 747 days in study 04/89. Among the 22 patients with a t(4;11) and/or MLL-AF-4 rearrangements, 17 achieved a CR and 8 are still in CCR, of whom 4 underwent an allogeneic BMT. Remission duration and overall survival did not differ significantly between pro-B ALL patients with 11q23 abnormalities and those with a normal karyotype or other structural abnormalities. These data indicate that intensification of postremission treatment may improve the prognosis of adult pro-B ALL, including patients with a t(4;11).
© 1998 by The American Society of Hematology.
ADVANCES IN immunophenotyping, cytogenetics, and molecular genetics and their combined application to characterize leukemic blasts have not only considerably improved our knowledge of the pathobiology of acute leukemias, but have also contributed to a refined classification of acute lymphoblastic leukemia (ALL) subtypes and identification of distinct clinicopathologic entities.1-5 Especially in B-lineage ALL, it has become evident that immunophenotypic subgroups mirror a high degree of genotypic diversity and that multiple, distinct molecular pathways are involved in ALL pathogenesis (reviewed in Cline1). This information has been useful to achieve a more precise distinction of biologically and clinically relevant subgroups, including immature CD10− B-cell precursor ALL, often associated with 11q23 translocations, and common or pre-B ALL with fusion transcripts of BCR/ABL due to a t(9;22) (reviewed in Raimondi6), as well as to adjust treatment protocols to the biological features of the leukemic cells involved.3-5
The most immature subtype of B-cell precursor ALL, pro-B ALL (also referred to as pre-pre-B ALL, early-early-B, early B-precursor ALL, null-ALL, B1, or pre-B1 ALL),7-15 accounting for approximately 5% of childhood and 10% of adult ALL cases, is characterized by the expression of CD19, cytoplasmic (cy) or surface membrane CD22, CD24, and cyCD79a, whereas CD10, cyIg M and surface (S) Ig are negative. In childhood ALL, the pro-B immunophenotype is often associated with unfavorable clinical features such as age less than 1 year, high leukocyte counts, massive hepatosplenomegaly, and central nervous system (CNS) disease at diagnosis,9 16-20 whereas relatively little is known about the correlation between pro-B ALL and clinical characteristics in adults.
More recently, significant associations between a CD10− B-cell precursor immunophenotype and abnormalities of chromosome 11, band q23, have been described, particularly in infant ALL (reviewed in Pui et al21 and Rubnitz et al22). Structural lesions involving chromosome 11, band q23, are among the most common cytogenetic abnormalities seen in hematopoietic malignancies. They occur in approximately 5% to 10% of ALL and comprise a heterogeneous collection of gene rearrangements, with most of them involving the MLL gene (also termedHRX, ALL-1, or Htrx1). The MLL gene has recently been cloned,23,24 and three regions of the MLL protein show a significant homology with the Drosophilatrithorax protein. The breakpoints in MLL are restricted to an 8.3-kb region of the gene that is involved in reciprocal translocations, with at least 25 chromosomal regions in a number of phenotypically distinct acute leukemias, including ALL, de novo acute myeloid leukemias (AML), and some types of chemotherapy-related AML.25-27 Although little is known about the normal physiological function of the 431-kD protein encoded by the MLL gene and the cellular mechanisms of MLL-induced leukemogenesis, the involvement of MLL in both lymphoid and myeloid disorders and its structural features suggest that it is involved in the regulation of differentiation pathways, both through direct DNA binding and via interactions with other transcription factors (reviewed in Rubnitz et al22).
Of all the various chromosomal loci participating in 11q23 translocations, the t(4;11)(q21;q23) is by far the best characterized structural lesion, with specific biological and clinical features. This chromosomal abnormality has been detected by standard cytogenetic techniques in 30% to 50% of infant ALL and in about 2% to 6% of older children or adult patients with ALL. A unique constellation of phenotypic and clinical characteristics has been associated with the t(4;11), regardless of the age of the patients (reviewed in Léglise et al,28 Pui,29 and Hilden et al30). These characteristics include a CD10−, CD19+, CD24− or weakly + B-cell precursor phenotype with a high frequency of myeloid-associated (ie, CD15 and/or CD65s) antigen expression, hyperleukocytosis, organomegaly, a high incidence of CNS leukemia, and a dismal prognosis, especially in infant ALL.18,19 31-43The characteristic antigen expression has been interpreted as evidence for the hypothesis that the transformation event in these leukemias involves a stem cell or an early committed progenitor cell with the potential for both lymphoid and myeloid differentiation.
As stated above, relatively little is known about the relationship of immunophenotypic features and 11q23 translocations involving the MLL gene for prognosis in adult patients with pro-B ALL compared with childhood ALL, because in most studies the prognostic relevance of immunophenotype and genotype could not be evaluated due to the small number of patients and heterogeneous treatment protocols.15,36 42-45
In the present study, we therefore studied immunophenotypic and genotypic features in 57 adults with pro-B ALL, the largest series reported to date, to elucidate the biological heterogeneity of this immature B-lineage ALL subtype, to correlate pro-B ALL with clinical characteristics, and to assess the prognostic value of pro-B ALL according to its genotypic features.
MATERIALS AND METHODS
Patients.
From March 1987 to March 1993, central immunophenotyping was successfully performed in 611 adult patients aged 15 to 65 years with newly diagnosed ALL (B-ALL not included), of whom 65 (10.6%) exhibited a pro-B ALL phenotype. All patients were enrolled in two successive clinical trials of the German multicenter study group for treatment of adult ALL (GMALL 03/87 and 04/89).
Morphology and cytochemistry.
Bone marrow and peripheral blood smears were stained using the May-Grünwald-Giemsa technique, and the morphological appearance of blasts was classified in accordance with the criteria of the French-American-British (FAB) Cooperative Group by H. Löffler and W. Gassmann at the central reference institution (Kiel, Germany).46 In addition, the following cytochemical reactions using standard procedures were performed: myeloperoxidase (MPO), naphthyl acetate esterase, acid phosphatase, dipeptidylaminopeptidase IV, and periodic acid Schiff reaction.
Immunophenotyping.
Immunophenotyping was performed at the central reference laboratory of the GMALL studies (Universitätsklinikum Benjamin Franklin, Free University of Berlin, Berlin, Germany). Leukemic cells from heparinized fresh bone marrow or peripheral blood samples were isolated by Ficoll-Hypaque (Pharmacia, Biotech, Freiburg, Germany) density gradient centrifugation, and cell-surface antigens were detected by a standard indirect immunofluorescence (IF) assay, as previously described.7 47 Nonspecific binding was avoided by adding heat-inactivated 10% rabbit serum (GIBCO BRL, Eggenstein, Germany) before immunostaining as well as in subsequent incubation steps with primary or secondary reagents. Fluorescent labelings of surface membrane antigens were immediately evaluated by flow cytometry using a FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, CA). Data acquisition in flow cytometry was performed with FACScan research or Lysis II software (Becton Dickinson). Isotype-matched nonreactive mouse monoclonal antibodies (MoAbs) with the same protein concentrations were used as negative controls in all experiments. Although the viability in percent of fresh leukemic cell suspensions usually exceeded 90%, dead cells were excluded from flow cytometric analysis with propidium iodide (Sigma Chemical Co, St Louis, MO) gating.
The following MoAbs, all available from commercial sources, were used: (1) T- and B-lineage–associated antigens: CD3/Leu-4, CD7/Leu-9, CD19/HD37, CD20/B1, and CD24/OKB2; (2) panmyeloid antigens: CD13/My7, CD33/My9, and CD65s/VIM-2; and (3) nonlineage-restricted antigens: CD10/J5, CD34/My10, and HLA-DR/OKIa1.
Coexpression of lymphoid and myeloid antigens was confirmed by standard two-color flow cytometric analysis using appropriate MoAbs directly conjugated to fluorescein isothiocyanate or phycoerythrin.
For cytoplasmic and nuclear staining, cytospin preparations of leukemic blasts were fixed in acetone (cyCD3, cyIgM) or methanol (terminal deoxynucleotidyl transferase [TdT]), subsequently assayed for direct (cyIgM) or indirect IF using Leu-4 MoAb (cyCD3) or heterologous antisera (goat antimouse IgM, rabbit anti-TdT antiserum; Supertechs, Bethesda, MD), and evaluated for IF using Zeiss microscopes (Zeiss, Oberkochen, Germany) equipped with epi-illumination and phase-contrast devices.
A sample was considered positive for surface antigens if more than 20% of the leukemic cells expressed fluorescence intensity more than 98% of the negative control cells. Positivity for TdT and cytoplasmic antigens was arbitrarily defined as more than 10% of the cells exhibiting nuclear or intracytoplasmic fluorescence as compared with negative controls.
Immunophenotypic subgroups of B-cell precursor ALL were defined as follows: pro-B ALL, TdT+ CD19+CD10− cyIgM−SIg−; common ALL, TdT+ CD19+CD10+ cyIgM− SIg−; pre-B ALL, TdT+ CD19+ CD10+cyIgM+ SIg−.14
Cytogenetics.
For cytogenetic analysis, performed in one of the three reference laboratories (Fonatsch, Lübeck, Germany; Heinze, Ulm, Germany; and Hossfeld, Hamburg, Germany), chromosomes were prepared directly and after 24, 48, and 72 hours of short-term cultivation. Methods used for cell harvest, chromosome preparation, and staining by G-banding technique are described elsewhere.33 Chromosome abnormalities were identified and assigned according to an International System for Human Cytogenetic Nomenclature.48
Molecular studies.
RNA was isolated from cryopreserved leukemia cells obtained at initial diagnosis. MLL-AF-4 fusion transcripts were analyzed by reverse transcription-polymerase chain reaction (RT-PCR) as described in detail elsewhere.49
Clinical investigations.
Clinical investigations were composed of physical examination and peripheral blood and bone marrow analyses. Lumbar puncture for cerebrospinal fluid (CSF) analysis was performed at diagnosis irrespective of whether neurological symptoms were present. Routine laboratory analyses included lactate dehydrogenase (LDH) levels. Furthermore, ultrasonography of the abdomen, x-ray of the chest, computer tomography of the chest and abdomen (if necessary), and echocardiography were performed.
Treatment schedules.
Stratification criteria applied to the definition of low- and high-risk patients and indications for allogeneic bone marrow transplantation (BMT) in first complete remission (CR) were previously reported.50 The criteria used for the definition of the high-risk group in GMALL studies 03/87 and 04/89 were as follows: age greater than 35 years, white blood cell (WBC) count greater than 30 × 109/L, time to CR greater than 4 weeks, pro-B ALL (formerly designated as null ALL), and Ph+ALL.
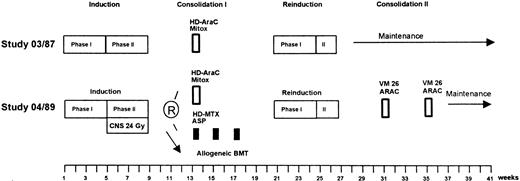
Treatment consisted of induction, reinduction, different consolidation regimens (depending on study and risk group), maintenance, and CNS prophylaxis (Fig 1).
Treatment strategies for high-risk patients of GMALL studies 03/87 and 04/89. The therapy protocols are given in detail in Table 1.
Treatment strategies for high-risk patients of GMALL studies 03/87 and 04/89. The therapy protocols are given in detail in Table 1.
The drugs and doses used in the treatment protocols are listed in Table 1. The induction and reinduction treatment was similar for all patients in GMALL studies 03/87 and 04/89 and has been described in detail elsewhere.51 Patients with large tumor mass or high initial white blood cell count (>25 × 109/L) received 1 week of preinduction treatment with 60 mg/m2 prednisolone orally (PO; days 1 through 7) and 2 mg vincristine intravenously (IV; day 1). The induction was composed of eight cytotoxic drugs administered sequentially in two phases over an 8-week period. During the first 4 weeks (phase I), the patients received 60 mg/m2prednisolone PO daily (days 1 through 28) plus 45 mg/m2daunorubicin and 2 mg vincristine IV weekly (days 1, 8, 15, and 22). L-asparaginase (5,000 U/m2) was administered IV daily (days 15 through 28). In the second 4 weeks (phase II), patients received two doses of 650 mg/m2 cyclophosphamide IV (days 29 and 57) together with 60 mg/m2 6-mercaptopurine PO daily (days 29 through 57) and four courses of 75 mg/m2 cytarabine IV (days 31 through 34, 38 through 41, 45 through 48, and 52 through 55). In study 04/89, one additional dose of cyclophosphamide was administered on day 43. Reinduction treatment was scheduled to begin week 21. The first 4-week cycle of reinduction was identical to phase I of induction, except for daunorubicin, which was substituted by 25 mg/m2 adriamycin, and L-asparaginase, which was omitted. During phase II of reinduction, patients received 650 mg/m2cyclophosphamide IV once (day 29), 60 mg/m2 thioguanine PO (day 29 through 42), and two cycles of 75 mg/m2 cytarabine (days 31 through 34 and 38 through 41).
Treatment Elements for High-Risk ALL in GMALL Studies 03/87 and 04/89
| Induction | |||
| Phase I | |||
| Prednisolone | 3 × 20 mg/m2 | PO | Days 1-28 |
| Vincristine | 2 mg | IV | Days 1, 8, 15, and 22 |
| Daunorubicin | 45 mg/m2 | IV | Days 1, 8, 15, and 22 |
| L-Asparaginase | 5,000 E/m2 | IV | Days 15-28 |
| Methotrexate | 15 mg | IT | Day 1 |
| Phase II | |||
| Cyclophospha mide | 650 mg/m2 | IV | Days 29, 43*, and 57 |
| Cytarabine | 75 mg/m2 | IV | Days 31-34, 38-41, 45-48, and 52-55 |
| 6-Mercaptopu rine | 60 mg/m2 | PO | Days 29-57 |
| Methotrexate | 15 mg | IT | Days 31, 38, 45, and 52 |
| Reinduction | |||
| Phase I | |||
| Prednisolone | 3 × 20 mg/m2 | PO | Days 1-28 |
| Vincristine | 2 mg | IV | Days 1, 8, 15, and 22 |
| Doxorubicin | 25 mg/m2 | IV | Days 1, 8, 15, and 22 |
| Methotrexate | 15 mg | IT | Day 1 |
| Cytarabine | 40 mg | IT | Day 1 |
| Dexamethasone | 4 mg | IT | Day 1 |
| Phase II | |||
| Cyclophospha mide | 650 mg/m2 | IV | Day 29 |
| Cytarabine | 75 mg/m2 | IV | Days 31-34, and 38-41 |
| Thioguanine | 60 mg/m2 | PO | Days 29-42 |
| Methotrexate | 15 mg | IT | Day 29 |
| Cytarabine | 40 mg | IT | Day 29 |
| Dexamethasone | 4 mg | IT | Day 29 |
| Consolidation cycles | |||
| HD cytarabine/ mitoxantrone (week 13) | |||
| Cytarabine | 3 g/m2 (03/87) | IV | Days 1-4 (12 hourly) |
| 1 g/m2 (04/89) | IV | Days 1-4 (12 hourly) | |
| Mitoxantrone | 10 mg/m2 (03/87) | IV | Days 2-6 |
| 10 mg/m2 (04/89) | IV | Days 2-5 | |
| HD methotrexate/ L-asparaginase (weeks 13, 15, and 17) | |||
| HD-Methotrexate | 1500 mg/m2 | IV | Day 1 |
| 1/10 within 30 min and 9/10 within 23.5 hours | |||
| L-Asparaginase | 10,000 IU/m2 | IV | Day 2 |
| Teniposide/ cytarabine (weeks 31 and 35) | |||
| Teniposide | 60 mg/m2 | IV | Days 1-5 |
| Cytarabine | 75 mg/m2 | IV | Days 1-5 |
| Induction | |||
| Phase I | |||
| Prednisolone | 3 × 20 mg/m2 | PO | Days 1-28 |
| Vincristine | 2 mg | IV | Days 1, 8, 15, and 22 |
| Daunorubicin | 45 mg/m2 | IV | Days 1, 8, 15, and 22 |
| L-Asparaginase | 5,000 E/m2 | IV | Days 15-28 |
| Methotrexate | 15 mg | IT | Day 1 |
| Phase II | |||
| Cyclophospha mide | 650 mg/m2 | IV | Days 29, 43*, and 57 |
| Cytarabine | 75 mg/m2 | IV | Days 31-34, 38-41, 45-48, and 52-55 |
| 6-Mercaptopu rine | 60 mg/m2 | PO | Days 29-57 |
| Methotrexate | 15 mg | IT | Days 31, 38, 45, and 52 |
| Reinduction | |||
| Phase I | |||
| Prednisolone | 3 × 20 mg/m2 | PO | Days 1-28 |
| Vincristine | 2 mg | IV | Days 1, 8, 15, and 22 |
| Doxorubicin | 25 mg/m2 | IV | Days 1, 8, 15, and 22 |
| Methotrexate | 15 mg | IT | Day 1 |
| Cytarabine | 40 mg | IT | Day 1 |
| Dexamethasone | 4 mg | IT | Day 1 |
| Phase II | |||
| Cyclophospha mide | 650 mg/m2 | IV | Day 29 |
| Cytarabine | 75 mg/m2 | IV | Days 31-34, and 38-41 |
| Thioguanine | 60 mg/m2 | PO | Days 29-42 |
| Methotrexate | 15 mg | IT | Day 29 |
| Cytarabine | 40 mg | IT | Day 29 |
| Dexamethasone | 4 mg | IT | Day 29 |
| Consolidation cycles | |||
| HD cytarabine/ mitoxantrone (week 13) | |||
| Cytarabine | 3 g/m2 (03/87) | IV | Days 1-4 (12 hourly) |
| 1 g/m2 (04/89) | IV | Days 1-4 (12 hourly) | |
| Mitoxantrone | 10 mg/m2 (03/87) | IV | Days 2-6 |
| 10 mg/m2 (04/89) | IV | Days 2-5 | |
| HD methotrexate/ L-asparaginase (weeks 13, 15, and 17) | |||
| HD-Methotrexate | 1500 mg/m2 | IV | Day 1 |
| 1/10 within 30 min and 9/10 within 23.5 hours | |||
| L-Asparaginase | 10,000 IU/m2 | IV | Day 2 |
| Teniposide/ cytarabine (weeks 31 and 35) | |||
| Teniposide | 60 mg/m2 | IV | Days 1-5 |
| Cytarabine | 75 mg/m2 | IV | Days 1-5 |
*Only administered in GMALL study 04/89 (see treatment schedules).
Consolidation treatment was different in studies 03/87 and 04/89. In GMALL study 03/87, high-risk patients received one cycle of high-dose (HD) cytarabine (3 g/m2, every 12 hours as a 3-hour infusion, days 1 through 4) plus 10 mg/m2 mitoxantrone IV (days 2 through 6). For patients older than 50 years, the dose of cytarabine was reduced to 1 g/m2. After reinduction, maintenance was started with daily oral doses of 60 mg/m2mercaptopurine and 20 mg/m2 methotrexate IV weekly from week 28 to 132, without further consolidation cycles. In GMALL study 04/89, younger patients (15 to 50 years of age) in the high-risk group were scheduled for allogeneic BMT after induction phase II if a HLA-compatible sibling donor was available. Patients without a donor were randomized to receive either one cycle of HD cytarabine and mitoxantrone (week 13), as described above, or three cycles of HD methotrexate and L-asparaginase (weeks 13, 15, and 17). The dosage of cytarabine was reduced to 1 g/m2, and mitoxantrone treatment was shortened (days 2 through 5). HD methotrexate was administered as a 24-hour infusion at the dose level of 1,500 mg/m2 (1/10 during the first 30 minutes and 9/10 during 23.5 hours) on day 1, followed by 10,000 U/m2L-asparaginase as a 1-hour infusion on day 2 and a leucovorin rescue. After reinduction, patients received two cycles of teniposide and cytarabine (weeks 31 and 35). Teniposide (60 mg/m2) and cytarabine (75 mg/m2) were each administered as a 1-hour infusion daily from day 1 to 5. Starting from week 39, maintenance treatment was administered up to week 142. In patients older than 50 years, consolidation consisted of two further cycles of teniposide and cytarabine (weeks 13 and 17) instead of the randomized consolidation, as described above.
CNS prophylaxis, consisting of 15 mg methotrexate, was administered intrathecally on days 1, 31, 38, 45, and 52 of induction and on day 1 of reinduction. During maintenance, patients received every 2 months intrathecal (IT) triple chemotherapy with 15 mg methotrexate, 40 mg cytarabine, and 4 mg dexamethasone, up to a total number of 12 administrations. In GMALL study 04/89, all patients received cranial irradiation with 24 Gy parallel to induction phase II. Intrathecal methotrexate was administered as described above. Intrathecal triple therapy started from days 1 and 29 of reinduction and was continued during maintenance, as described above. The triple combination was also administered intrathecally on day 1 of the cycles with teniposide and cytarabine.
In GMALL study 03/87, all patients with pro-B ALL were treated in accordance with the high-risk protocol, whereas, in study 04/89, elderly patients (>50 years) were stratified into the standard-risk group.50
Statistical analysis.
The statistical analysis was performed by the Biometric Centre for Therapy Studies (Ü. Aydemir, Munich, Germany) and N. Gökbuget (Frankfurt, Germany). The influence of entrance parameters on the achievement of CR was evaluated by the χ2-test. Median values were compared using the Wilcoxon-Mann Whitney test. Survival was defined as the time from the beginning of the therapy to the date of the last review or death in all evaluable patients. Probability of continuous CR (CCR) was determined from the date of CR to the date of last review or BMT in censored patients and to the date of relapse or death in CR in patients with events. The probability of surviving and remaining in CR was estimated by the Kaplan and Meier method.52 The univariate comparison between survival curves was performed by the log-rank test.53
The results were updated in May 1997, with a median follow-up time for censored patients for study ALL 03/87 of 2,547 days and for study ALL 04/89 of 2,051 days.
RESULTS
Clinical characteristics of the patients.
Of the 65 pro-B ALL patients from 28 different centers enrolled in two consecutive studies (GMALL 03/87 and 04/89), 57 patients were evaluable. Reasons for exclusion were refusal of the treatment protocol, psychotic depressive disorder, and pro-B ALL as second neoplasm. The clinical characteristics of the evaluable patients are shown in Table 2. Pro-B ALL patients in GMALL study 04/89 had a higher median WBC count compared with study 03/87 (85.3 v 14.5 × 109/L; P = .007). Thirty-eight percent of the patients in GMALL study 03/87 as compared with 12% in study 04/89 had initial infections (P = .03). There was a higher proportion of patients older than 50 years in GMALL study 03/87 (44%) and a higher median age (42.5 years) compared with study 04/89 (17% and 28 years, respectively). However, these differences did not reach statistical significance. The other clinical characteristics did not differ significantly between both studies.
Initial Clinical Characteristics of 57 Patients With Pro-B ALL
| Patient Characteristics . | ALL-Study 03/87 N = 16 (%) . | ALL-Study 04/89 N = 41 (%) . | P Value . | Total N = 57 (%) . |
|---|---|---|---|---|
| Sex | ||||
| Male | 7 (44) | 20 (49) | NS | 27 (47) |
| Female | 9 (56) | 21 (51) | 30 (53) | |
| Age (yr) | ||||
| Median (range) | 42.5 (17-61) | 28 (16-62) | NS | 30 (16-62) |
| ≤20 | 2 (12) | 6 (15) | 8 (14) | |
| 21-50 | 7 (44) | 28 (68) | NS | 35 (61) |
| >50 | 7 (44) | 7 (17) | 14 (25) | |
| Bleeding | 6 (38) | 15 (37) | NS | 21 (37) |
| Infections | 6 (38) | 5 (12) | .03 | 11 (19) |
| Peripheral lymph nodes | 7 (44) | 19 (46) | NS | 26 (46) |
| Hepatomegaly | 8 (50) | 14 (34) | NS | 22 (39) |
| Splenomegaly | 4 (25) | 20 (49) | NS | 24 (42) |
| CNS involvement | 1 (6) | 3 (7) | NS | 4 (7) |
| Mediastinal mass | 1 (6) | 1 (2) | NS | 2 (4) |
| WBC (×109/L) | ||||
| Median (range) | 14.5 (1.3-185) | 85.3 (0.5-562) | .007 | 30.6 (0.5-562) |
| ≤30 | 10 (63) | 18 (44) | NS | 28 (49) |
| >30 | 6 (37) | 23 (56) | 29 (51) | |
| Hb (g/dL) | ||||
| ≤8 | 6 (37) | 10 (24) | NS | 16 (28) |
| >8 | 10 (63) | 31 (76) | 41 (72) |
| Patient Characteristics . | ALL-Study 03/87 N = 16 (%) . | ALL-Study 04/89 N = 41 (%) . | P Value . | Total N = 57 (%) . |
|---|---|---|---|---|
| Sex | ||||
| Male | 7 (44) | 20 (49) | NS | 27 (47) |
| Female | 9 (56) | 21 (51) | 30 (53) | |
| Age (yr) | ||||
| Median (range) | 42.5 (17-61) | 28 (16-62) | NS | 30 (16-62) |
| ≤20 | 2 (12) | 6 (15) | 8 (14) | |
| 21-50 | 7 (44) | 28 (68) | NS | 35 (61) |
| >50 | 7 (44) | 7 (17) | 14 (25) | |
| Bleeding | 6 (38) | 15 (37) | NS | 21 (37) |
| Infections | 6 (38) | 5 (12) | .03 | 11 (19) |
| Peripheral lymph nodes | 7 (44) | 19 (46) | NS | 26 (46) |
| Hepatomegaly | 8 (50) | 14 (34) | NS | 22 (39) |
| Splenomegaly | 4 (25) | 20 (49) | NS | 24 (42) |
| CNS involvement | 1 (6) | 3 (7) | NS | 4 (7) |
| Mediastinal mass | 1 (6) | 1 (2) | NS | 2 (4) |
| WBC (×109/L) | ||||
| Median (range) | 14.5 (1.3-185) | 85.3 (0.5-562) | .007 | 30.6 (0.5-562) |
| ≤30 | 10 (63) | 18 (44) | NS | 28 (49) |
| >30 | 6 (37) | 23 (56) | 29 (51) | |
| Hb (g/dL) | ||||
| ≤8 | 6 (37) | 10 (24) | NS | 16 (28) |
| >8 | 10 (63) | 31 (76) | 41 (72) |
Abbreviation: NS, not significant.
Morphology and cytochemistry.
Of the 53 evaluable patients with pro-B ALL, 79% disclosed a L2 morphology as compared with 66% of common ALL patients analyzed in parallel. No clear-cut differences could be observed between pro-B and common ALL with regard to cytoplasmic or nuclear features and cytochemical stainings. In none of the patients were MPO-positive blasts found.
Immunophenotyping.
Although the total number of patients varied for each marker tested, mainly because of insufficient cell numbers, the diagnostically relevant B-, T-, and myeloid-lineage, as well as non–lineage-restricted antigens could be analyzed in the vast majority of the patient population.
By definition, none of the patient samples expressed CD10, whereas leukemic blasts from all patient samples tested for CD19 (N = 53) expressed this antigen. Expression of CD24 was found in 35 of 53 patients (66%), and weak expression (20% to 40% positive cells) of CD20 was found in 3 of 56 patients (5%). In none of the 57 patients was evidence for coexpression of the T-lineage–associated antigen CD7 found.
Of the panmyeloid antigens analyzed, a coexpression of CD65s was observed in 24 of 56 patients (43%), whereas a coexpression of CD13 and CD33 occurred much less frequently (10% and 14%, respectively). In all patients, coexpression of B- and myeloid-lineage–associated antigens was confirmed by two-color IF studies or marked numerical overlap of percentage of cells expressing the respective antigens.
HLA-DR was strongly expressed by leukemic blasts from all 57 patients, and TdT-positivity was found in 51 of 55 patients (93%). CD34 positivity was found in 29 of 50 patients (58%).
Cytogenetic and molecular studies.
Of the 57 evaluable patients, adequate cytogenetic analyses could be performed in 33 patients and molecular studies could be performed in 18 patients. In 12 patients, both cytogenetic and molecular data were available.
Nine of the 33 patients with adequate cytogenetic studies had only normal metaphases. Eighteen patients (54.5%) had a t(4;11)(q21;q23), including 1 patient with the derivative chromosome der (11) involved in a translocation t(11;17)(p11;q11) (Table3).
Characteristics of Patients With Pro-B ALL and t(4;11) and/or MLL-AF-4 Rearrangements Ordered by Age
| Patient No. . | Age (yr) . | WBC (×109/L) . | Karyotype . | MLL-AF-4 . | CD24* . | CD65s* . |
|---|---|---|---|---|---|---|
| 1 | 22 | 16.8 | 46, XY, t(4;11)(q21;q23) | R | 24 | 50 |
| 2 | 22 | 146.0 | 46, XY, t(4;11)(q21;q23) | ND | 29 | 40 |
| 3 | 24 | 562.0 | 45, XY, der(2q) | R | 1 | 8 |
| 4 | 24 | 11.6 | 46, XY, t(4;11)(q21;q23) | R | 3 | 20 |
| 5 | 24 | 373.0 | 46, XY, t(4;11)(q21;q23) | ND | 4 | 30 |
| 6 | 24 | 184.0 | 46, XY, t(4;11)(q21;q23) | R | 17 | 37 |
| 7 | 25 | 198.0 | ND | R | 20 | 30 |
| 8 | 25 | 346.0 | 46, XY, t(4;11)(q21;q23) | ND | 10 | 70 |
| 9 | 26 | 0.8 | ND | R | 20 | 9 |
| 10 | 28 | 355.0 | 46, XX, t(4;11)(q21;q23) | ND | 23 | 17 |
| 11 | 28 | 175.0 | 46, XY, t(4;11)(q21;q23) | ND | 26 | 17 |
| 12 | 30 | 228.0 | 46, XX, t(4;11)(q21;q23) | R | 2 | 45 |
| 13 | 30 | 246.9 | 46, XY, t(4;11)(q21;q23) | R | 9 | 25 |
| 14 | 31 | 168.5 | 46, XY, t(4;11)(q21;q23), i(7)(q10) | R | 7 | 30 |
| 15 | 36 | 67.0 | Normal | R | 0 | 83 |
| 16 | 40 | 11.0 | 46, XX, t(4;11)(q21;q23)/48, XX, t(4;11)(q21;q23), +mar, +mar | ND | 40 | 10 |
| 17 | 41 | 168.0 | 46, XX, t(4;11)(q21;q23) | R | ND | 25 |
| 18 | 49 | 163.0 | 46, XX, t(4;11)(q21;q23) | R | 0 | 46 |
| 19 | 50 | 421.0 | 46, XX, t(4;11)(q21;q23)/48-53, XX, +X, +1, t(4;11)(q21;q23), inc [cp6] | ND | 4 | 74 |
| 20 | 53 | 18.6 | 46, XY, der(4) t(4;11)(q21;q23), der(11) t(11;17)(p11;q11) t(4;11)(q21;q23), −17, +mar | ND | 25 | 2 |
| 21 | 56 | 73.4 | 46, XX, t(4;11)(q21;q23) | ND | 43 | 0 |
| 22 | 62 | 9.4 | 46, XY, t(4;11)(q21;q23) | ND | 44 | 8 |
| Patient No. . | Age (yr) . | WBC (×109/L) . | Karyotype . | MLL-AF-4 . | CD24* . | CD65s* . |
|---|---|---|---|---|---|---|
| 1 | 22 | 16.8 | 46, XY, t(4;11)(q21;q23) | R | 24 | 50 |
| 2 | 22 | 146.0 | 46, XY, t(4;11)(q21;q23) | ND | 29 | 40 |
| 3 | 24 | 562.0 | 45, XY, der(2q) | R | 1 | 8 |
| 4 | 24 | 11.6 | 46, XY, t(4;11)(q21;q23) | R | 3 | 20 |
| 5 | 24 | 373.0 | 46, XY, t(4;11)(q21;q23) | ND | 4 | 30 |
| 6 | 24 | 184.0 | 46, XY, t(4;11)(q21;q23) | R | 17 | 37 |
| 7 | 25 | 198.0 | ND | R | 20 | 30 |
| 8 | 25 | 346.0 | 46, XY, t(4;11)(q21;q23) | ND | 10 | 70 |
| 9 | 26 | 0.8 | ND | R | 20 | 9 |
| 10 | 28 | 355.0 | 46, XX, t(4;11)(q21;q23) | ND | 23 | 17 |
| 11 | 28 | 175.0 | 46, XY, t(4;11)(q21;q23) | ND | 26 | 17 |
| 12 | 30 | 228.0 | 46, XX, t(4;11)(q21;q23) | R | 2 | 45 |
| 13 | 30 | 246.9 | 46, XY, t(4;11)(q21;q23) | R | 9 | 25 |
| 14 | 31 | 168.5 | 46, XY, t(4;11)(q21;q23), i(7)(q10) | R | 7 | 30 |
| 15 | 36 | 67.0 | Normal | R | 0 | 83 |
| 16 | 40 | 11.0 | 46, XX, t(4;11)(q21;q23)/48, XX, t(4;11)(q21;q23), +mar, +mar | ND | 40 | 10 |
| 17 | 41 | 168.0 | 46, XX, t(4;11)(q21;q23) | R | ND | 25 |
| 18 | 49 | 163.0 | 46, XX, t(4;11)(q21;q23) | R | 0 | 46 |
| 19 | 50 | 421.0 | 46, XX, t(4;11)(q21;q23)/48-53, XX, +X, +1, t(4;11)(q21;q23), inc [cp6] | ND | 4 | 74 |
| 20 | 53 | 18.6 | 46, XY, der(4) t(4;11)(q21;q23), der(11) t(11;17)(p11;q11) t(4;11)(q21;q23), −17, +mar | ND | 25 | 2 |
| 21 | 56 | 73.4 | 46, XX, t(4;11)(q21;q23) | ND | 43 | 0 |
| 22 | 62 | 9.4 | 46, XY, t(4;11)(q21;q23) | ND | 44 | 8 |
Abbreviations: R, rearranged; ND, not done.
Percentage of positive lymphoblasts.
Six patients had other structural or numerical abnormalities. Two of these patients had a standard t(9;22)(q34;q11), with 1 of them showing additional aberrations such as dup(1)(q23q32), del(16)(q11) and i(17)(q10).54 In both patients with a Ph chromosome, coexpression of myeloid antigens (CD33 and/or CD13) on leukemic blasts was detected by immunophenotyping. Other cytogenetic abnormalities included 1 patient with hyperdiploidy (56 chromosomes), 1 with complex aberrations, 1 with a dic(7;9)(p11;p11), and 1 with a der(2q).
Molecular studies performed by RT-PCR showed MLL-AF-4 transcripts in 12 of 18 patients analyzed. Of the 10 patients in our study with a t(4;11) and/or evidence of MLL-AF-4 rearrangements, for whom both cytogenetic and molecular studies were available, the two methods were in agreement in 8 cases, whereas molecular studies showed MLL-AF-4 transcripts in 2 patients, with 1 showing a normal karyotype and 1 a derivative chromosome 2 by cytogenetic analysis (Table 3).
Correlation of surface antigen expression with genotypic features and initial clinical characteristics in patients with chromosome 11q23 abnormalities.
The initial characteristics of 22 patients ordered by age with a pro-B ALL and a t(4;11) and/or a MLL-AF-4 rearrangement are summarized in Table 3. Age ranged from 22 to 62 years. The presence of a t(4;11) or of a MLL rearrangement occurred more frequently in male patients and, in most cases, was associated with very high leukocyte counts. Coexpression of CD65s on leukemic blasts was found in 14 of 22 patients; in 2 additional cases, there was a small subpopulation of leukemic cells disclosing CD65s positivity. In none of the 22 patients, lymphoblasts demonstrated a clear coexpression of the other panmyeloid antigens tested (ie, CD13, CD33). In contrast to other B-lineage ALL subgroups, such as common, pre-B and B-ALL, the percentage of CD19 positive blasts (>70% in all but 1 case) was much higher than that for CD24 in all patients, with most cases showing low levels (20% to 40% CD24+ cells) or, occasionally, missing CD24 expression.
Clinical features and response to induction therapy according to cytogenetic and/or molecular analyses.
For comparison of the main pretherapeutic features and response to induction therapy, the 57 patients were divided into three subgroups based on the availability and the results of cytogenetic and/or molecular analyses: (1) patients with a t(4;11) and/or a MLL-AF-4 rearrangement; (2) patients with a normal karyotype and other cytogenetic abnormalities without MLL-AF-4 rearrangement; and (3) patients for whom cytogenetic and/or molecular results were not available (Table 4). Comparisons between patients with a t(4;11) and/or a MLL-AF-4 rearrangement and patients with a normal karyotype or other cytogenetic abnormalities showed the following significant differences: patients with a t(4;11) and/or a MLL-AF-4 rearrangement were more often in the younger age group (P = .007) and had less often initial infections (P = .01), and their leukemic blasts disclosed more often a coexpression of CD65s (P = .03). Additionally, there was a male predominance and a higher median WBC count in patients with a t(4;11) and/or a MLL-AF-4 rearrangement that, however, did not have statistical significance. The other clinical characteristics and the CR rate did not differ significantly between patients with a t(4;11) and/or a MLL-AF-4 rearrangement and patients with a normal karyotype or other cytogenetic abnormalities.
Characteristics of Patients and Response to Induction Treatment in Pro-B ALL Subtypes According to Cytogenetic and Molecular Analyses
| Characteristic . | t(4;11) and/or MLL-AF-4 Rearrangement N = 22 (%) . | Normal Karyotype or Other Cytogenetic Abnormalities N = 13 (%) . | P Value3-150 . | Cytogenetic and Molecular Analyses Not Available N = 22 (%) . |
|---|---|---|---|---|
| Sex | ||||
| Male | 14 (64) | 5 (38) | NS | 8 (36) |
| Female | 8 (36) | 8 (62) | 14 (64) | |
| Age (yr) | ||||
| Median (range) | 29 (22-62) | 39 (16-59) | NS | 33 (16-62) |
| ≤20 | 0 | 3 (23) | 5 (23) | |
| 21-50 | 19 (86) | 5 (38) | .007 | 11 (50) |
| >50 | 3 (14) | 5 (38) | 6 (27) | |
| Bleeding | 12 (55) | 3 (23) | NS | 6 (27) |
| Infection | 1 (5) | 5 (38) | .01 | 5 (23) |
| Peripheral lymph nodes | 10 (45) | 5 (38) | NS | 11 (50) |
| Hepatomegaly | 9 (41) | 6 (46) | NS | 7 (32) |
| Splenomegaly | 13 (59) | 5 (38) | NS | 6 (27) |
| CNS involvement | 2 (9) | 1 (8) | NS | 1 (5) |
| Mediastinal mass | 0 | 0 | 2 (9) | |
| WBC (×109/L) | ||||
| Median (range) | 168.3 (0.8-562.0) | 30.6 (5.2-545.0) | NS | 16.1 (0.5-489.0) |
| ≤30 | 6 (27) | 6 (46) | NS | 16 (73) |
| >30 | 16 (73) | 7 (54) | 6 (27) | |
| Hb (g/dL) | ||||
| ≤8 | 10 (45) | 6 (46) | NS | 10 (45) |
| >8 | 12 (55) | 7 (54) | 12 (55) | |
| Coexpression of myeloid antigens3-151 | ||||
| CD13 | 0 | 3 (27) | NA | 2 (16) |
| CD33 | 0 | 4 (36) | NA | 3 (18) |
| CD65s | 14 (64) | 3 (25) | .03 | 7 (32) |
| CR rate | 17 (77) | 11 (85) | NS | 14 (64) |
| Characteristic . | t(4;11) and/or MLL-AF-4 Rearrangement N = 22 (%) . | Normal Karyotype or Other Cytogenetic Abnormalities N = 13 (%) . | P Value3-150 . | Cytogenetic and Molecular Analyses Not Available N = 22 (%) . |
|---|---|---|---|---|
| Sex | ||||
| Male | 14 (64) | 5 (38) | NS | 8 (36) |
| Female | 8 (36) | 8 (62) | 14 (64) | |
| Age (yr) | ||||
| Median (range) | 29 (22-62) | 39 (16-59) | NS | 33 (16-62) |
| ≤20 | 0 | 3 (23) | 5 (23) | |
| 21-50 | 19 (86) | 5 (38) | .007 | 11 (50) |
| >50 | 3 (14) | 5 (38) | 6 (27) | |
| Bleeding | 12 (55) | 3 (23) | NS | 6 (27) |
| Infection | 1 (5) | 5 (38) | .01 | 5 (23) |
| Peripheral lymph nodes | 10 (45) | 5 (38) | NS | 11 (50) |
| Hepatomegaly | 9 (41) | 6 (46) | NS | 7 (32) |
| Splenomegaly | 13 (59) | 5 (38) | NS | 6 (27) |
| CNS involvement | 2 (9) | 1 (8) | NS | 1 (5) |
| Mediastinal mass | 0 | 0 | 2 (9) | |
| WBC (×109/L) | ||||
| Median (range) | 168.3 (0.8-562.0) | 30.6 (5.2-545.0) | NS | 16.1 (0.5-489.0) |
| ≤30 | 6 (27) | 6 (46) | NS | 16 (73) |
| >30 | 16 (73) | 7 (54) | 6 (27) | |
| Hb (g/dL) | ||||
| ≤8 | 10 (45) | 6 (46) | NS | 10 (45) |
| >8 | 12 (55) | 7 (54) | 12 (55) | |
| Coexpression of myeloid antigens3-151 | ||||
| CD13 | 0 | 3 (27) | NA | 2 (16) |
| CD33 | 0 | 4 (36) | NA | 3 (18) |
| CD65s | 14 (64) | 3 (25) | .03 | 7 (32) |
| CR rate | 17 (77) | 11 (85) | NS | 14 (64) |
Abbreviations: NS, not significant; NA, not applicable.
P values refer to the comparison between patients with a t(4;11) and/or MLL-AF-4 rearrangement versus patients with a normal karyotype or other cytogenetic abnormalities.
Missing data for 8 patients (CD13), 6 patients (CD33), and 1 patient (CD65s), respectively.
Treatment results.
Table 5 summarizes the overall results of the induction therapy and the subsequent course of adult patients with pro-B ALL in GMALL studies 03/87 and 04/89. The CR rate did not differ significantly between both studies. One patient in GMALL study 03/87 and 6 in study 04/89 failed to achieve a CR due to treatment failure, and 8 patients died during induction therapy within 56 days (early death) because of tumor lysis syndrome (N = 1), infection (N = 5), hepatic failure (N = 1), or unknown cause (N = 1). Of the 12 patients who achieved a CR in GMALL study 03/87, 10 relapsed or died in CR and 2 patients remain in CR. One of the 2 patients remaining in CCR received autologous BMT and the other was treated using HD cytarabine and mitoxantrone as consolidation. Of the 30 patients who achieved a CR in GMALL study 04/89, 16 (median age, 25 years; range, 16 to 51 years) remained in CCR between 883 and 2,886 days. Two patients died in CR and 12 patients relapsed. Of the 16 patients remaining in CCR, 11 received consolidation therapy with HD cytarabine and mitoxantrone (N = 7), with HD methotrexate and L-asparaginase (N = 2), or with teniposide and cytarabine (N = 2). Five of the CCR patients received allogeneic (N = 4) or autologous (N = 1) BMT. Both patients with autologous BMT had received consolidation therapy with HD cytarabine and mitoxantrone before BMT.
Overall Results in Adult Pro-B ALL Patients
| Treatment Results . | ALL-Study 03/87 . | ALL-Study 04/89 . |
|---|---|---|
| Study period | 1987-1989 | 1989-1993 |
| Patients enrolled | 18 | 47 |
| Patients evaluable | 16 | 41 |
| Median age in years (range) | 42.5 (17-61) | 28 (16-62) |
| CR | 12 (75%) | 30 (73%) |
| Failure | 1 (8%) | 6 (15%) |
| Early death | 3 (19%) | 5 (12%) |
| CCR | 2 (17%) | 16 (53%) |
| HD AraC/mitoxantrone | 1 | 7 |
| HD AraC/mitoxantrone and autologous BMT | 1 | 1 |
| Allogeneic BMT | 0 | 4 |
| HD MTX/L-asp; VM26/AraC | 0 | 4 |
| Relapse | 8 (66%) | 12 (40%) |
| Before HD AraC/mitoxantrone | 1 | 4 |
| HD AraC/mitoxantrone | 4 | 0 |
| Allogeneic BMT | 0 | 1 |
| HD MTX/L-asp; VM26/AraC | 3 | 7 |
| Death in CR | 2 (17%) | 2 (7%) |
| Before HD AraC/mitoxantrone | 1 | 0 |
| HD AraC/mitoxantrone | 1 | 0 |
| Allogeneic BMT | 0 | 2 |
| Treatment Results . | ALL-Study 03/87 . | ALL-Study 04/89 . |
|---|---|---|
| Study period | 1987-1989 | 1989-1993 |
| Patients enrolled | 18 | 47 |
| Patients evaluable | 16 | 41 |
| Median age in years (range) | 42.5 (17-61) | 28 (16-62) |
| CR | 12 (75%) | 30 (73%) |
| Failure | 1 (8%) | 6 (15%) |
| Early death | 3 (19%) | 5 (12%) |
| CCR | 2 (17%) | 16 (53%) |
| HD AraC/mitoxantrone | 1 | 7 |
| HD AraC/mitoxantrone and autologous BMT | 1 | 1 |
| Allogeneic BMT | 0 | 4 |
| HD MTX/L-asp; VM26/AraC | 0 | 4 |
| Relapse | 8 (66%) | 12 (40%) |
| Before HD AraC/mitoxantrone | 1 | 4 |
| HD AraC/mitoxantrone | 4 | 0 |
| Allogeneic BMT | 0 | 1 |
| HD MTX/L-asp; VM26/AraC | 3 | 7 |
| Death in CR | 2 (17%) | 2 (7%) |
| Before HD AraC/mitoxantrone | 1 | 0 |
| HD AraC/mitoxantrone | 1 | 0 |
| Allogeneic BMT | 0 | 2 |
Abbreviations: CR, complete remission; CCR, continuous complete remission; HD, high dose; AraC, cytarabine; BMT, bone marrow transplantation; MTX, methotrexate; L-asp, L-asparaginase; VM26, teniposide.
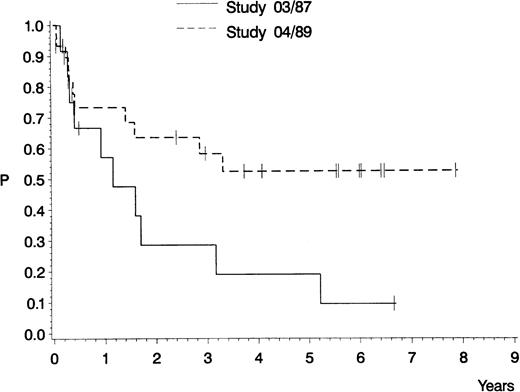
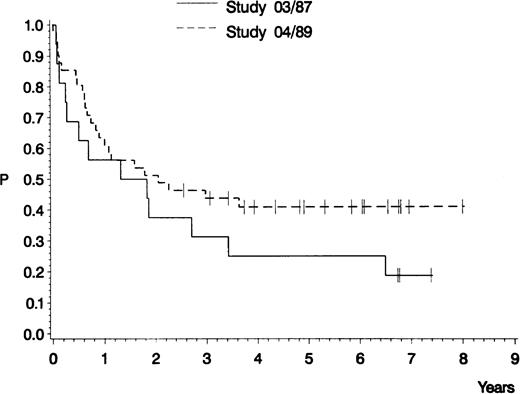
The estimated probability of CCR and overall survival is shown in Figs 2 and3, respectively. For the 12 CR patients in GMALL study 03/87, the median remission duration is 420 days and the probability of being in CCR at 5 years is 10%. For the 30 CR patients in GMALL study 04/89, the median remission duration has not yet been reached and the probability of being in CCR at 5 years is 52% (study 03/87 v 04/89: P = .04). The estimated median survival is 571 days for all 16 patients in study GMALL 03/87, compared with 747 days for all 41 patients in study 04/89 (P = .21).
Probability of continuous complete remission of 12 adult patients with pro-B ALL treated in GMALL study 03/87 (0.10; median, 420 days) and of 30 adult patients with pro-B ALL treated in GMALL study 04/89 (0.52; median, not yet reached). P (log-rank) = .04.
Probability of continuous complete remission of 12 adult patients with pro-B ALL treated in GMALL study 03/87 (0.10; median, 420 days) and of 30 adult patients with pro-B ALL treated in GMALL study 04/89 (0.52; median, not yet reached). P (log-rank) = .04.
Probability of survival of 16 adult patients with pro-B ALL treated in GMALL study 03/87 (0.19; median, 571 days) and of 41 adult patients with pro-B ALL treated in GMALL study 04/89 (0.41; median, 747 days). P (log-rank) = .21.
Probability of survival of 16 adult patients with pro-B ALL treated in GMALL study 03/87 (0.19; median, 571 days) and of 41 adult patients with pro-B ALL treated in GMALL study 04/89 (0.41; median, 747 days). P (log-rank) = .21.
Probability of CCR and overall survival proved to be dependent on age, with a better outcome for the younger age group (probability of being in CCR, 0.45; probability of survival, 0.41) compared with older patients (0.13 and 0.07, respectively). However, these differences only had statistical significance for survival (P = .006) when both studies were analyzed together. When looking at different age groups in GMALL studies 03/87 and 04/89 with regard to their treatment outcome, the median remission duration (not yet reached) and probability of being in CCR (59%) in the younger age group were better for 26 CR patients in study 04/89 when compared with 8 CR patients in study 03/87 (median, 334 days; probability of CCR, 16%). However, this difference was not significant (P = .06), probably due to the small number of patients.
Of the 11 patients in the younger age group receiving consolidation therapy with HD cytarabine and mitoxantrone in GMALL studies 03/87 and 04/89, 8 remained in CCR more than 3 years. Of the 7 patients receiving consolidation with HD methotrexate and L-asparaginase in GMALL study 04/89, 2 are still in CCR. Nine patients from both studies underwent BMT (allogeneic N = 7, autologous N = 2), and 6 of them remain in CCR (allogeneic N = 4, autologous N = 2). The median age of the patient groups receiving either different consolidation regimens or BMT is comparable (HD cytarabine plus mitoxantrone, 22 years; HD methotrexate plus L-asparaginase, 26 years; and BMT, 24 years). The median remission duration for consolidation with HD cytarabine and mitoxantrone has not been reached (probability of being in CCR, 68%) compared with 576 days (probability of being in CCR, 29%) for consolidation with HD methotrexate and L-asparaginase (P = .07).
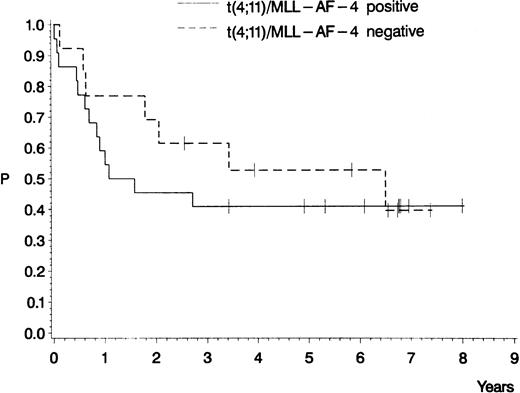
Of the 57 patients with pro-B ALL, 22 had a t(4;11) and/or molecular evidence of MLL-AF-4 fusion transcripts, of whom 17 (77%) achieved a CR. Fifteen of the patients who achieved a CR were in the younger age group, with 8 of them still being in CCR. The patients with a t(4;11) and/or MLL-AF-4 transcripts did not differ with respect to the estimated probability of CCR (40%) and overall survival (41%) from patients with a normal karyotype or with other cytogenetic abnormalities (probability of CCR, 45%; overall survival, 40%;P = .46 and .53, respectively; Figs4 and 5). Pro-B ALL patients whose leukemic blasts disclosed a coexpression of myeloid-associated antigens, mainly CD65s, did not differ significantly regarding duration of remission and survival from patients with a myeloid-antigen–negative pro-B ALL (data not shown).
Probability of CCR for pro-B ALL patients treated in GMALL studies 03/87 and 04/89 according to the results of the cytogenetic and molecular analyses. Seventeen patients with a t(4;11) and/or MLL-AF-4 fusion transcripts (0.40; median, 576 days) and 11 patients with a normal karyotype or with other cytogenetic abnormalities (0.45; median, 1,906 days). P (log-rank) = .46.
Probability of CCR for pro-B ALL patients treated in GMALL studies 03/87 and 04/89 according to the results of the cytogenetic and molecular analyses. Seventeen patients with a t(4;11) and/or MLL-AF-4 fusion transcripts (0.40; median, 576 days) and 11 patients with a normal karyotype or with other cytogenetic abnormalities (0.45; median, 1,906 days). P (log-rank) = .46.
Probability of survival for pro-B ALL patients treated in GMALL studies 03/87 and 04/89 according to the results of the cytogenetic and molecular analyses. Twenty-two patients with a t(4;11) and/or MLL-AF-4 fusion transcripts (0.41; median, 484 days) and 13 patients with a normal karyotype or other cytogenetic abnormalities (0.40; median, 2,373 days). P (log-rank) = .53.
Probability of survival for pro-B ALL patients treated in GMALL studies 03/87 and 04/89 according to the results of the cytogenetic and molecular analyses. Twenty-two patients with a t(4;11) and/or MLL-AF-4 fusion transcripts (0.41; median, 484 days) and 13 patients with a normal karyotype or other cytogenetic abnormalities (0.40; median, 2,373 days). P (log-rank) = .53.
For high-risk patients with a common or pre-B ALL, the median remission duration is 309 days and the probability of being in CCR at 4 years 18% in GMALL study 03/87, as compared with a median remission duration of 317 days and a probability of being in CCR at 4 years of 20% in study 04/89. In GMALL study 03/87, there was no difference between the CCR probability for pro-B ALL versus common and pre-B ALL patients (19% v 18% at 4 years; P = .99), whereas in study 04/89, the CCR probability for pro-B ALL was superior to common and pre-B ALL (52% v 20% at 4 years; P = .006).
DISCUSSION
In contrast to childhood ALL, few studies on cell-biological features and treatment outcome in adults with CD10− pro-B ALL have yet been reported. The present prospective study, including 57 adult patients with pro-B ALL treated on two consecutive multicenter trials, is the largest report published to date. Our results as to the frequency of pro-B ALL (∼10%) are in agreement with other recent studies of adult ALL. More importantly, we observed a high incidence (∼50%) of a t(4;11) and/or MLL-AF-4 fusion transcripts with characteristic associations between immunophenotype, genotype, and clinical features in adult pro-B ALL, comparable with previous findings in childhood, especially infant ALL. Furthermore, the treatment outcome observed in study ALL 04/89 is the first report in adult patients showing that intensification of postremission treatment by HD cytarabine and mitoxantrone or allogeneic BMT can lead to a substantial improvement of prognosis in this high-risk patient population.
According to our criteria, 57 of 611 evaluable patients (9%) could be assigned to the pro-B immunophenotype. Thus, the frequency of pro-B ALL observed in the studies ALL 03/87 and 04/89 is twice as much when compared with the data reported in childhood ALL, indicating a higher proportion of cases in adult ALL being arrested at this very immature stage of B-lymphoid differentiation.12,20 Our findings are in line with other recent studies in adult ALL patients applying a similar panel of MoAbs to the immunophenotypic characterization of leukemic blasts.10,11,13 However, few of these studies provided detailed results as to the antigenic profile, including myeloid-antigen coexpression, as well as immunophenotypic-genotypic associations of this subgroup, and in only one study was the treatment outcome of pro-B ALL reported.13
The results of our detailed immunologic marker analyses underline the diagnostic value of CD19 for lineage affiliation in immature B-cell precursor ALL. In view of the strong expression levels of CD19 in all cases investigated, the application of other, more recently identified pan-B markers such as CD79a may be restricted to those rare cases without CD19 expression.55 In the vast majority of adult ALL patients with a t(4;11) and/or molecular evidence of MLL-AF-4 fusion transcripts, leukemic blasts disclosed a typical antigenic profile (ie, CD10−, CD19+, CD24− or weakly +, CD65s+). In our experience, these features, especially the missing or weak expression of CD24 as compared with CD19, and the coexpression of CD65s, usually associated with negativity of other panmyeloid antigens (eg, CD13, CD33), are highly predictive for the cytogenetic and/or molecular demonstration of MLL rearrangements, mostly due to a t(4;11), or, more rarely, other 11q23 aberrations. This statement is in line with our data from a relatively large series of pro-B ALL patients composed of children and adults49,56 that documented a significant association of the MLL-AF-4 recombination with coexpression of CD65s and/or CD15, as well as other previous studies in childhood and adult ALL based on cytogenetic and/or molecular analyses.15,17-19,36,39,40,42 43
The recently generated MoAb 7.1, which recognizes the chondroitin sulfate proteoglycan molecule NG2 expressed by malignant hematopoietic cells with abnormalities in chromosome band 11q23 but not by normal hematopoietic cells, may be useful in the future for screening ALL patients with pro-B ALL for whom cytogenetic and molecular analyses are not available, both at diagnosis and during follow-up for detection of minimal residual disease (MRD).57 58
In our study, 22 of 35 adults with adequate genotypic studies disclosed a t(4;11) and/or MLL-AF-4 rearrangements, the molecular hallmark of a t(4;11). Considering the overall frequency of pro-B ALL in adult ALL and the usual absence of a t(4;11) and MLL-AF-4 transcripts in other B-lineage ALL subgroups,49 our results match well with a recent large cytogenetic study performed on 443 adult patients with ALL indicating an incidence of 4% for ALL associated with a t(4;11).15 However, it should be taken into account that, in our series, adequate cytogenetic studies could only be performed in 58% of patients, and molecular analyses were retrospectively performed in a relatively small proportion of these patients selected only by availability of cryopreserved cell samples. In view of the typical antigenic profile (weak or missing expression of CD24, coexpression of CD65s, and absence of other panmyeloid antigens such as CD13 and CD33) observed in the additional 6 patients without cytogenetic and molecular studies, in our results the true incidence of a t(4;11) in adult pro-B ALL is probably underestimated. Furthermore, our study did not include a systematic survey, eg, by Southern blot analysis, of other 11q23 abnormalities that have been recently described to occur in infant ALL in up to 25% of cases based on cytogenetic and molecular studies40 and in about 7% of adult ALL cases based on cytogenetic analyses.15
The results of our molecular analyses demonstrating an MLL-AF-4 rearrangement in 1 patient with cytogenetic abnormalities not involving chromosome 11 and in 1 patient with a normal karyotype are in accordance with recent sudies, indicating that cytogenetic studies may fail to detect 11q23 translocations.24,39,40,49,56 In contrast, very few studies have yet reported on patients with a t(4;11) who disclose a germline configuration of the MLL gene on the molecular level.40 Taken together, our data in adult ALL patients and other studies, mainly comprising infants with ALL, provide a compelling reason to test MLL rearrangements by molecular analyses in all newly diagnosed CD10− B-lineage ALL patients entered into treatment protocols.
Contradictory findings have been reported on the main clinical risk factors such as age and WBC count, as well as on other clinical characteristics such as sex distribution, organomegaly, and initial CNS manifestation within the group of adult pro-B ALL patients. Our results, indicating that pro-B ALL patients are slightly older and usually have higher WBC counts as compared with other B-lineage ALL subgroups, are in line with a recent report of the French LALA87 study13 including 45 patients with pro-B ALL. Additionally, there is a slight female predominance and evidence of organomegaly in about 40% of patients. More importantly, the analysis of clinical characteristics according to the cytogenetically and/or molecularly defined subgroups showed a much higher WBC count and a higher proportion of male patients within the pro-B ALL subgroup characterized by a t(4;11) and/or fusion transcripts of MLL-AF-4. Moreover, patients with 11q23 abnormalities were younger at diagnosis compared with those without these genetic abnormalities. Our results concerning the mean age, mean WBC counts, and sex ratio are comparable with a recent review summarizing the clinical data of children and adults with ALL and a t(4;11).28 Other recent studies on the correlation between cytogenetic abnormalities and clinical characteristics in adult ALL patients also uniformly observed very high WBC counts in ALL cases associated with a t(4;11).15,42 In one of these studies, which was based, however, only on 6 cases with a t(4;11), the investigators suggested that patients greater than 50 years of age may be twice as likely as children to have t(4;11) ALL.42 In our study, which was restricted to adult patients between 15 and 65 years of age, no clue as to an increased incidence of a t(4;11) in the older age group was found.
Our results, which demonstrate a CR rate of about 75% for pro-B ALL patients in studies GM-ALL 03/87 and 04/89, are in line with other studies that did not observe substantial differences in remission rates for childhood or adult B-cell precursor ALL subgroups. However, previous studies have reported a worse prognosis for both children and adults whose leukemic blasts express an immature pro-B phenotype, which in adult series are usually referred to as null-ALL.7,13,16,17 20 As pointed out above, this inferior treatment outcome can be, especially in infant ALL, explained by the association of this immunophenotype with adverse biological (eg, 11q23 rearrangements) and clinical features (eg, hyperleukocytosis), occurring in up to 50% of patients.
In this regard, the treatment outcome of pro-B ALL patients in GMALL study 04/89 is remarkable for several reasons.
Firstly, remission duration and overall survival of pro-B ALL patients could be substantially improved in GMALL study 04/89 as compared with the preceding GMALL trials.7,59 This may be, at least partially, explained by the intensification of postremission therapy, either by the administration of HD cytarabine and mitoxantrone or by allogeneic BMT in first CR. The inferior outcome of pro-B-ALL patients in GMALL study 03/87 may be due to the higher proportion of elderly patients older than 50 years of age (see Table 2) who possibly did not benefit from an intensive consolidation with HD cytarabine and mitoxantrone,60 and, accordingly, the lower proportion of patients who underwent BMT (8% v 27%). Our results in GMALL study 04/89, implying an improvement of prognosis for pro-B ALL patients due to more intensive therapy, are corroborated by the results of the ongoing GMALL study 05/93, including 47 patients with pro-B ALL who received identical consolidation with HD cytarabine and mitoxantrone as in study 04/89. In this study, comparable with the results of study 04/89, a CR rate of 81% and a relatively high probability of CCR (0.55 at 2 years) could be achieved (Hoelzer et al, unpublished observations). Interestingly, recent studies with the 3-(4,5-dimethyl-thiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay indicate a high antileukemic activity of cytarabine as compared with corticosteroids, L-asparaginase, and anthracyclines in leukemic blasts from pro-B ALL patients (R. Pieters, personal communication, April 1997), thus supporting our treatment results in studies 04/89 and 05/93.
High-risk patients with a common or pre-B ALL phenotype, as compared with pro-B ALL, had a similar probability of CCR in study GMALL 03/87 and a significantly inferior probability of CCR in study 04/89. These data suggest that this subgroup of B-lineage ALL, which, however, includes a large number of Ph+ patients, did not benefit from an intensification of consolidation treatment.
Secondly, the division of pro-B ALL patients into different subgroups according to the genotypic features of their leukemic blasts allowed us to demonstrate that a portion of adult patients with a t(4;11) and/or MLL-AF-4 transcripts may achieve long-term remission and probably cure, after having received intensive postremission treatment. Our recent observations, applying RT-PCR analyses to detect MLL-AF-4 rearrangements in 5 adults with pro-B ALL in CR, all of whom exhibited MLL-AF-4 transcripts at diagnosis and lacked evidence of minimal residual disease 8 to 52 months after diagnosis, are in line with this statement.49 These results are in contrast with previous reports, mainly based on surveys of literature,28,29,34 or comprising a relatively small number of patients with, usually, heterogeneous treatment.31,32,42,43 These reports unequivocally suggested that, of patients with a t(4;11), adults had the worst prognosis, with median survivals of 7 months,31even with contemporary intensive treatment strategies, thus justifying the use of BMT or innovative treatment approaches.34
Thirdly, as opposed to the French LALA87 study group that recently published their results in 45 adult patients with CD10− B-lineage ALL,13 our data strongly suggest that the distinction between CD10− pro-B ALL and CD10+ common or pre-B ALL provides useful information for tailoring treatment strategies according to specific subtypes of ALL. Adult patients with pro-B ALL and 11q23 translocations, in our series exclusively t(4;11), represent a distinct clinicopathologic entity that can be recognized easily based on its immunophenotypic and genotypic features. Obviously, further studies, applying cytogenetic and, especially, molecular analyses, are needed to better characterize the biological and clinical features of adult pro-B ALL patients whose leukemic blasts lack 11q23 translocations, display 11q23 rearrangements not caused by a t(4;11), or show deletions and inversions affecting the 11q23 region but do not involve MLL rearrangements. Recent results from the Groupe Francais de Cytogénétique Hématologique suggested that adult patients with breakpoints on 11q23 not involving a t(4;11) or deletions of 11q23 had the same poor outcome as patients with a t(4;11).15 In contrast, a recent study of 17 childhood ALL cases with a deletion or inversion affecting the 11q23 region without molecular evidence of MLL gene rearrangements suggested that these structural chromosomal abnormalities do not share the unfavorable prognosis of 11q23 translocations and, therefore, represent clinically and biologically different entities.61
In conclusion, our results in a large series of consecutively studied adult pro-B ALL patients suggest that current intensive therapies including consolidation with HD cytarabine and mitoxantrone or allogeneic BMT may improve the prognosis of this B-lineage ALL subgroup despite its high-risk genetic and clinical features. Moreover, our data demonstrate that, in contrast to former results, long-term remission duration can be achieved in a subset of pro-B ALL patients. In the future, the combined application of immunophenotyping as well as cytogenetic and molecular studies will be essential for a more precise distinction of the biological heterogeneity within this group of patients and, hopefully, will contribute to individually adjusted treatment strategies.
Supported by the Bundesministerium für Forschung und Technologie, Federal Republic of Germany, Contract No. 01ZP88045 and by Deutsche Krebshilfe, Federal Republic of Germany, Contract No. M84/92Ho1.
Presented in part at the 1994 annual meeting of the American Society of Hematology.
Address reprint requests to Wolf-Dieter Ludwig, MD, Robert-Rössle-Klinik, Department of Hematology, Oncology, and Tumor Immunology, Universitätsklinikum Charité, Medizinische Fakultät der Humboldt-Universität zu Berlin, Lindenberger Weg 80, D-13125 Berlin, Germany; e-mail:ludwig@rrk-berlin.de.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal