Abstract
The Epstein-Barr virus (EBV)-encoded latent membrane protein (LMP-1) is required for viral transformation and functions to protect cells from apoptotic cell death, in part, by induction of antiapoptotic genes, including Bcl-2 and A20. We have used antisense oligodeoxynucleotides targeted to LMP-1 as a strategy to suppress LMP-1 expression and thereby inhibit its functions. We have shown that levels of LMP-1 protein in EBV-positive lymphoblastoid cell lines can be reduced by in vitro treatment with unmodified oligodeoxynucleotides targeted to the first five codons of the LMP-1 open-reading frame. Furthermore, suppression of LMP-1 was associated with molecular and phenotypic effects that included downregulation of the LMP-1–inducible antiapoptotic genes, Bcl-2 and Mcl-1, inhibition of proliferation, stimulation of apoptosis, and enhancement of sensitivity to the chemotherapeutic agent, etoposide. These effects were largely sequence-specific and observed in EBV-positive, but not EBV-negative cell lines. These studies suggest that lowering expression of LMP-1 in EBV-associated malignancy might have therapeutic effects and might synergize with other antitumor agents.
© 1998 by The American Society of Hematology.
EPSTEIN-BARR VIRUS (EBV) is associated with several human lymphoid malignancies, including endemic Burkitt’s lymphoma, acquired immunodeficiency syndrome (AIDS)-related lymphoma, and lymphoproliferative disease in transplant recipients, as well as nasopharyngeal carcinoma.1 The biologic activity of EBV that causally links it to lymphomagenesis is its capacity to growth-transform resting B cells to immortalized lymphoblastoid cells that proliferate indefinitely and harbor the virus in a latent state.2,3 Latent EBV infection is characterized by restricted expression of viral gene products, including six nuclear antigens (Epstein-Barr nuclear antigens [EBNAs]-1, -2, -3a, -3b, -3c, and -LP) and two transmembrane proteins (latent membrane protein [LMP]-1 and -2) that function cooperatively to initiate and maintain growth transformation.4 5 Although the precise role of each of these latent gene products in transformation is not fully understood, they mediate their transforming functions, in part, by constitutively activating cellular genes that are involved in physiologic B-cell activation, proliferation, and survival.
Although transformation of B cells by EBV requires the expression of at least five latent viral genes (EBNA-1, EBNA-2, EBNA-3a, EBNA-3c, and LMP-1) and thus cannot be mediated by a single viral gene,3,6-9 LMP-1 most closely mimics a classical oncogene. Transfection of LMP-1 into immortalized rat fibroblasts induces full phenotypic transformation, rendering them tumorigenic in vivo,10,11 and, in human epithelial cells, LMP-1 expression inhibits differentiation.12 Expression of LMP-1 in B cells confers a phenotype resembling activated lymphocytes, including induction of adhesion molecules, homotypic aggregation, increased cell size, and entry into cell cycle.13,14 In addition, LMP-1 confers a survival advantage to EBV-infected B cells by protecting cells from apoptosis.15,16 Gene transfer studies in EBV-negative Burkitt cells have demonstrated that the antiapoptotic effects of LMP-1 in B cells are mediated, in part, by induction of the antiapoptotic cellular gene, Bcl-2.15,16 In addition, LMP-1 induces expression of other antiapoptotic genes, including A20 and the homolog of Bcl-2, Mcl-1.17 18 Thus, LMP-1 is a multifunctional effector of EBV-mediated transformation, modulating not only cell-surface phenotype and cell growth, but also cell death.
Because LMP-1 is essential for immortalization by EBV and plays a critical role in preventing apoptosis, suppression of LMP-1 expression in EBV-immortalized lymphoblastoid cells should have significant effects on cell growth and survival. To test this hypothesis, we examined the effects of antisense oligodeoxynucleotides targeted to LMP-1 sequence in EBV-immortalized lymphoblastoid cells. We observed suppression of LMP-1 protein levels by antisense targeted to codons one to five of the LMP-1 open-reading frame. Furthermore, suppression of LMP-1 was associated with inhibition of proliferation, downregulation of Bcl-2 and its homolog Mcl-1, induction of apoptosis, and increased sensitivity to etoposide-mediated cell death.
MATERIALS AND METHODS
Cell lines.
X50-7 and 11-23 are EBV-immortalized lymphoblastoid cell lines derived by infecting umbilical cord lymphocytes with the B958 and FF41 strains of EBV, respectively. Cells from these two lines are uniformly positive for EBV as determined by anticomplement immunofluorescence, and express EBNA-1, EBNA-2, and LMP-1 by immunoblotting. BJAB and Louckes are EBV-negative Burkitt B-cell lines. X50-7 was generously provided by George Miller (Yale University, New Haven, CT); BJAB and Louckes were provided by W.P. Summers (Yale University). 11-23 was derived in our laboratory. All cells were maintained in RPMI-1640 plus 7.5% fetal calf serum.
Oligodeoxynucleotides.
Fifteen-mer unmodified oligodeoxynucleotides were custom-made and purchased from Macromolecular Resources (Colorado State University, Fort Collins). The appropriate sequence for LMP-1 antisense corresponding to the complementary sequence of base pairs +1 to +15 of LMP-1 was derived from the published sequence of the LMP-1 gene of the B958 strain of EBV.19 Three oligodeoxynucleotide sequences were used in the described experiments, as follows: LMP-1 antisense (5′-AAG GTC GTG TTC CAT-3′ corresponding to base pairs +1 to +15 of the LMP-1 open-reading frame) and LMP-1 scrambled antisense sequences, designated SS1 and SS2 (5′-ACG TCA TGC TAG TGT-3′ and GTC AGT ACT GCA TTG-3′, respectively, representing random scrambling of the LMP-1 antisense sequence). The lyophilized oligodeoxynucleotides were resuspended in water just before use and used at a final concentration of 5, 10, or 50 μmol/L.
Incubation of cells with oligodeoxynucleotides.
Oligodeoxynucleotides were added directly to cell cultures in log phase of growth (2 to 4 × 105 cells/mL) in RPMI-1640 plus 7.5% fetal calf serum at a final concentration of 5 or 50 μmol/L; at 24-hour intervals, the culture medium was replaced with fresh medium and oligodeoxynucleotide. Alternatively, to enhance uptake and reduce the amount of oligodeoxynucleotide, for some experiments, cells were cultured in the presence of commercially available liposomes (LIPOFECTIN reagent; GIBCO-BRL, Gaithersburg, MD) in serum-free artificial medium (Opti-MEM I; GIBCO-BRL) for the first 24 hours of exposure to oligodeoxynucleotides. Cells were first washed in serum-free RPMI-1640 twice and resuspended in Opti-MEM with LIPOFECTIN reagent (50 μg/mL) and oligodeoxynucleotide (10 or 50 μmol/L). For untreated controls, cells were cultured with liposomes in serum-free medium in the absence of oligodeoxynucleotides. After 24 hours of culture, the Opti-MEM was replaced with fresh medium (RPMI-1640 with 7.5% fetal calf serum, as indicated) containing oligodeoxynucleotide at a concentration of 10 or 50 μmol/L. Cells were exposed to oligodeoxynucleotide for 24, 30, 48, or 72 hours, as specified. The Opti-MEM, LIPOFECTIN reagent, and oligodeoxynucleotide were prepared according to the manufacturerer’s protocol (GIBCO-BRL).
Cellular proliferation assays.
Cells (8 × 104) were plated in triplicate in 200 μL of medium in microtiter wells and cultured for 48 hours with and without oligodeoxynucleotide (10 μmol/L) using liposomes to enhance oligodeoxynucleotide uptake during the first 24 hours of culture, as described earlier. During the last 16 hours of culture, each well was pulsed with 1 μCi of [3H]thymidine (73 Ci/mmol). Cells were harvested with a multiple automated sample harvester (Cambridge Technology, Cambridge, MA), and incorporation of [3H]thymidine was measured by standard scintillation counting and expressed as the mean ± SD of triplicate assays. The two-tailed unpaired t test was used to determine significance of differences in [3H]thymidine incorporation between antisense oligodeoxynucleotide- and scrambled oligodeoxynucleotide-treated cells.
Immunoblotting.
The preparation of cell extracts, electrophoresis, and transfer were performed as described previously.20 In brief, cells were obtained after 24, 48, or 72 hours of culture with and without oligodeoxynucleotides, as described earlier. A quantity of 2 × 105 viable cells was used for each condition; cell viability was determined by trypan blue exclusion, exactly as previously described.20 For some experiments, if there were sufficient numbers of viable cells, samples were aliquoted and run in duplicate sets on the same gel. Total protein from whole-cell lysates was resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocellulose paper. The immunoblotting procedure was performed according to the manufacturer’s protocol for the chemiluminescent detection of proteins using the Phototope-HRP Western Blot Detection Kit (New England Biolabs, Beverly, MA). The following primary antibodies were used: for LMP-1 detection, a cocktail of four mouse monoclonal antibodies to LMP-1 was purchased from DAKO (Glostrup, Denmark); for Bcl-2 detection, mouse monoclonal antibody was purchased from Alexis (San Diego, CA); for Mcl-1 detection, affinity-purified rabbit polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); for EBV nuclear antigen 1 (EBNA-1) detection, human serum previously characterized as containing high titer of antibody to EBNA-1 was generously provided by James Jones (National Jewish Center, Denver, CO); for poly-(ADP-ribose)-polymerase (PARP), rabbit polyclonal antibody was purchased from Boehringer Mannheim (Indianapolis, IN). Relative amounts of protein were quantitated by laser scanning densitometry analysis of the protein bands (Molecular Dynamics, Sunnyvale, CA).
Apoptosis assays.
To detect apoptosis, immunoblotting assays were performed as described earlier to detect PARP, a 113-kD protein substrate for apoptosis-specific proteases from the interleukin-1β–converting enzyme (ICE) family, and its fragments; apoptosis was detected by the appearance of an 89-kD PARP fragment.21-23 In addition, a flow cytometric assay based on quantitating DNA breaks was used to measure apoptosis.24 This method uses terminal deoxynucleotide transferase (TdT) and Br-dUTP to label exposed 3′OH DNA ends in fixed cells; BrdU-tagged DNA is then quantitated by flow cytometry using fluorescein-conjugated anti-BrdU antibody. This assay was performed according to the manufacturer’s protocol using the APO-BRDU kit (Pharmingen, San Diego, CA). The percentage of cells induced to undergo apoptosis by antisense treatment was calculated as follows: (percentage of apoptotic antisense-treated cells − percentage of apoptotic untreated cells)/(100 − percentage of apoptotic untreated cells) × 100.
Drug treatments and cytotoxicity assays.
Etoposide (Sigma, St Louis, MO) was prepared as a 100-mmol/L stock in dimethyl sulfoxide (DMSO) and stored at −20°C. Before use, dilutions of the stock solution were made into 30% DMSO. The drug was added to cell cultures in log phase of growth (2 × 105cells/mL) plated in triplicate in RPMI-1640 plus 7.5% fetal calf serum with or without oligodeoxynucleotide (50 μmol/L) at a final concentration of 2, 20, or 200 μmol/L with a final concentration of DMSO in all cultures that did not exceed 0.3%; control cultures received equivalent DMSO treatment without etoposide. After 24 hours of exposure to drug, cell viability was determined by trypan blue exclusion as previously described.20 The percentage of viable cells at each concentration of drug was determined and expressed as the mean ± SD of triplicate samples.
RESULTS
Antisense oligodeoxynucleotides to LMP-1 suppress LMP-1, Bcl-2, and Mcl-1 proteins.
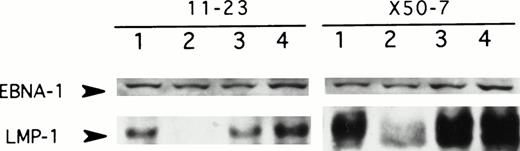
To determine whether antisense oligodeoxynucleotides to LMP-1 can suppress LMP-1 protein expression, EBV-positive lymphoblastoid cell lines were exposed to three different unmodified 15-mer oligodeoxynucleotide sequences (+1 to +15, +16 to +30, and +8 to +22 of the LMP-1 open-reading frame). LMP-1 expression was assessed by immunoblotting after 48 hours of exposure to oligodeoxynucleotides. The antisense sequence complementary to the first five codons reproducibly suppressed LMP-1 expression in two different lymphoblastoid cell lines and was used in all subsequent experiments (Fig1). Although we observed some variability in the degree of LMP-1 suppression from experiment to experiment, protein levels were consistently diminished by more than 70% relative to untreated cells (range, 70% to >95%). Suppression of LMP-1 was a sequence-specific effect, since the antisense oligodeoxynucleotide consistently inhibited LMP-1 expression by no less than 54% relative to the control oligodeoxynucleotide sequences (range, 54% to >95%). The control oligodeoxynucleotides either had no effect on LMP-1 expression (SS1; Fig 1, lane 4) or, in some experiments, a modest inhibitory effect (<40%) relative to untreated cells in the presence of cationic liposomes (SS2; Fig 2A, lane 3). Furthermore, suppression of LMP-1 by antisense was protein-specific as well, since expression of the viral protein EBNA-1 (Fig 1), as well as other nonspecific proteins detected on long exposure of the autoradiographs (data not shown), was unaffected by antisense treatment. Dose-response studies established that unmodified oligodeoxynucleotide at a concentration of 50 μmol/L consistently suppressed LMP-1 expression (Fig 1). However, with the addition of cationic liposomes (LIPOFECTIN) during the first 24 hours of exposure to antisense, the amount of oligodeoxynucleotide required for reproducible suppression of LMP-1 could be reduced to 10 μmol/L (Fig 2).
LMP-1 antisense oligodeoxynucleotide treatment suppresses LMP-1 protein levels. Western blot analysis of LMP-1 and EBNA-1 protein expression in 2 EBV-positive lymphoblastoid cell lines, 11-23 and X50-7, was performed after exposure to unmodified antisense oligodeoxynucleotide targeted to LMP-1 (codons 1 through 5) for 48 hours. Lane 1, untreated control; lane 2, LMP-1 antisense-treated (50 μmol/L); lane 3, LMP-1 antisense-treated (5 μmol/L); lane 4, control oligodeoxynucleotide (SS1)-treated (50 μmol/L). Duplicate sets of samples for each condition were immunoblotted for LMP-1 or EBNA-1, as described in the Methods.
LMP-1 antisense oligodeoxynucleotide treatment suppresses LMP-1 protein levels. Western blot analysis of LMP-1 and EBNA-1 protein expression in 2 EBV-positive lymphoblastoid cell lines, 11-23 and X50-7, was performed after exposure to unmodified antisense oligodeoxynucleotide targeted to LMP-1 (codons 1 through 5) for 48 hours. Lane 1, untreated control; lane 2, LMP-1 antisense-treated (50 μmol/L); lane 3, LMP-1 antisense-treated (5 μmol/L); lane 4, control oligodeoxynucleotide (SS1)-treated (50 μmol/L). Duplicate sets of samples for each condition were immunoblotted for LMP-1 or EBNA-1, as described in the Methods.
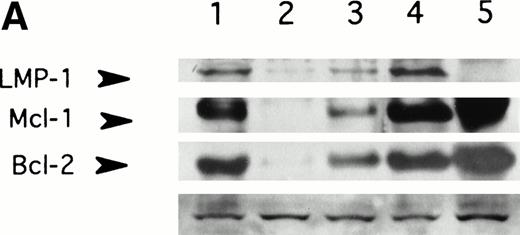
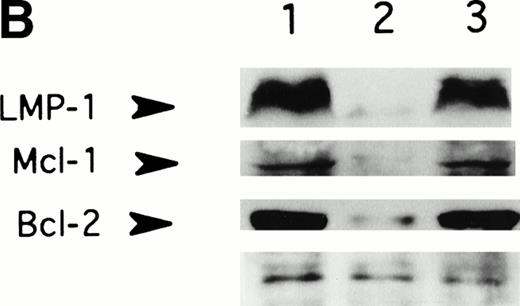
LMP-1 antisense oligodeoxynucleotide treatment suppresses LMP-1–inducible antiapoptotic genes, Bcl-2 and Mcl-1. Western blot analysis of LMP-1, Bcl-2, and Mcl-1 expression in the EBV-positive lymphoblastoid cell line, X50-7, was performed after exposure to oligodeoxynucleotide (10 μmol/L in the presence of liposomes) for 48 (A) or 72 (B) hours. Lanes 1, untreated control cultured in liposomes; lanes 2, antisense-treated; lanes 3, control oligodeoxynucleotide (SS2)-treated; lane 4, untreated control cultured without liposomes; lane 5, untreated EBV-negative B-cell line, Louckes. Blots were sequentially exposed to anti–LMP-1 antibody, anti–Bcl-2 antibody, and anti–Mcl-1 antibody. Bottom panel shows a representative nonspecific high–molecular-weight band seen on prolonged exposure of the blot using LMP-1 antibody, confirming approximately equivalent amounts of protein loaded per lane.
LMP-1 antisense oligodeoxynucleotide treatment suppresses LMP-1–inducible antiapoptotic genes, Bcl-2 and Mcl-1. Western blot analysis of LMP-1, Bcl-2, and Mcl-1 expression in the EBV-positive lymphoblastoid cell line, X50-7, was performed after exposure to oligodeoxynucleotide (10 μmol/L in the presence of liposomes) for 48 (A) or 72 (B) hours. Lanes 1, untreated control cultured in liposomes; lanes 2, antisense-treated; lanes 3, control oligodeoxynucleotide (SS2)-treated; lane 4, untreated control cultured without liposomes; lane 5, untreated EBV-negative B-cell line, Louckes. Blots were sequentially exposed to anti–LMP-1 antibody, anti–Bcl-2 antibody, and anti–Mcl-1 antibody. Bottom panel shows a representative nonspecific high–molecular-weight band seen on prolonged exposure of the blot using LMP-1 antibody, confirming approximately equivalent amounts of protein loaded per lane.
LMP-1 has been implicated in the regulation of several cellular genes that control cell proliferation and cell death.13-18 In gene-transfer experiments, LMP-1 has been shown to induce expression of the antiapoptotic gene, Bcl-2, and its homolog, Mcl-1, and to block apoptosis in serum-starved EBV-negative Burkitt cell lines.15,16 18 To determine whether antisense-mediated suppression of LMP-1 caused a reduction in the levels of Bcl-2 or Mcl-1 proteins, LMP-1 antisense-treated cells were assayed for Bcl-2 and Mcl-1 expression by immunoblotting. Exposure to LMP-1 antisense for 48 and 72 hours was associated with suppression of both Bcl-2 and Mcl-1 compared with untreated and control oligodeoxynucleotide-treated cells (Fig 2). There was no evidence of global suppression of protein expression by LMP-1 antisense, since multiple nonspecific protein bands observed on long exposure of the autoradiograph were unaffected by oligodeoxynucleotide treatment. There was no effect of LMP-1 antisense on Bcl-2 or Mcl-1 expression in two different EBV-negative cell lines examined (data not shown).
Antisense to LMP-1 inhibits proliferation of EBV-positive lymphoblastoid cell lines.
To determine whether antisense-mediated suppression of LMP-1 affected cellular proliferation, EBV-immortalized lymphoblastoid cell lines were exposed to LMP-1 antisense or control oligodeoxynucleotides in the presence of liposomes (LIPOFECTIN) and assayed for proliferation by [3H]thymidine incorporation. In two different EBV-positive lymphoblastoid cell lines, LMP-1 antisense treatment for 48 hours significantly decreased proliferation compared with untreated cells or cells treated with control scrambled oligodeoxynucleotides, as shown in one of four representative experiments (Fig3). In contrast to the antiproliferative effect of LMP-1 antisense in EBV-positive cells, there was no significant difference in proliferation between antisense-treated and control oligodeoxynucleotide-treated cells in two different EBV-negative cell lines. These results suggested the antiproliferative effect of LMP-1 antisense oligodeoxynucleotide was sequence-specific and linked to the presence of EBV.
LMP-1 antisense oligodeoxynucleotide treatment inhibits proliferation of EBV-positive lymphoblastoid cells. Proliferation was assayed by [3H]thymidine incorporation in 2 EBV-positive lymphoblastoid cell lines (11-23 and X50-7) and an EBV-negative B-cell line (BJAB) after exposure to oligodeoxynucleotide (10 μmol/L in liposomes) for 48 hours. Values represent the mean ± SD of triplicate assays. (□) Antisense-treated; (▪) control oligodeoxynucleotide (SS1)-treated; (▨) untreated control. P values for the mean difference between antisense and SS1-treated cells are as follows: 11-23, P < .002; X50-7, P = .0003; BJAB, P> .8. P values for the mean difference between SS1-treated and untreated cells are as follows: 11-23, P > .1; X50-7,P > .08; BJAB, P > .06.
LMP-1 antisense oligodeoxynucleotide treatment inhibits proliferation of EBV-positive lymphoblastoid cells. Proliferation was assayed by [3H]thymidine incorporation in 2 EBV-positive lymphoblastoid cell lines (11-23 and X50-7) and an EBV-negative B-cell line (BJAB) after exposure to oligodeoxynucleotide (10 μmol/L in liposomes) for 48 hours. Values represent the mean ± SD of triplicate assays. (□) Antisense-treated; (▪) control oligodeoxynucleotide (SS1)-treated; (▨) untreated control. P values for the mean difference between antisense and SS1-treated cells are as follows: 11-23, P < .002; X50-7, P = .0003; BJAB, P> .8. P values for the mean difference between SS1-treated and untreated cells are as follows: 11-23, P > .1; X50-7,P > .08; BJAB, P > .06.
LMP-1 antisense stimulates apoptosis.
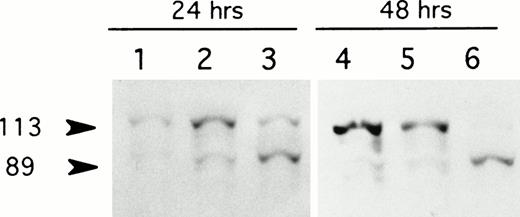
LMP-1 has been implicated in the suppression of apoptosis of EBV-immortalized cells by upregulating several antiapoptotic cellular genes, including Bcl-2, Mcl-1, and A20.15-18 Thus, suppression of LMP-1 (and Bcl-2 and Mcl-1) expression in lymphoblastoid cell lines would be predicted to induce apoptotic cell death under conditions of serum deprivation. The antiproliferative effects of LMP-1 antisense treatment supported the possibility that apoptosis would be stimulated by antisense mediated suppression of LMP-1. To determine whether LMP-1 antisense treatment promoted apoptotic cell death, EBV-positive lymphoblastoid cells were exposed to LMP-1 antisense or control oligodeoxynucleotides in serum-free medium in the presence of liposomes and assayed for PARP cleavage by immunoblotting at 24 and 48 hours. After 24 hours of exposure to antisense, there was a minimal decrease in the ratio of the 113-kD PARP to the 89-kD cleavage product relative to control oligodeoxynucleotide-treated or untreated cells; at 48 hours, the 113-kD PARP was substantially diminished in association with a further increase in the level of the 89-kD subfragment (Fig4). A decrease in the ratio of the 113-kD PARP to the 89-kD subfragment product was not observed in the control oligodeoxynucleotide-treated cells at 48 hours. These results suggested that LMP-1 antisense treatment for 48 hours triggered apoptotic cell death under conditions of serum deprivation.
LMP-1 antisense oligodeoxynucleotide treatment induces cleavage of PARP. Western blot analysis of PARP expression in the EBV-positive lymphoblastoid cell line, X50-7, was performed after exposure to unmodified oligodeoxynucleotide in serum-free medium with liposomes for 24 and 48 hours. Lanes 1 and 4, untreated controls; lanes 2 and 5, control oligodeoxynucleotide (SS1)-treated; lanes 3 and 6, LMP-1 antisense-treated. The 113-kD PARP and its 89-kD cleavage product are indicated (arrows).
LMP-1 antisense oligodeoxynucleotide treatment induces cleavage of PARP. Western blot analysis of PARP expression in the EBV-positive lymphoblastoid cell line, X50-7, was performed after exposure to unmodified oligodeoxynucleotide in serum-free medium with liposomes for 24 and 48 hours. Lanes 1 and 4, untreated controls; lanes 2 and 5, control oligodeoxynucleotide (SS1)-treated; lanes 3 and 6, LMP-1 antisense-treated. The 113-kD PARP and its 89-kD cleavage product are indicated (arrows).
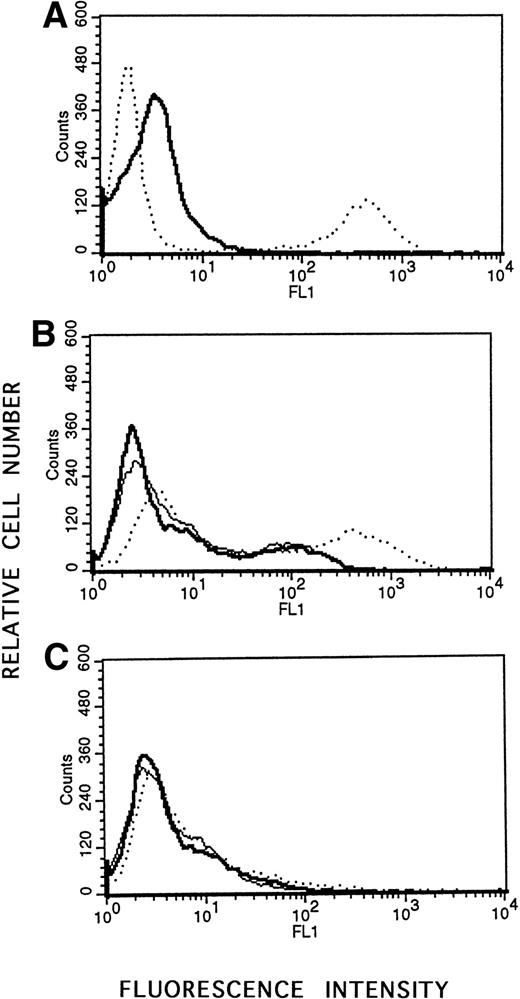
To corroborate the finding that antisense to LMP-1 induced apoptosis, a flow cytometric assay based on quantitation of DNA fragmentation was used to quantitate the percentage of cells undergoing apoptosis as a result of LMP-1 antisense treatment. Lymphoblastoid cells (X50-7) or Louckes cells in log phase of growth were exposed to antisense or control oligodeoxynucleotide in the presence of liposomes in serum-free medium for 24 to 48 hours and assayed for apoptosis. Although we observed background spontaneous apoptosis in the untreated cells cultured in serum-free medium with liposomes that appeared to increase with duration of culture, the percentage of apoptotic cells in antisense-treated cultures was consistently increased relative to untreated controls or cells treated with control oligodeoxynucleotide in multiple experiments (range, 22% to 84%; Table 1 and Fig 5). Treatment with the control scrambled oligodeoxynucleotide did not increase apoptosis relative to untreated control cultures (Table 1 and Fig 5). Furthermore, LMP-1 antisense treatment of an EBV-negative cell line (Louckes) did not stimulate apoptotic cell death by this assay (Fig 5). These findings provided further support that antisense directed to LMP-1 protein not only decreased LMP-1 protein levels, but also stimulated the apoptotic pathway in EBV-immortalized lymphoblastoid cells.
Percentage of EBV-Positive Lymphoblastoid Cells Induced to Undergo Apoptosis by Treatment With Antisense Oligodeoxynucleotide Targeted to LMP-1
| Duration of Antisense Treatment (h) . | % Apoptotic Cells . | % AntisenseInduced Apoptosis* . | ||
|---|---|---|---|---|
| Untreated . | SS1Treated . | AntisenseTreated . | ||
| 24 | 21 | — | 65 | 56 |
| 24 | 20 | 22 | 46 | 33 (31) |
| 24 | 17 | 16 | 87 | 84 (84) |
| 30 | 35 | — | 49 | 22 |
| 30 | 47 | — | 61 | 26 |
| 48 | 48 | 39 | 70 | 42 (51) |
| Duration of Antisense Treatment (h) . | % Apoptotic Cells . | % AntisenseInduced Apoptosis* . | ||
|---|---|---|---|---|
| Untreated . | SS1Treated . | AntisenseTreated . | ||
| 24 | 21 | — | 65 | 56 |
| 24 | 20 | 22 | 46 | 33 (31) |
| 24 | 17 | 16 | 87 | 84 (84) |
| 30 | 35 | — | 49 | 22 |
| 30 | 47 | — | 61 | 26 |
| 48 | 48 | 39 | 70 | 42 (51) |
*The percentage antisense-induced apoptosis is calculated relative to untreated controls and scrambled oligodeoxynucleotide (SS1)-treated cells (value shown in parentheses).
Flow cytometric analysis of apoptosis using the APO-BRDU kit. Cells were incubated with Br-dUTP in the presence of TdT enzyme to incorporate Br-dUTP into exposed 3′-OH ends. Quantitation of Br-dUTP sites was determined by flow cytometric analysis using fluorescein-conjugated anti-BrdU monoclonal antibody. Positive (apoptotic) and negative (nonapoptotic) control HL60 cells, treated with camptothecin or untreated, were provided by the company. (A) Negative control (—); positive control (· · ·). (B) EBV-positive X50-7 cells treated with LMP-1 antisense oligodeoxynucleotide (· · ·); treated with control SS1 oligodeoxynucleotide (—); or untreated (). (C) EBV-negative Louckes line treated with LMP-1 antisense oligodeoxynucleotide (· · ·); treated with control SS1 oligodeoxynucleotide (—); or untreated ().
Flow cytometric analysis of apoptosis using the APO-BRDU kit. Cells were incubated with Br-dUTP in the presence of TdT enzyme to incorporate Br-dUTP into exposed 3′-OH ends. Quantitation of Br-dUTP sites was determined by flow cytometric analysis using fluorescein-conjugated anti-BrdU monoclonal antibody. Positive (apoptotic) and negative (nonapoptotic) control HL60 cells, treated with camptothecin or untreated, were provided by the company. (A) Negative control (—); positive control (· · ·). (B) EBV-positive X50-7 cells treated with LMP-1 antisense oligodeoxynucleotide (· · ·); treated with control SS1 oligodeoxynucleotide (—); or untreated (). (C) EBV-negative Louckes line treated with LMP-1 antisense oligodeoxynucleotide (· · ·); treated with control SS1 oligodeoxynucleotide (—); or untreated ().
LMP-1 antisense treatment increases sensitivity to the cytotoxic drug, etoposide.
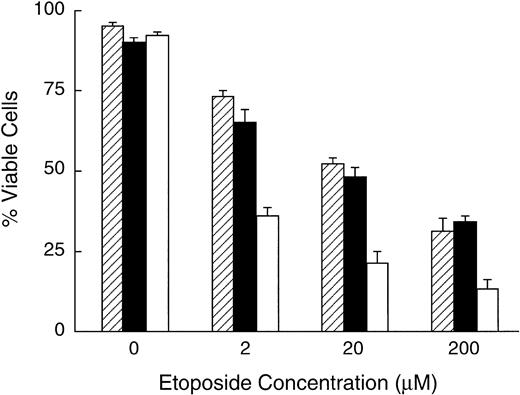
Previous gene-transfer studies have shown that Bcl-2 expression can block apoptosis induced by chemotherapeutic agents in vitro.22 25-28 The demonstration that antisense to LMP-1 suppressed not only LMP-1, but also the antiapoptotic proteins, Bcl-2 and Mcl-1, suggested that antisense treatment may sensitize cells to the cytotoxic effect of chemotherapeutic agents. To determine whether LMP-1 antisense treatment enhanced sensitivity to the DNA topoisomerase II inhibitor, etoposide, oligodeoxynucleotide-treated and untreated lymphoblastoid cells were exposed to increasing concentrations of etoposide and assayed for viability after 24 hours of exposure. Due to excessive toxicity of etoposide in the presence of liposomes and absence of serum, cells were cultured without cationic liposomes using oligodeoxynucleotide at a concentration of 50 μmol/L for these experiments. Under these conditions, in the presence of serum, we observed no significant effect on cell viability of LMP-1–targeted antisense oligodeoxynucleotide treatment for 24 hours, in the absence of etoposide, as determined by trypan blue exclusion. In the presence of etoposide, cell viability diminished with increasing concentrations of drug in untreated and oligodeoxynucleotide-treated cells. However, the concentration of etoposide that reduced viability by 50% was approximately 10-fold less for antisense-treated cells compared with either control scrambled oligodeoxynucleotide-treated or untreated cells, as shown in one of four representative experiments (Fig6). These results indicated that suppression of LMP-1 using antisense oligodeoxynucleotides enhanced susceptibility of lymphoblastoid cells to the cytotoxic effects of etoposide. This effect was sequence-specific, since etoposide sensitivity of cells treated with control oligodeoxynucleotides was similar to untreated cells (Fig 6).
LMP-1 antisense oligodeoxynucleotide treatment enhances sensitivity to etoposide. X50-7 cells were exposed to etoposide in the presence or absence of oligodeoxynucleotide (50 μmol/L in the presence of serum) for 24 hours and assayed for viability by trypan blue exclusion. Values represent the mean ± SD of triplicate samples. (□) LMP-1 antisense-treated; (▪) control oligodeoxynucleotide (SS1)-treated; (▨) untreated control. P values for the mean difference between antisense-treated and SS1-treated cells in the presence of etoposide are as follows: 2 μmol/L, P < .002; 20 μmol/L, P < .001; 20 μmol/L, P = .0002.
LMP-1 antisense oligodeoxynucleotide treatment enhances sensitivity to etoposide. X50-7 cells were exposed to etoposide in the presence or absence of oligodeoxynucleotide (50 μmol/L in the presence of serum) for 24 hours and assayed for viability by trypan blue exclusion. Values represent the mean ± SD of triplicate samples. (□) LMP-1 antisense-treated; (▪) control oligodeoxynucleotide (SS1)-treated; (▨) untreated control. P values for the mean difference between antisense-treated and SS1-treated cells in the presence of etoposide are as follows: 2 μmol/L, P < .002; 20 μmol/L, P < .001; 20 μmol/L, P = .0002.
DISCUSSION
The EBV-encoded latent protein, LMP-1, is expressed in the majority of EBV-associated neoplasms, including posttransplant lymphoproliferative disease, AIDS-associated lymphoma, and nasopharyngeal carcinoma.29-31 Although the precise role of LMP-1 in the genesis of these neoplasms is unclear, expression of LMP-1 is an absolute requirement for immortalization of B cells by EBV.8 Furthermore, LMP-1 confers the properties of a classical oncogene when expressed in rodent fibroblasts or human epithelial cells.10-12 In addition to the profound phenotypic properties associated with expression of LMP-1 in lymphoid and nonlymphoid cells, this viral protein has been directly implicated in protecting cells from apoptotic cell death.15,16Gene-transfer experiments in a variety of cell lines have shown that expression of LMP-1 induces the antiapoptotic cellular genes, Bcl-2, Mcl-1, and A20.15-18 Furthermore, LMP-1 protects cells from a variety of apoptotic stimuli, including serum withdrawal and p53 activation.15,16 32 The induction of antiapoptotic genes by LMP-1 presumably underlies the protective effects of LMP-1 on sensitivity to apoptosis.
Because LMP-1 is expressed in the majority of EBV-associated neoplasms, but not in normal cells, and has critical functions in promoting cell survival, it represents a logical target for modulation by antisense strategies. Our studies have now demonstrated that short-term, daily treatment of EBV-positive lymphoblastoid cells in vitro with unmodified antisense oligodeoxynucleotides targeted to the first five codons of the LMP-1 open-reading frame results in reproducible suppression of LMP-1 protein. This effect was sequence-specific, since control oligodeoxynucleotides containing the same base content as the antisense sequence did not consistently alter LMP-1 levels. The effect was also protein-specific, since we did not observe global suppression of protein levels in antisense-treated cells. LMP-1 protein has a very short half-life,33 and it was possible to observe suppression of LMP-1 protein as early as 24 hours of antisense treatment (data not shown); more dramatic suppression was observed after 48 and 72 hours of exposure to antisense. Due to the instability of unmodified oligodeoxynucleotides and their associated transient effects in tissue culture, we used relatively high concentrations of antisense (50 μmol/L in the absence of liposomes) and treated cells at 24-hour intervals. However, using nuclease-resistant, phosphorothioated antisense oligodeoxynucleotides that are more stable in vivo, we have elicited similar effects on LMP-1 levels and cellular proliferation using lower concentrations of oligodeoxynucleotide (5 to 10 μmol/L) added at 36-hour intervals in the absence of liposomes (data not shown).
Importantly, suppression of LMP-1 with antisense was associated with molecular and phenotypic effects that can be predicted from the known functions on LMP-1. These effects included downregulation of the LMP-1-inducible antiapoptotic genes Bcl-2 and Mcl-1, inhibition of proliferation, stimulation of the apoptotic pathway of cell death under conditions of serum deprivation, and enhanced sensitivity to the chemotherapeutic agent, etoposide. The stimulation of apoptosis and enhanced sensitivity to etoposide likely derived, in part, from suppression of Bcl-2, since previous studies have shown that Bcl-2 protects EBV-negative B- and pre-B-lymphoid cells from apoptosis induced by serum deprivation or cytotoxic drug treatment.15 25 The effects of LMP-1 antisense were not observed in EBV-negative lymphoid cells, confirming that these events likely derive from sequence-specific suppression of LMP-1. Surprisingly, we observed minimal nonspecific toxic effects of control scrambled oligodeoxynucleotides on proliferation, apoptosis, or viability after exposure to etoposide. This may be attributed, in part, to the short duration of exposure and the use of unmodified oligodeoxynucleotides.
The prognosis of EBV-associated lymphomas that occur in the setting of AIDS or organ transplantation remains poor, and there is a compelling need for novel, nontoxic therapeutic approaches. Because these malignancies are EBV-dependent, there is sound rationale for targeting critical viral products in the design of alternative therapies. Previously, we have demonstrated that the latent viral gene product, EBNA-1, can be suppressed using an antisense strategy.20However, EBNA-1 is a stable protein, and prolonged exposure to antisense oligodeoxynucleotides was required to elicit significant downregulation of protein levels and biologic effects in vitro. We have now identified a second critical viral gene product, LMP-1, that is suppressible by antisense oligodeoxynucleotides. Since LMP-1 is expressed in the majority of EBV-associated neoplasms, and, importantly, has a short half-life, it represents an ideal target for suppression by antisense oligodeoxynucleotides as a potential treatment strategy. Our studies confirm that a relatively short continuous exposure to LMP-1 antisense in vitro (<72 hours) elicits biologic effects. Furthermore, the observation that LMP-1 antisense treatment modulates susceptibility to etoposide has important potential clinical implications. Since phosphorothioated oligodeoxynucleotides have proven to be remarkably nontoxic in animals and humans when administered as continuous infusions,34-36 it may be possible to use LMP-1–targeted antisense as a nontoxic agent to enhance the chemosensitivity of these tumors, thereby increasing treatment efficacy. Indeed, Bcl-2–targeted antisense oligodeoxynucleotide treatment has recently been shown to enhance chemosensitivity, not only in vitro, but also in vivo in a murine model of melanoma.34Further studies using combinations of LMP-1–targeted antisense in combination with chemotherapeutic agents both in vitro and in vivo using the murine SCID model of EBV-associated lymphomas should yield further information regarding the validity and feasibility of this approach for clinical applications.
ACKNOWLEDGMENT
We gratefully acknowledge James Jones (National Jewish Center, Denver, CO) for providing EBNA-1–positive human serum.
Supported by Public Health Services Grant No. CA 67396.
Address reprint requests to Jill Lacy, MD, Department of Internal Medicine, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06520.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. LMP-1 antisense oligodeoxynucleotide treatment inhibits proliferation of EBV-positive lymphoblastoid cells. Proliferation was assayed by [3H]thymidine incorporation in 2 EBV-positive lymphoblastoid cell lines (11-23 and X50-7) and an EBV-negative B-cell line (BJAB) after exposure to oligodeoxynucleotide (10 μmol/L in liposomes) for 48 hours. Values represent the mean ± SD of triplicate assays. (□) Antisense-treated; (▪) control oligodeoxynucleotide (SS1)-treated; (▨) untreated control. P values for the mean difference between antisense and SS1-treated cells are as follows: 11-23, P < .002; X50-7, P = .0003; BJAB, P> .8. P values for the mean difference between SS1-treated and untreated cells are as follows: 11-23, P > .1; X50-7,P > .08; BJAB, P > .06.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/5/10.1182_blood.v92.5.1721/4/m_blod41708003x.jpeg?Expires=1769143818&Signature=XUx-B3-1ilNDLLq5CrJ3SbHi4Kq~xZV3mZOCpyydDSUD4UfLWoxZy9zcJU95C7IifXvkIN6wx-D-pyiyqThtk3yFsb87R43lJ1xeeqI0krLRNZuxhIdH9KMYHEAwEZrLcTLIvrO~KcJneTFa6o3Ch9FaH2QihzWH5DO5wcDLKebKhKitWRYYssbRd2vzdI~gJgXb4AstJ8LKpgUQsqUwUWZ8ow21bQNrEwVQyn2UDmsf6ckcYNEbm7FdgK6iDV4jmHt7-IhzO~5bQysJpsaFvJZqRBkXTwUqsJYxC7M4nEwbNf4PucbqzCSRmS5yQMapSSIDzMpksK9D-ja6XRSaIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal