Abstract
Erythrocyte protein 4.1 (P4.1) is an 80-kD cytoskeletal protein that is important for the maintenance of the structural integrity and flexibility of the red blood cell membrane. Limited chymotryptic digestion of erythroid P4.1 yields 4 structural domains corresponding to the 30-, 16-, 10-, and 22/24-kD domains. Using a yeast two-hybrid system, we isolated cDNA clones encoding pICln that specifically interacts with the 30-kD domain of P4.1. In this report, we show that the carboxyl-terminus (amino acid residues 103-237) of pICln binds to the 30-kD domain of P4.1 in a yeast two-hybrid system. The direct association between the 30-kD domain of P4.1 and pICln was further confirmed by the following findings: (1) the S35-methione–labeled pICln specifically bound to both GST/P4.1-80 (80 kD) and GST/P4.1-30 (30 kD) fusion proteins, but not to the proteins that lack the 30-kD domain; (2) coimmunoprecipitation analysis of the cell extracts from transfected SiHa cells showed that pICln and P4.1 associate in transfected cells. It was reported that pICln can form a complex with actin and may play a role involved in cellular volume regulation. The direct association between P4.1 and pICln suggests that pICln may link P4.1-bound cytoskeletal elements to an unidentified volume-sensitive chloride channel.
© 1998 by The American Society of Hematology.
PROTEIN 4.1 (P4.1) IS originally described as an 80-kD protein isolated from the cytoskeletal preparations of mature red blood cells. Its function is believed to stabilize a skeletal network complex containing spectrin and actin and to anchor them to the overlying plasma membrane via interactions with integral membrane proteins, such as glycophorin and band 3.1 Structural defects of these membrane skeletal components may profoundly alter red blood cell morphology and reduce membrane mechanical strength, leading to membrane fragmentation and hemolytic anemia.1
Recently, numerous P4.1 isoforms have been identified in erythroid as well as in nonerythroid cells.2-4 These isoforms vary in size and exhibit considerable structural and functional diversity. Although the function of nonerythroid P4.1 has not been well characterized, it is apparently not strictly confined to the cell membrane. The immunoreactive epitopes of P4.1 have been identified in cytoplasmic, nuclear, perinuclear regions, stress fibers, Golgi apparatus, and centrosome.5-10 The heterogeneity of these P4.1 isoforms can be generated by complex alternative splicing of P4.1 pre-mRNA4,11 and by posttranslational modifications of P4.1 proteins.12 The P4.1 gene, located at human chromosome 1p33-34.2,13 comprises at least 23 exons (13 constitutive exons and 10 alternative exons) that are interrupted by 22 introns.14,15 Interestingly, different P4.1 isoforms expressed in restricted tissues might possess different functions. For example, a P4.1 isoform (80 kD) produced predominantly in mature red blood cells contains an exon 16-encoded peptide (21 amino acids) that is necessary for formation of the ternary complex with spectrin and actin,16 17 whereas a lymphoid P4.1 isoform (135 kD), generated by selective use of an exon 2′-encoded translation initiation site, appears to be located within the nucleus of nonerythroid cells.
Limited chymotryptic digestion of erythrocyte P4.1 (80 kD) generates 4 structural domains18: (1) the amino-terminal domain (30 kD) is a protease-resistant domain that mediates membrane-cytoskeleton interaction; (2) a 16-kD hydrophilic domain possesses a PKC phosphorylation site; (3) a 10-kD domain contains an exon-16 encoded peptide that is important for interaction with spectrin-actin complexes16,17; and (4) a carboxy-terminal domain (22/24 kD) whose functions have not yet been well-characterized.
Among these 4 domains, the 30-kD domain shows a substantial sequence homology to members of the P4.1 superfamily that can be subdivided into 5 gene families: band 4.1 (P4.1), talin, PTPH1 (protein tyrosine phosphatase), ERM (radixin/moesin/merlin), and NBL4 (novel band 4.1-like proteins).19 Considering that the sequence of the 30-kD domain is homologous to the P4.1 superfamily and that it may mediate membrane-cytoskeleton interactions via integral membrane proteins (eg, glycophorin C/D and band 3), it is reasonable to speculate that all other P4.1 members should be located underneath the membrane and bound to integral membrane proteins via its homologous domain. Indeed, the ERM family members were reported to be highly concentrated at the specialized region of plasma membranes where actin filaments are densely associated with plasma membranes.20Moreover, a high sequence homology was found between the 30-kD domain of P4.1 (P4.1-30), protein tyrosine phosphatases,21 and the human homologue of the Drosophila discs large tumor suppressor protein (hdlg),22 which suggests that P4.1-30 may be involved in the regulation of cell growth and cell-cell contact by affecting cytoskeleton/membrane interaction.
For a better understanding of the physiological roles of P4.1-30, it is necessary to know how many and what types of P4.1-30–associated proteins are included. In this study, we took advantage of the yeast two-hybrid system to identify P4.1-associated proteins. Our results show that the 30 kD of P4.1 directly binds to pICln, a protein that induces an outwardly rectifying, nucleotide-sensitive chloride current,23 which implicates the possible role of P4.1 involved in volume regulation.
MATERIALS AND METHODS
Yeast two-hybrid screen.
The 30-kD domain of human P4.1 (P4.1-30) was fused to the GAL4 DNA binding domain (GAL4-DB) in vector pAS2-1 (Clontech, Palo Alto, CA) to generate pAS/P4.1-30, which was used as the bait in the screen. Likewise, a human lymphocyte cDNA library purchased from Clontech was constructed in the GAL4-AD vector (pACT) to produce fusions of the random proteins encoded by the library cDNAs and the GAL4 transcriptional activation domain. The 2 types of plasmids were then cotransformed into yeast Y190 strain. The transformants were plated on SD minimal medium containing 25 mmol/L 3-amino-1,2,4-triazole (3-AT), but lacking leucine (−Leu), tryptophan (−Trp), and histidine (−His). Yeast genetic techniques and media composition were as described by the manufacturer (Clontech). Primary transformants (ie, Leu+, Trp+, His+) were further tested for expression of the second reporter gene (lacZ) using colony-lift assay or liquid assay for β-galactosidase activity as described by the manufacturer (Clontech).
To narrow down the region of P4.1 that binds to pICln, constructs encoding various portions of human P4.1 were fused to the sequence encoding the GAL4 DNA-binding domain of pAS2-1. Yeast cells (Y187) were simultaneously transformed with GAL4 DB-P4.1 fusion plasmids and a GAL4-AD-pICln fusion plasmid, followed by plating on SD minimal medium lacking leucine and tryptophan. The positive transformants were assayed as described above.
Plasmids construction and in vitro binding assay.
The human cDNAs that span the coding region of the 80-kD (residues 1-622), the 30-kD (residues 1-283), and the 10+24-kD (residues 378-622) domains of P4.1 were inserted inframe into a pGEX-2T expression vector (Pharmacia, Uppsala, Sweden) to generate glutathione-S-transferase (GST)/P4.1 fusion proteins. Overexpression and affinity purification of GST fusion proteins were performed as previously described.24
The full-length human pICln cDNA originally isolated from a yeast two-hybrid screen (Fig 1) was constructed in a pGEM4 vector (Promega, Madison, WI). Synthetic sense-capped mRNA were generated from the SP6 promoter. The pICln mRNAs were translated in a rabbit reticulocyte system (Promega) in the presence of S35-methionine to radiolabel newly synthesized proteins as described.4 Equal portions of the labeled pICln protein were incubated with affinity-purified GST/P4.1 fusion proteins previously coupled to Glutathione sepharose beads for 1 hour at 4°C in the presence ofEscherichia coli extract. After incubation, the immobilization of the pICln-P4.1 complex was washed 4 times with bead binding buffer (50 mmol/L potassium phosphate, pH 7.5, 150 mmol/L KCl, 1 mmol/L MgCl2, 10% glycerol, 1% Triton X-100, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF]). The bound protein complex was subjected to analysis on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by autoradiography as described.4
Cell transfection, immunoprecipitation, and Western blot analysis.
The cDNA encoding the full-length human pICln was inserted in frame into a CMV promoter-driven pEGFP-C1 expression vector (Clontech). SiHa cells (a human cervical carcinoma cell line, ATCC HTB35) were transiently transfected with or without GFP-tagged pICln cDNA by electroporation as described.25 Fourty-eight hours later, the cells were collected and lysed in a MAP buffer (20 mmol/L Tris-HCl, pH 8.0, 137 mmol/L NaCl, 1 mmol/L EGTA, 1% Triton X-100, 10% glycerol, 1.5 mmol/L MgCl2, 100 μmol/L Na3VO4, 50 mmol/L NaF, 1 mmol/L PMSF, 50 μg/mL aprotinin, and 50 μmol/L leupeptin). For in vitro binding (Fig 5), the cell extracts were incubated with affinity-purified GST/P4.1 fusion protein coupled to Glutathione sepharose beads for 2 hours at 4°C with gentle mixing. The bound complex was washed 4 times with MAP buffer and analyzed by 10% SDS-PAGE. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and incubated with a polyclonal antibody against GFP (Clontech). The immunoreactive polypeptides were then detected with peroxidase-conjugated goat antirabbit IgG (Promega) using the Western Exposure Chemiluminescent Detection system (Pierce, Rockford, IL), as described.25
For Western blot analysis (Fig 6A), the cell extracts prepared from the SiHa cells transfected with or without GFP-pICln DNA or with GFP vector alone were immunoblotted with a monoclonal antibody against GFP. For the immunoprecipitation experiment (Fig 6B), whole cell lysates precleared by protein A sepharose beads were immunoprecipitated with anti–P4.1-80 antibody4 for 2 hours at 4°C, followed by incubation with the protein A-Sepharose beads for another 1 hour. Immunoprecipitates were washed 4 times with the MAP buffer and analyzed by SDS-PAGE. After transfer to a PVDF membrane, the immunoreactive proteins were detected by a mouse monoclonal antibody against GFP (Clontech).
RESULTS
Screening for proteins that interact with the 30-kD domain of protein 4.1 (P4.1-30).
To identify proteins that physically interact with the 30-kD domain of P4.1, we performed a yeast two-hybrid screen. The P4.1-30 cDNA, fused in frame to the Gal4 DNA-binding domain, was used as a bait to screen a human lymphocyte cDNA library fused to a Gal4 activation domain. Interaction of 2 proteins in this system allows for growth on medium lacking histidine and for expression of β-galactosidase.
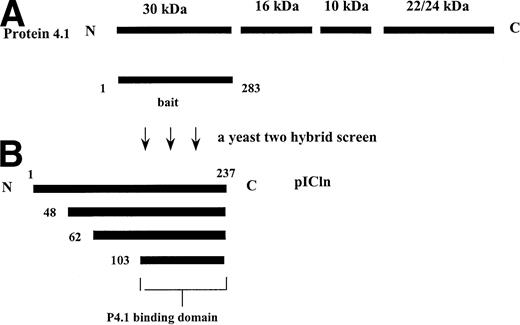
A total of 1 × 106 colonies were screened and nearly 70 positive clones were isolated. The plasmid DNAs were transformed into E coli and subjected to DNA sequencing. A search of the GenBank database showed that at least 3 known genes (pICln, CD44, and 14-3-3 β) were recovered from this screening. Among these, the pICln clones with variable lengths were independently isolated 31 times. Interestingly, all pICln clones contained a common carboxyl region starting from amino acid residues 103 to 237 (Fig 1), which implicates that this region contains the binding site for P4.1-30. In addition to pICln, 2 other known genes, CD44 and 14-3-3β, were also isolated. The interaction of P4.1-30 and 14-3-3β could be a false result, because the intact P4.1 (P4.1-80) did not bind to 14-3-3β both in vitro and in vivo (unpublished data). The direct association between P4.1-30 and CD44 is currently under investigation.
Structure of protein 4.1 (A) and summary of the pICln clones isolated from the yeast two-hybrid screen using the 30-kD domain of P4.1 as a bait (B).
Structure of protein 4.1 (A) and summary of the pICln clones isolated from the yeast two-hybrid screen using the 30-kD domain of P4.1 as a bait (B).
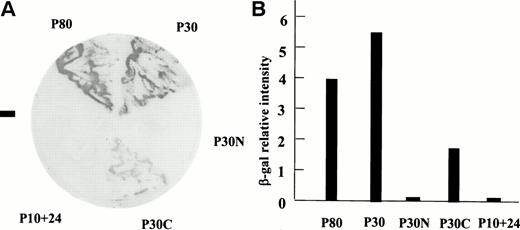
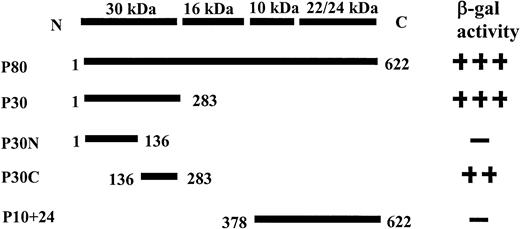
To determine the region of P4.1 that interacts with pICln, constructs encoding the 80-kD (P80), 30-kD (P30), the N-terminal portion of the 30-kD (P30N), the C-terminal portion of the 30-kD (P30C), and 10+24-kD (P10+24) domains were cotransformed into yeast with full-length pICln. Figure 2A shows that pICln interacted with full-length P4.1 (P80), P30, and P30C, but not with P10+24 or the N-terminal portion of 30-kD domain (P30N). These results were further evident with the more sensitive liquid culture assay using ONPG as substrate (Fig 2B). A summary of this study is depicted in Fig 3. We conclude that the C-terminal portion of the 30-kD domain (residues 136-283) contains the binding site for pICln.
Protein 4.1 interacts with pICln protein in the two-hybrid screen. Interaction between various portions of protein 4.1 and pICln using a colony-lift assay (A) or a liquid assay for β-galactosidase activity (B). “−” in (A) is a negative control transformation by both a pLAM5′-1 plasmid encoding a GAL4 DNA-BD/human lamin C hybrid and a GAL4-AD-pICln DNA.
Protein 4.1 interacts with pICln protein in the two-hybrid screen. Interaction between various portions of protein 4.1 and pICln using a colony-lift assay (A) or a liquid assay for β-galactosidase activity (B). “−” in (A) is a negative control transformation by both a pLAM5′-1 plasmid encoding a GAL4 DNA-BD/human lamin C hybrid and a GAL4-AD-pICln DNA.
Protein 4.1 binds to pICln in vitro.
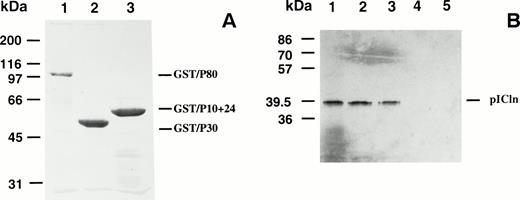
To confirm the direct interaction between P4.1 and pICln, the association was further analyzed by an in vitro binding assay. GST-tagged P4.1 protein, either full-length (P80) or the truncated forms (P30 and P10+24), was expressed and purified (Fig 4A). Immobilized GST-tagged P4.1 proteins were incubated with S35-methionine–labeled pICln and retention on the beads was analyzed by SDS-PAGE as described in the Materials and Methods. As shown in Fig 4B, pICln bound strongly to the full-length P4.1 (P80) (lane 2) and P30 (lane 3), but not to P10+24 (lane 4) or GST beads alone (lane 5). These results are consistent with that of the yeast two-hybrid experiments (Fig 2).
(A) Purified bacterially produced GST/protein 4.1 fusion proteins in E coli were shown by Coomassie blue staining. (B) Binding of GST/protein 4.1 to pICln in vitro. S35-methionine–labeled pICln (lane 1) was incubated with affinity-purified GST/P80 (lane 2), GST/P30 (lane 3), GST/P10+24 (lane 4), or GST sepharose beads (lane 5). After incubation, the bound protein complexes were analyzed by SDS-PAGE and autoradiography.
(A) Purified bacterially produced GST/protein 4.1 fusion proteins in E coli were shown by Coomassie blue staining. (B) Binding of GST/protein 4.1 to pICln in vitro. S35-methionine–labeled pICln (lane 1) was incubated with affinity-purified GST/P80 (lane 2), GST/P30 (lane 3), GST/P10+24 (lane 4), or GST sepharose beads (lane 5). After incubation, the bound protein complexes were analyzed by SDS-PAGE and autoradiography.
Protein 4.1 and pICln form a complex in transfected cells.
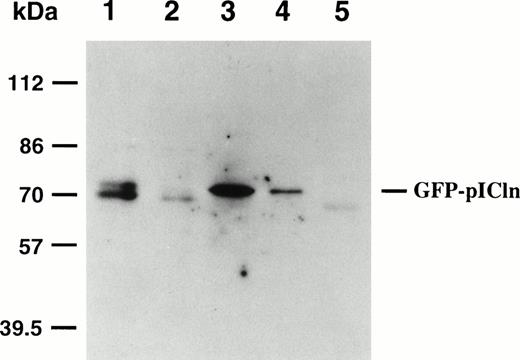
We next investigated whether P4.1 binds to pICln in mammalian cells. The pICln was tagged with GFP and transiently transfected into SiHa cells. The cell extract was prepared 48 hours after transfection and incubated with immobilized GST-tagged P4.1 proteins. The bound substances were analyzed by SDS-PAGE and immunoblotted with a polyclonal antibody against GFP. A single band was detected in transfected cell extracts incubated with GST/P80 (Fig 5, lane 3) and GST/P30 (lane 4), but not with GST/P10+24 (lane 5), which indicated the direct association of pICln with P80 and P30. The lower band found in the GFP-pICln transfected cell extract (lane 1) could be a nonspecific reacted band, which was also detected in the cell extract from nontransfected SiHa cells (lane 2).
Interaction between GFP-pICln and GST/P4.1 proteins. SiHa cells were electroporated with a GFP-pICln cDNA. The whole cell lysates prepared from transfected cells (lane 1) were incubated with GST/P80 (lane 3), GST/P30 (lane 4), or GST/P10+24 (lane 5) fusion proteins previously coupled to glutathione sepharose beads. The bound complexes were electrophoresed, transferred to the membrane, and analyzed by a polyclonal antibody against GFP. Lane 2 is a negative control. The cell lysate was prepared from nontransfected SiHa cells and analyzed by anti-GFP antibody.
Interaction between GFP-pICln and GST/P4.1 proteins. SiHa cells were electroporated with a GFP-pICln cDNA. The whole cell lysates prepared from transfected cells (lane 1) were incubated with GST/P80 (lane 3), GST/P30 (lane 4), or GST/P10+24 (lane 5) fusion proteins previously coupled to glutathione sepharose beads. The bound complexes were electrophoresed, transferred to the membrane, and analyzed by a polyclonal antibody against GFP. Lane 2 is a negative control. The cell lysate was prepared from nontransfected SiHa cells and analyzed by anti-GFP antibody.
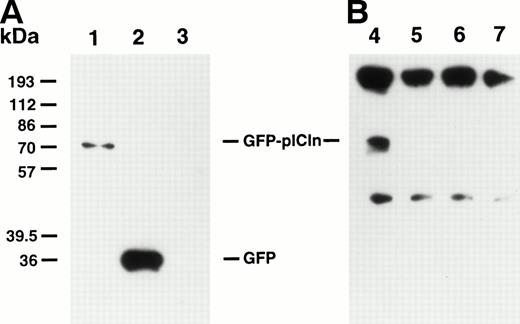
The direct association between pICln and P4.1 in transfected cells was further analyzed by immunoprecipitation study (Fig 6B). The cell lysates prepared from the GFP vector or GFP-tagged pICln-transfected cells were first immunoprecipitated with a polyclonal antibody against intact P4.1 (80 kD)4 and the Western blots were probed with a monoclonal antibody against GFP. Figure 6B shows that GFP-tagged pICln formed a complex with endogeneous P4.1 (lane 4). However, no such complex was detected in cells transfected with the GFP vector alone (lane 5) or in untransfected SiHa cells (lane 6). Taken together, our studies demonstrate that pICln binds to P4.1 both in vitro and in transfected cells.
Protein 4.1 binds to pICln in transfected cells. SiHa cells were electroporated with a GFP-pICln cDNA or a GFP vector alone. (A) Whole cell lysates (∼10 μg) were immunoblotted with a monoclonal antibody against GFP. Lane 1, GFP-pICln–transfected cells; lane 2, GFP vector DNA-transfected cells; lane 3, untransfected SiHa cells. Immunoblot analysis demonstrated that GFP-pICln was expressed in a substantial amount in transfected SiHa cells (lane 1). (B) Whole cell lysates were immunoprecipitated with a polyclonal anti-P4.1 antibody (anti-P80). The proteins were separated by SDS-PAGE, transferred to a membrane, and probed with a monoclonal antibody against GFP. Lane 4, GFP-pICln–transfected cells; lane 5, GFP vector DNA-transfected cells; lane 6, untransfected SiHa cells; lane 7, a negative control (GFP-pICln–transfected cell lysates immunoprecipitated with anti-P80 antibody and reprobed with an irrelevant anti-Flag monoclonal antibody). We did not add β-mercaptoethanol (a chemical reagent that can break a protein disulfide bond) into the sample buffer; therefore, most Ig molecules remained as intact molecules (upper bands), whereas only some dissociated into Ig heavy chain subunits (lower bands).
Protein 4.1 binds to pICln in transfected cells. SiHa cells were electroporated with a GFP-pICln cDNA or a GFP vector alone. (A) Whole cell lysates (∼10 μg) were immunoblotted with a monoclonal antibody against GFP. Lane 1, GFP-pICln–transfected cells; lane 2, GFP vector DNA-transfected cells; lane 3, untransfected SiHa cells. Immunoblot analysis demonstrated that GFP-pICln was expressed in a substantial amount in transfected SiHa cells (lane 1). (B) Whole cell lysates were immunoprecipitated with a polyclonal anti-P4.1 antibody (anti-P80). The proteins were separated by SDS-PAGE, transferred to a membrane, and probed with a monoclonal antibody against GFP. Lane 4, GFP-pICln–transfected cells; lane 5, GFP vector DNA-transfected cells; lane 6, untransfected SiHa cells; lane 7, a negative control (GFP-pICln–transfected cell lysates immunoprecipitated with anti-P80 antibody and reprobed with an irrelevant anti-Flag monoclonal antibody). We did not add β-mercaptoethanol (a chemical reagent that can break a protein disulfide bond) into the sample buffer; therefore, most Ig molecules remained as intact molecules (upper bands), whereas only some dissociated into Ig heavy chain subunits (lower bands).
DISCUSSION
Protein 4.1 is the prototype of a superfamily of proteins including P4.1, ezrin, moesin, merlin (a candidate tumor suppressor), talin, and nonreceptor tyrosine phosphatase (PTP-MEG, PTPH1). All members of this superfamily share a highly conserved region to the N-terminal of the 30 kD of P4.1, whose function is poorly understood.19Recently, several studies have shown that the 30 kD of P4.1 contains the binding sites that interact with glycophorin C, p55, band 3, calmodulin, and dlg/hdlg.26-30 In this study, we found that the 30-kD domain of P4.1 also mediates its binding to pICln, a protein involved in volume regulation. Taken together, these results lead us to propose that the 30-kD domain and its homologue in other members of the P4.1 superfamily may act as binding sites for protein-protein interaction. At the present time, neither the binding affinity of these P4.1-associated proteins with P4.1 nor the regulation of how P4.1 interacts with its associated proteins is completely clear. A detailed analysis of particular amino acids of this 30-kD domain involved in protein-protein interaction may provide some useful information to elucidate its binding association.
The finding that pICln interacts with the 30-kD domain of P4.1 was quite informative. The maintenance of a constant cell volume is essential for the normal function and survival of animal cells. The cell volume regulatory response to hypotonic stress is initiated by activation of K+ and Cl− channels with resultant water loss from the intracellular compartment.31 32 Although this phenomenon has been known for decades, the molecular identities of the proteins responsible for the volume control machinery have been largely unknown.
pICln, originally identified in Madin Darby canine kidney (MDCK) epithelial cells,23 is a 235-amino acid protein that induces an outwardly rectifying, nucleotide-sensitive chloride current when expressed in Xenopus oocytes.23 pICln has been proposed to make a channel by dimerizing and forming a β-barrel pore.23 Expression of mammalian pICln in Xenopus oocytes produced a prominent chloride current23displaying features similar to the swelling-induced chloride current seen in many cell types (epithelial cells, atrial and ventricular myocytes, etc).33-35 This effect was originally explained by assuming that pICln was a plasma membrane-spanning protein that constituted the anion channel itself.23 However, Krapivinsky et al36 reported that pICln was a soluble cytosolic protein and did not colocalize with the plasma membrane. These findings led them to hypothesize that pICln could be a cytosolic regulator that regulates an unidentified volume-sensitive anion channel.36 Recently, several studies showed that pICln is present predominantly in the cytosol (only 5% to 10% in the membrane fraction)37,38 and that hypotonic stress may induce translocation of the pICln from the cytosol into the membrane.38 Buyse et al39 proposed a possible model to explain how pICln works. They suggested that pICln may form a dimer, perhaps in a β-barrel configuration, after a hypotonic shock. This form may then insert into the membrane and act like a channel or regulate a preexisting anion channel.
Taking into account all of the evidence described above, whether pICln serves as a plasma-membrane anion channel or a cytosolic regulator of a channel still remains controversial. Clearly, more experimental work is required to resolve this discrepancy. Nevertheless, our current data demonstrate a direct association between P4.1 and pICln that provides a new direction to reconsider how pICln functions.
It has been shown that the volume-regulatory response may require dynamic changes in actin filament organization.40 Actin filaments could play a role in the regulation of cell swelling possibly by two distinct mechanisms.41 One is involved in the coordinated interaction of actin and actin-binding proteins to all plasma membrane linkage. In shark rectal gland cells, disruption of the actin-membrane organization is correlated with increased swelling.41 The other is via regulation of ion transport proteins that are activated by cell swelling. Using F-actin stabilizing (phalloidin) and destabilizing (dihydrocytochalasins) drugs, it was shown that F-actin regulates swelling-activated Cl−current and regulatory volume decrease (RVD).42 P4.1 has been reported to be an actin-associated protein that binds to the spectrin-actin complex via its central 10-kD domain.43 Our current finding also indicates that P4.1 binds to pICln through its 30-kD domain. Thus, P4.1 may act as a adaptor protein that bridges the spectrin-actin skeleton to a protein (pICln) involved in volume regulation. If pICln is in fact a regulatory protein rather than a channel protein, then this type of linkage may connect actin-bound cytoskeletal elements to an unidentified volume-sensitive chloride channel.
The mechanisms that regulate the volume-sensitive chloride channels are essentially unknown. The identified protein-protein interaction between P4.1, pICln, and spectrin-actin complex makes P4.1 a candidate protein of such volume-regulatory schemes. Awareness of such interaction may provide the means in the future to show the molecular mechanism between the membrane-cytoskeletal complex and signal transduction pathways in controlling volume regulation.
Supported by a grant (NSC87-2314-B001-012) from the National Science Council of the Republic of China and an Institutional grant from Academia Sinica.
Address reprint requests to Tang K. Tang, PhD, Institute of Biomedical Sciences, Academia Sinica, 128 Yen-Chiu-Yuan Rd, Sec. 2, Taipei, Taiwan, 115, Republic of China; e-mail: tktang@ibms.sinica.edu.tw.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal