Abstract
Scott syndrome is a rare inherited bleeding disorder in which platelets and other blood cells fail to promote normal assembly of the membrane-stabilized proteases of the plasma coagulation system. The defect in Scott blood cells is known to reflect inability to mobilize phosphatidylserine from inner plasma membrane leaflet to the cell surface in response to an elevation of Ca2+ at the endofacial surface. To gain insight into the molecular basis of this membrane defect, we examined the expression in Scott cells of plasma membrane proteins that have been implicated to participate in the accelerated transbilayer movement of plasma membrane PL. By both reverse transcriptase-polymerase chain reaction (RT-PCR) and functional assay, the level of expression of the multidrug resistance (MDR)1 and MDR3 P-glycoproteins in immortalized B-lymphoblast cell lines from the patient with Scott syndrome were indistinguishable from matched cell lines derived from normal controls. Whereas the plasma membrane of Scott cells are insensitive to activation of the plasma membrane PL scramblase pathway, it had been shown that PL scramblase protein isolated from detergent-solubilized Scott erythrocytes exhibits normal function when incorporated into proteoliposomes (Stout JG, Basse F, Luhm RA, Weiss HJ, Wiedmer T, Sims PJ: J Clin Invest 99:2232, 1997). Consistent with this finding in Scott erythrocytes, we found that Scott lymphoblasts expressed normal levels of PL scramblase mRNA and protein, and that the deduced sequence of PL scramblase in Scott cells is identical to that of normal controls. These data suggest that the defect in Scott syndrome is related either to aberrant posttranslational processing of the PL scramblase polypeptide or to a defect or deficiency in an unknown cofactor that is required for normal expression of plasma membrane PL scramblase function in situ, or alternatively, reflects the presence of a detergent-dissociable inhibitor of this pathway.
© 1998 by The American Society of Hematology.
PLASMA MEMBRANE phospholipids (PL) are normally asymmetrically distributed, with phosphatidylcholine (PC) and sphingomyelin located primarily in the outer leaflet, and the aminophospholipids, phosphatidylserine (PS), and phosphatidylethanolamine (PE) restricted to the cytoplasmic leaflet.1,2 This membrane lipid asymmetry is maintained at least in part through the activity of a Mg2+- and adenosine triphosphate (ATP)-dependent aminophospholipid translocase that selectively transports PS and PE from outer to inner membrane leaflet.3-5 Upon an increase in intracellular Ca2+ due to either cell activation, cell injury, or apoptosis, rapid bidirectional movement of the plasma membrane PL between leaflets is observed, resulting in exposure of PS and PE at the cell surface.1,6-8 This exposure of plasma membrane amino PL has been shown to promote assembly and activation of several key enzymes of the coagulation and complement systems, and to accelerate the clearance of injured or apoptotic cells by the reticuloendothelial system, suggesting that Ca2+-induced remodeling of plasma membrane PL is central to both vascular hemostasis and cellular clearance.1,9-12 The molecular mechanism(s) underlying this intracellular Ca2+-initiated remodeling of the transbilayer distribution of plasma membrane PL remains poorly understood. We recently identified and cloned an integral plasma membrane protein,PL scramblase, that mediates Ca2+-dependent, bidirectional movement of PL between membrane leaflets, mimicking the action of Ca2+ at the endofacial surface of the plasma membrane.13-17 PL scramblase was shown to be expressed in erythrocytes, platelets, endothelium and a variety of other cells and tissues that are known to expose plasma membrane PS in response to elevated cytosolic Ca2+. Furthermore, we demonstrated that the level of expression of PL scramblase in the plasma membrane determines the extent to which PS becomes exposed at the cell surface upon an increase in cytosolic Ca2+.17
Scott syndrome is a rare bleeding disorder in which platelets and other blood cells show a diminished capacity to mobilize PS to the cell surface, resulting in impaired assembly and activation of the surface-catalyzed coagulation enzyme complexes that are required for normal plasma clotting.18-23 The plasma membranes of Scott cells contain normal amounts of PS and other PL, and exhibit normal aminophospholipid translocase activity.19,24 Nevertheless, the plasma membrane of these cells are unresponsive to elevations in [Ca2+] at the endofacial surface, which in normal cells evokes the accelerated movement of all PL between inner and outer plasma membrane leaflets. Recent evidence indicates that Scott syndrome is an autosomal recessive trait affecting all hematologic lineages that results in abrogation of normal Ca2+-stimulated transbilayer movement of plasma membrane PL.23,25,26However, the defective gene giving rise to this disorder remains to be identified. It had been observed that upon activation by thrombin plus collagen, Scott syndrome platelets showed reduced protein tyrosine phosphorylation, implying a potential enzyme defect affecting one or more intracellular protein tyrosine kinases.27Nevertheless, a subsequent study demonstrated that the reduced protein phosphorylation observed in Scott syndrome cells is likely an epiphenomenon relating to the aberrant stability of the plasma membrane PL distribution in these cells and is not causally related to the disorder.28 Recently, Toti et al29 reported that Epstein-Barr virus (EBV)-transformed B lymphocytes from a patient with Scott syndrome lacked expression of multidrug resistance (MDR) genes MDR1 and MDR3. They proposed that deficiency in the expression of the MDR proteins was responsible for the aberrant properties of the plasma membrane of Scott cells, and they proposed that the phenotype observed in Scott syndrome resulted through mutation in an unlinked gene coding for a regulatory protein that is required for normal MDR gene expression.
In this study, we report that EBV-transformed B lymphocytes from a patient with Scott syndrome exhibit normal expression of both PL scramblase and MDR genes. Furthermore, cDNA sequence encoding PL scramblase in Scott syndrome cells is identical to that we previously reported for cells exhibiting normal PL scramblase function.15
MATERIALS AND METHODS
Materials.
Fetal bovine serum, RPMI 1640, rhodamine 123 (Rh123), and verapamil were purchased from Sigma, (St Louis, MO). PCR2.1 vector and T-A Cloning kit were from Invitrogen (Carlsbad, CA). All restriction enzymes were from New England BioLabs, Inc (Beverly, MA). ExpressHyb, Klentaq Polymerase, and Advantage reverse transcriptase-polymerase chain reaction (RT-PCR) kits were from Clontech Laboratories (Palo Alto, CA). α-32P-deoxycytidine triphosphate (dCTP) was purchased from Dupont (Wilmington, DE). Random Primed DNA Labeling Kit was from Boehringer Mannheim (Indianapolis, IN), Hybond-N Nylon membrane from Amersham (Arlington Heights, IL), and SuperSignal ULTRA Chemiluminescence Kit from Pierce Chemical Co (Rockford, IL).
B-cell lines.
EBV-transformed B-lymphoblast cell lines were established from peripheral B lymphocytes of a single patient with Scott syndrome (patient MS; now deceased) as previously described.26 The clinical and cellular abnormalities identified in this patient have been reviewed in detail by Weiss.18,19 Three independent transformations of B lymphocytes were performed using venous blood obtained on separate occasions from MS, and from three separate normal volunteers. The lymphoblasts were cultured in RPMI 1640 containing 10% fetal bovine serum. All three cell lines derived from the patient with Scott syndrome exhibited a marked defect in capacity to expose PS at the cell surface upon ionophore-induced increase in intracellular Ca2+.26
32P-labeling of cDNA probes.
cDNA of PL scramblase and β-actin were labeled with 1 mCi of α-32P-dCTP using random-primed DNA labeling kit. The labeled probes were separated from free α-32P-dCTP by filtration through S-400 spin columns. The specific radioactivity of the PL probes for PL scramblase and β-actin was 4.6 × 108 and 8 × 108 dpm/μg, respectively.
Isolation of RNA and Northern blot.
Total cellular RNA was isolated from 4 × 107 Scott or normal control B lymphoblasts by the guanidinium thiocyanate cell lysis method using a Total RNA isolation kit (Ambion, Austin, TX). A total of 10 μg of RNA from Scott or normal B lymphoblasts were loaded to each lane of an agarose gel, separated by electrophoresis, and transferred to a nylon membrane. The membrane was prehybridized with ExpressHyb solution at 68°C for 30 minutes and hybridized with ExpressHyb containing 5 ng/mL 32P-labeled PL scramblase cDNA probe at 68°C for 1 hour, then washed, and exposed to x-ray film. After development, the membranes were stripped and hybridized with 32P-labeled β-actin cDNA probe using identical conditions.
RT-PCR.
A total of 1 μg RNA was reverse transcribed using Moloney murine leukemia virus transcriptase and oligo (dT) primers. cDNA representing 50 ng RNA was then subjected to PCR for 35 cycles in a final volume of 100 μL using 2.5 U of Klentaq polymerase to ensure high-fidelity amplification. The primers to amplify PL scramblase, MDR1, MDR3, and β2-microglobulin cDNA sequences were identical to those previously described by us15 and by Toti et al,29 respectively. After an initial denaturation of 2 minutes at 94°C, each cycle consisted of 1 minute at 94°C, 1 minute at 55°C, and 2 minutes at 68°C. PCR products were separated on a 2% agarose gel. Bands were visualized by ethidium bromide staining and photographed.
cDNA cloning and sequencing.
After RT-PCR and electrophoresis, PL scramblase cDNA derived from Scott B lymphoblasts was cut from the gel, purified with Wizard kit, and directly cloned into T-A cloning vector pCR2.1. Escherichia coli strain INVαF’ was transformed, and the presence of a 954-bp cDNA insert in the plasmid isolated from a single colony was confirmed by digestion with EcoRI. The plasmid was purified by Qiagen Kit. DNA was sequenced on an ABI DNA Sequencer Model 373 Stretch (Applied Biosystems, Foster City, CA) using PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit (Perkin Elmer-Cetus, Norwalk, CT). To avoid any PCR-mediated errors, two batches of independent RT-PCR products were separately cloned and each sequenced in duplicate.
Western blot.
B lymphoblasts from the patient with Scott syndrome or normal controls were lysed (60 minutes at 4°C) with 2% (vol/vol) NP-40 in Hanks’ balanced salt solution (HBSS) containing 5 mmol/L EDTA, 50 mmol/L benzamidine, 50 mmol/L N-ethyl maleimide, 1 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L leupeptin. The lysates were centrifuged (250,000g, 30 minutes, 4°C), and the supernatants denatured (100°C, 5 minutes) in 10% (wt/vol) sodium dodecyl sulfate (SDS) sample buffer containing 2% β-mercaptoethanol. After SDS-polyacrylamide gel electrophoresis (PAGE) (lysate from 1 × 106 cells per lane) and transfer to nitrocellulose, the blocked membrane was incubated with 10 μg/mL of rabbit anti-PL scramblase-E306-W318, a specific antibody raised against the C-terminus of PL scramblase.15 The blots were incubated with horseradish peroxidase-conjugated goat antirabbit IgG and developed by SuperSignal ULTRA chemiluminescence.
Dye efflux assay for MDR1 P-glycoprotein (Pgp) activity.
B lymphoblasts from a patient with Scott syndrome or normal controls were suspended in RPMI 1640 complete medium at 106 cells/mL and incubated in presence of 5 μg/mL Rh123 for 10 minutes at 37°C. After washing, the rhodamine-loaded cells were suspended in RPMI 1640 complete medium and incubated in the presence or absence of the MDR1 Pgp inhibitor verapamil (50 μmol/L final concentration) at 37°C, 5% CO2 for up to 3 hours. Aliquots of 0.5 mL were removed at times 0 hour and 3 hours, the cells were recovered by centrifugation (735g, 1 minute), and suspended in 0.5 mL HBSS. Single cell fluorescence was quantified by flow cytometry monitoring cell-associated Rh123 fluorescence in the FL1 channel (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, CA).
RESULTS
Expression of MDR genes in Scott B lymphoblasts.
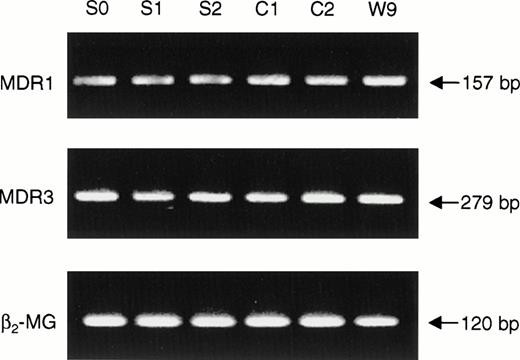
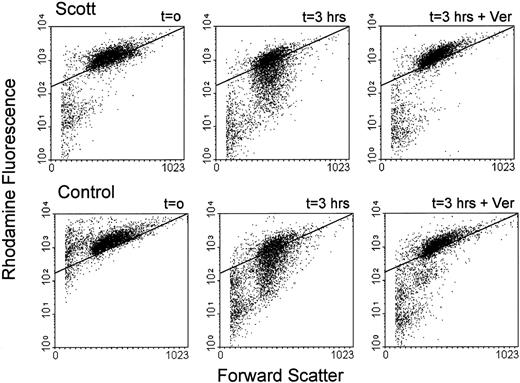
Toti et al29 recently reported that the genes MDR1 and MDR3 were not expressed in EBV-transformed lymphoblasts from a patient with Scott syndrome and inferred a role of this family of proteins both in surface exposure of PS and in the plasma membrane defect manifest in Scott syndrome. Therefore, it was of interest to investigate MDR gene expression in the immortalized B cells obtained from the single other individual (MS) who was well documented to exhibit the Scott syndrome.18-22 RT-PCR was performed on RNA isolated from each of three Scott and three normal control EBV-transformed B-lymphoblast cell lines, using oligonucleotides specific for MDR1 and MDR3, respectively. As shown in Fig 1, transcripts of both the MDR1 and MDR3 genes were detected in all cell lines analyzed. Although absolute amounts of each of these mRNAs varied slightly among the individual cell lines, we observed no consistent differences in the levels expressed by Scott versus normal control cells. To confirm the presence of functional MDR1 Pgp in these cells, B lymphoblasts were loaded with the fluorescent dye Rh123, and efflux of Rh123 was quantified by flow cytometry. As shown in Fig 2, Rh123 was efficiently extruded from both Scott and normal control cells, as evidenced by a time-dependent decrease in cell-associated fluorescence upon incubation of the Rh123-loaded cells in dye-free medium. Although the rate of dye efflux varied somewhat from cell line to cell line, each of the three Scott B-lymphoblast cell lines tested was capable of actively extruding Rh123, and no significant difference in MDR1 Pgp activity was discerned between the Scott and normal control cell lines (data not shown). In all cases, the efflux of Rh123 from the B lymphoblasts (Scott or control) was completely inhibited when dye-loaded cells were incubated in the presence of the MDR1 Pgp inhibitor, verapamil. These data confirm the presence of functional MDR1 Pgp in the B lymphoblasts obtained from this patient with Scott syndrome and suggest that the abnormality in transbilayer mobilization of plasma membrane PL that is characteristic of these cells must relate to a defect or deficiency in another plasma membrane PL transporting protein.
Expression of MDR genes in Scott and normal control EBV-transformed B lymphocytes. RNA was extracted from three EBV-transformed B-lymphoblast cell lines derived from a patient with Scott syndrome (S0, S1, S2) and three normal volunteers (C1, C2, W9), and RT-PCR performed with primers specific for MDR1, MDR3, and β2-microglobulin (control gene), respectively. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. See Materials and Methods for details.
Expression of MDR genes in Scott and normal control EBV-transformed B lymphocytes. RNA was extracted from three EBV-transformed B-lymphoblast cell lines derived from a patient with Scott syndrome (S0, S1, S2) and three normal volunteers (C1, C2, W9), and RT-PCR performed with primers specific for MDR1, MDR3, and β2-microglobulin (control gene), respectively. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. See Materials and Methods for details.
Flow cytometric analysis of MDR1 Pgp activity in B lymphoblasts. B lymphoblasts from a patient with Scott syndrome and normal controls were loaded with Rh123 (10 minutes, 37°C), washed and reincubated in Rh123-free medium in the presence (3 hours + Ver) or absence (3 hours) of 50 μmol/L verapamil. Aliquots were removed at t = 0 hour and 3 hours, and analyzed by flow cytometry. Dot plots are shown for one normal control (C2; lower panels) and one Scott lymphoblast cell line (S2; upper panels). An arbitrary gate is drawn to facilitate comparison between samples. All three Scott and three normal control cell lines displayed MDR1 activity in the absence of verapamil, although differences in activity between cell lines were observed (not shown).
Flow cytometric analysis of MDR1 Pgp activity in B lymphoblasts. B lymphoblasts from a patient with Scott syndrome and normal controls were loaded with Rh123 (10 minutes, 37°C), washed and reincubated in Rh123-free medium in the presence (3 hours + Ver) or absence (3 hours) of 50 μmol/L verapamil. Aliquots were removed at t = 0 hour and 3 hours, and analyzed by flow cytometry. Dot plots are shown for one normal control (C2; lower panels) and one Scott lymphoblast cell line (S2; upper panels). An arbitrary gate is drawn to facilitate comparison between samples. All three Scott and three normal control cell lines displayed MDR1 activity in the absence of verapamil, although differences in activity between cell lines were observed (not shown).
Expression of the PL scramblase gene in Scott B lymphoblasts.
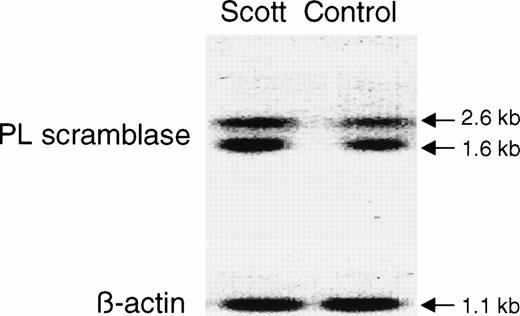
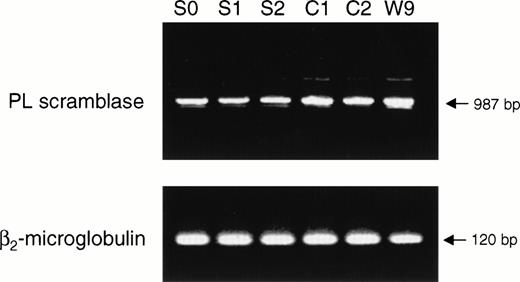
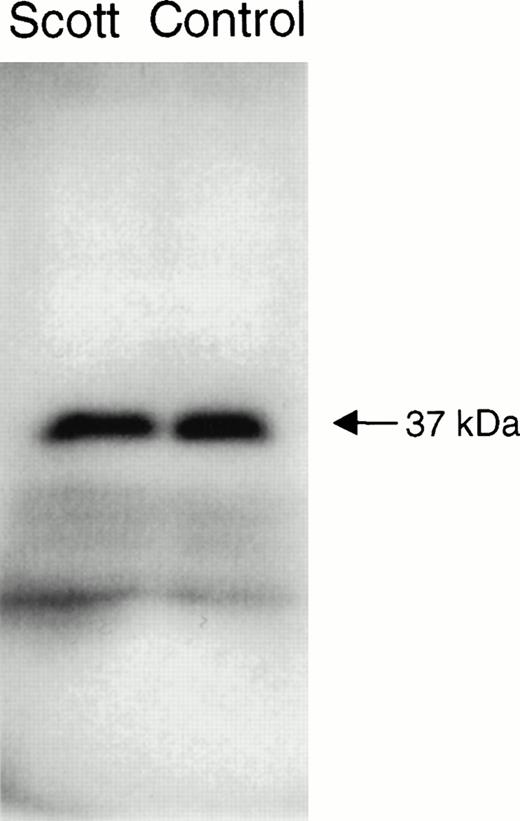
The functional abnormality in the blood cells of patients with Scott syndrome has been shown to specifically relate to a defective response of the plasma membrane to an elevation of Ca2+ at the endofacial surface. Whereas intracellular Ca2+ normally initiates collapse of the plasma membrane PL asymmetry by stimulating the rapid transbilayer movement of all plasma membrane PL, in Scott syndrome cells, the plasma membrane remains unresponsive to such elevation in intracellular Ca2+, and these cells retain their PL asymmetry despite entry of Ca2+ into the cytosol in the circumstance of either plasma membrane injury or cell activation. This implied that Scott cells are either deficient or defective in the protein that is responsible for mediating Ca2+-induced transbilayer movement of the plasma membrane PL. PL scramblase is an endofacially-oriented plasma membrane protein that has been shown to mediate rapid transbilayer movement of all plasma membrane PL upon binding Ca2+, mimicking the action of Ca2+ at the internal surface of the plasma membrane. The similarity of the function exhibited by PL scramblase to the observed defect in Scott syndrome cells, suggested that this syndrome was likely related to an abnormality specifically affecting this protein. To gain further insight into the origin of the functional defect in the Scott syndrome cells, we quantified the level of PL scramblase expression and obtained the cDNA sequence of the PL scramblase that is expressed in the aberrant Scott cell lines for comparison to that in normal cells (Figs 3 and 4). Expression of PL scramblase RNA was examined in three different Scott and three normal control B-lymphoblast cell lines. Northern blot with PL scramblase cDNA probe showed two transcripts of approximately 1.6 and 2.6 kb in both Scott (S2) and normal control (W9) B lymphoblasts (Fig 3), in agreement with what we previously observed in a number of other tissues and cell lines examined.15,17 RT-PCR performed with the same B-lymphoblast cell lines indicated the presence of PL scramblase cDNA in Scott cells of the same size as that found in normal control lymphoblasts (Fig 4). Furthermore, Western blotting with antibody directed against the C-terminal peptide of PL scramblase15 showed the presence of ∼ 37 kD PL scramblase protein at comparable amounts in both Scott and normal control B lymphoblasts (Fig 5). Complete identity in the predicted amino acid sequence of PL scramblase expressed in Scott syndrome cells to that previously reported for wild-type human PL scramblase (GenBank accession number AF008445) was also confirmed by sequencing of cDNA obtained from duplicate RT-PCR reactions performed for three different Scott B-lymphoblast cell lines. Thus, as demonstrated for the MDR1 and MDR3 Pgps, the phenotype in Scott syndrome also cannot be attributed to mutation in the PL scramblase gene.
Northern blot analysis of PL scramblase in Scott and normal B lymphoblasts. RNA was extracted from Scott and normal control B lymphoblasts, and Northern blot performed with32P-labeled PL scramblase cDNA probe as described in Materials and Methods. Results are shown for Scott S2 and normal control W9 (upper panel). Comparable amounts of transcripts were observed for all cell lines tested. Lower panel shows Northern blot of β-actin.
Northern blot analysis of PL scramblase in Scott and normal B lymphoblasts. RNA was extracted from Scott and normal control B lymphoblasts, and Northern blot performed with32P-labeled PL scramblase cDNA probe as described in Materials and Methods. Results are shown for Scott S2 and normal control W9 (upper panel). Comparable amounts of transcripts were observed for all cell lines tested. Lower panel shows Northern blot of β-actin.
RT-PCR analysis of PL scramblase in Scott and normal B lymphoblasts. RNA was extracted from three Scott (S0, S1, S2) and three normal control (C1, C2, W9) B-lymphoblast cell lines and RT-PCR performed with PL scramblase-specific primers. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. See Materials and Methods for details. RT-PCR of β2-microglobulin as control gene is also shown.
RT-PCR analysis of PL scramblase in Scott and normal B lymphoblasts. RNA was extracted from three Scott (S0, S1, S2) and three normal control (C1, C2, W9) B-lymphoblast cell lines and RT-PCR performed with PL scramblase-specific primers. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. See Materials and Methods for details. RT-PCR of β2-microglobulin as control gene is also shown.
Western blot of PL scramblase in Scott and normal B lymphoblasts. Lysates of Scott and normal B lymphoblasts were subjected to SDS-PAGE, transferred to nitrocellulose, and the blot was developed with 10 μg/mL of rabbit anti-PL scramblase-E306-W318 raised against the C-terminal peptide of PL scramblase. See Materials and Methods for details.
Western blot of PL scramblase in Scott and normal B lymphoblasts. Lysates of Scott and normal B lymphoblasts were subjected to SDS-PAGE, transferred to nitrocellulose, and the blot was developed with 10 μg/mL of rabbit anti-PL scramblase-E306-W318 raised against the C-terminal peptide of PL scramblase. See Materials and Methods for details.
DISCUSSION
The multidrug resistance Pgps MDR1 and MDR3 are members of the superfamily of ATP-binding cassette transporters. Whereas MDR1 Pgp is thought to primarily function to extrude cytotoxic agents from the cell, MDR3 Pgp has no drug-pumping activity, but there is strong evidence that MDR3 Pgp is a PC-specific flippase.30,31 Mice deficient in mdr2, the homologue to human MDR3, display a defect in PL secretion into the bile.32 Evidence for MDR1 Pgp-mediated transport of PC and PE, but not PS, from inner to outer plasma membrane leaflet has also been presented.33,34 Toti et al29 recently reported the lack of expression of both MDR1 and MDR3 genes in EBV-transformed B lymphocytes derived from a patient with Scott syndrome and inferred a link between transbilayer transport of PS and the MDR family of Pgp. In this study, we present evidence that both MDR1 and MDR3 Pgp are normally expressed in another well-documented case of Scott syndrome. Our results suggest that neither MDR1 nor MDR3 plays a role in Ca2+-induced surface exposure of PS, the transbilayer PL transport activity that is deficient in Scott syndrome. Although the possibility exists that the patient with Scott syndrome described by Toti et al is deficient in an as yet unknown member of the MDR family, it is also noteworthy that the activity of these Pgps is ATP-, but not Ca2+-dependent. It is also worth considering that expression of MDR genes has been reported to vary between individuals, with differentiation stage and age. For instance, Pilarski et al35 reported that 50% to 80% of normal blood T or B lymphocytes were positive for cell surface MDR1 Pgp, with the proportion of Pgp+ cells decreased in young children and individuals over age 60. Furthermore, the possibility exists that MDR expression in the study by Toti et al was decreased upon EBV-transformation of B lymphocytes and does not reflect the level of MDR Pgp expression in the circulating blood cells of this patient, as neither analysis by Northern blot nor by MDR1 Pgp function apparently yielded conclusive results.29 36
There is now strong evidence that cell surface exposure of PS that occurs upon cell activation or injury is mediated by the Ca2+-activated PL scramblase, a plasma membrane protein that mediates rapid transbilayer movement of all plasma membrane PL. Although Scott cells are characterized by an apparent functional defect in this Ca2+-activated plasma membrane PL scramblase pathway, we had previously shown that Scott erythrocytes contain a protein that exhibits normal PL scramblase function, after it is extracted from the erythrocyte with detergent and reconstituted into liposomal membranes.14 This implied that the gene defect in Scott syndrome affecting the PL scramblase pathway is only manifest in situ, and that the protein functions normally after exposure to detergent and reconstitution with exogenous PL. Our current data also indicate that Scott syndrome cells express normal levels of PL scramblase mRNA and protein, and that the deduced sequence of the expressed polypeptide is identical to that of PL scramblase found in normal cells.15 Thus, the molecular identity of the defect leading to the aberrant phenotype of the Scott syndrome cell remains elusive. Among the possibilities now being pursued are (1) the presence in Scott cells of an inhibitor of the PL scramblase pathway that dissociates from the protein upon solubilization in detergent; (2) a deficiency in a protein that normally interacts with PL scramblase and is necessary for its function in situ; (3) a defect in Scott cells in a posttranslational modification of the PL scramblase polypeptide affecting either its affinity for Ca2+ or the expression of its PL mobilizing function. In this context, we recently showed that the activity of PL scramblase is dependent upon thioesterification of one or more cytoplasmic cysteinyls with fatty acid.37Nevertheless, preliminary experiments in Scott syndrome lymphoblasts suggest that the PL scramblase expressed in these cells is normally palmitoylated (data not shown).
Supported in part by National Heart, Lung and Blood Institute Grant No. HL36946 from the National Institutes of Health (to P.J.S. and T.W.) and a Grant-In-Aid from the American Heart Association (Grant No. 95013720 to T.W.). Q.Z. is the recipient of a Research Fellowship Award from the American Heart Association, Wisconsin Affiliate (Grant No. 96-F-Post-50).
Address reprint requests to Therese Wiedmer, PhD, Blood Research Institute, The Blood Center of Southeastern Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: twiedmer@bcsew.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal