To the Editor:
Budd-Chiari syndrome is characterized by the hepatic venous outflow obstruction. A multifactorial interaction between the genetic and circumstantial risk factors may be responsible for this kind of disorder. Among these, myeloproliferative syndromes are reported to be the commonest cause of Budd-Chiari syndrome. Other causes include thrombophilia states, oral contraceptives, and cancer.1
Until recently, the major known genetic defects detected for repeated venous thrombosis were deficiencies of protein C, protein S, and antithrombin III, which together accounted for 5% to 10% of these type of cases. The defect in the anticoagulation response to activated protein C has been detected as a new mechanism for thrombophilia, which was subsequently linked to a single point mutation on the factor V gene, resulting in Arg506-Gln substitution in the activated protein C cleavage site.2 Since then, various thrombotic events like deep vein thrombosis, pulmonary embolism, preeclampsia, and pulmonary infarction have been studied for factor V Leiden mutations and reported.3 However, there is not much information on the frequency of these mutations in Budd-Chiari syndrome except for a few isolated case reports.4 This prompted us to report the present findings.
Nineteen Budd-Chiari cases (age ranging from 13 to 45 years; males, 8; females, 11), in whom hepatic doppler ultrasonography showed occluded hepatic veins followed by subsequent histological confirmation, were screened for the presence of factor V Leiden mutations by polymerase chain reaction (PCR). Except for three, all the remaining patients had a negative family history of thrombosis.
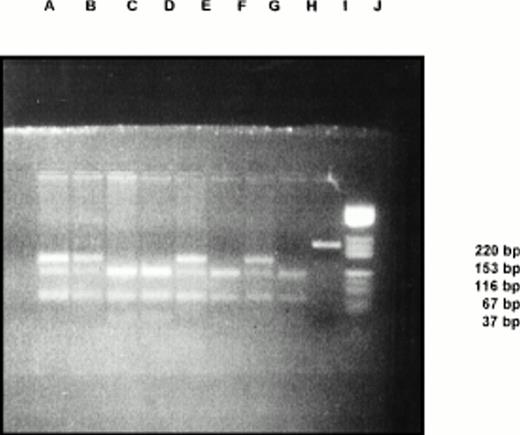
The DNA extracted from blood leukocytes was amplified using a set of primers (Cybersyn, Lenni, PA), followed by Mnl 1 digestion. The digested fragments were run in a 3% low-melting agarose gel for 2 hours. Five were heterozygous for factor V Leiden mutations (26%) (Fig 1). None were found to be homozygous in this group. All other tests such as protein C, protein S, and antithrombin III were normal in these cases.
Thus, approximately one fourth of our Budd-Chiari cases were shown to have this genetic defect, ie, CGA → CAA(Arg506Gln) in the factor V gene. However, it is possible theoretically that the mutation may not always be Arg506Gln. The information about the prevalence of this defect in our population was inadequate, but a preliminary study on north Indian population had a factor V Leiden allele frequency of 1.2%.5 When compared with this, it was found that the defect had a high frequency in cases of Budd-Chiari, suggesting the causal relationship between the two.
Because Budd-Chiari cases show severe hepatocellular insufficiency, the coagulation tests for factor V Leiden may not have been informative in determining the thrombophilia status. Nevertheless, the detection of factor V Leiden mutation by a simple PCR followed by enzyme digestion is economical and less time consuming, with 100% specificity. With this high frequency of factor V Leiden mutations and comparatively low cost of DNA analysis, it may be suggested that this mutation be examined in all the Budd-Chiari cases.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal