Abstract
Early recommendations on prophylactic transfusion of thrombocytopenic patients involved a standard platelet dose of about 0.5 × 1011/10 kg body weight. Given the lack of data supporting this dose, we prospectively studied the dose response to platelet transfusions in adults and children with hematologic malignancies. Each patient received, in similar clinical conditions, a medium, high, and very high dose of fresh (< 24 hours old) ABO-compatible platelets, in the form of apheresis platelet concentrates (APC). For the adults, the medium dose was defined as APC containing between 4 and 6 × 1011 platelets, the high dose between 6 and 8 × 1011, and the very high dose > 8 × 1011; for the children, the three doses corresponded to 2 to 4, 4 to 6, and > 6 × 1011 platelets. The end points were the platelet increment, platelet recovery, and the transfusion interval, and the results were compared with a paired t-test. Sixty-nine adults and 13 children could be assessed. Recoveries in the adults were similar with the three doses (from 28% to 30%), but the high and very high doses led to a significantly better platelet increment (52 and 61 × 109/L, respectively) than the medium dose (33 × 109/L, P < .01). The main difference was in the transfusion interval, which increased with the dose of platelets transfused, from 2.6 days with the medium dose to 3.3 and 4.1 days with the high and very high doses, respectively (P< .01). The positive effect of the high dose was observed regardless of pretransfusional clinical status, but was more marked in patients with no clinical factors known to impair platelet recovery. In these patients, a platelet dose of 0.07 × 1011 per kg of body weight led to a transfusion interval of more than 2 days in 95% of cases. In patients with clinical factors favoring platelet consumption, the proportion of transfusions yielding an optimal platelet increment and transfusion interval increased with the dose of platelets.The platelet dose-effect was also significant in the children, in whom the high and very high doses led to 1.5-fold to twofold higher posttransfusion platelet counts and transfusion intervals. We conclude that transfusion of high platelet doses can reduce the number of platelet concentrates required by thrombocytopenic patients and significantly reduce donor exposure.
© 1998 by The American Society of Hematology.
PLATELET SUPPORT ENABLES aggressive myelosuppressive radiation therapy and chemotherapy to be administered safely.1 Despite the recent cloning of the gene encoding thrombopoietin, the major growth factor for the megakaryocytopoietic lineage, and trials of its use in vivo,2 platelet transfusions will remain vital for many years to support thrombocytopenic patients. Platelet transfusion practices have been the subject of numerous editorials, guidelines, and consensus conferences.3-7 Many reports have either supported or argued against the use of therapeutic versus prophylactic platelet transfusion8,9 and discussed the advantages of apheresis platelet concentrates (APC) relative to platelet concentrates prepared from whole blood, as well as the threshold platelet count for prophylactic transfusion10 and the influence of compatibility.11 Most of these reports also discussed the cost/efficacy and risk/benefit ratios of various transfusion strategies. In contrast, very few investigators have analyzed platelet dose requirements in thrombocytopenic patients. Based on the expected posttransfusion recovery and life span of transfused platelets, a dose of around 0.5 × 1011 platelets/10 kg body weight has long been recommended12 and has been the rule in many institutions. Many years ago we adopted a policy based on the collection of APC containing high doses of apheresis platelets and their transfusion undivided. This resulted in the transfusion of platelet doses higher than those usually given. As expected, we obtained higher posttransfusion platelet counts, but also a longer transfusion interval. To validate this high-dose strategy, we conducted a prospective study comparing three platelet doses in thrombocytopenic patients treated for hematologic malignancies and then attempted to define the required platelet dose in these patients.
MATERIALS AND METHODS
Patients
Consecutive patients managed in our institution from December 1992 to October 1995 and likely to require several platelet transfusions after induction chemotherapy for acute myeloid leukemia (AML) or conditioning for allogenic bone marrow transplantation (BMT) were included in the study. Adult and children (defined as patients under 15 years of age and weighing less than 50 kg) were analyzed separately.
Pretransfusion Evaluation
Age, sex, and diagnosis were recorded before transfusion. Pretransfusion factors likely to affect platelet recovery were also documented and included temperature, spleen size, intravenous administration of amphotericin B on the day of the transfusion, and graft-versus-host disease (GVHD) or veno-occlusive disease (VOD) in patients undergoing allogenic BMT. Patients were deemed infected in case of microbiologically documented infection or persistent temperature above 38°C during the transfusion protocol. VOD was diagnosed according to Jones et al.13 Acute GVHD was diagnosed and graded on the basis of the usual criteria.14
Anti-HLA antibodies were detected using the standard NIH microlymphocytotoxicity test (LCT) with a panel of samples from 30 selected donors carrying the most frequent HLA antigens. Patients were screened at the time of induction and then once a week while receiving platelet transfusions. In case of platelet refractoriness and negative LCT, anti-HLA antibodies were tested for by using an antiglobulin-augmented lymphocytotoxicity assay15 and a platelet immunofluorescence test (PIFT) was performed.
Platelet Transfusion Protocol
Patients received single-donor APC obtained on one of three cell separators [Spectra (Cobe), AS104 (Fresenius), and Vivacell (Dideco)]. All platelets transfused were fresh (less than 24 hours old) and ABO-compatible. APC were leukocyte-depleted to reach a target residual leukocyte count below 1 × 106. Leukocytes were automatically depleted during the collection procedure on the Spectra (Cobe) and AS 104 (Fresenius) cell separators; APC collected on the Vivacell (Dideco) cell separator were leukocyte-depleted by centrifugation and filtration as previously described.16The number of platelets collected was measured in each APC (Model JT, Coulter Electronic), after leukocyte depletion.
Patients were transfused prophylactically whenever their platelet counts fell to between 10 and 20 × 109/L. All fresh APC were received in the afternoon, after testing; thus, taking into account the fall in platelet count during the day and the rate of platelet consumption in the previous days, the platelet count required in the morning to plan a transfusion was approximately between 15 and 25 × 109/L. Three different platelet doses were transfused to each patient; in adults, the medium dose was defined as transfusion of APC containing between 4 and 6 × 1011platelets, the high dose between 6 and 8 × 1011, and the very high dose above 8 × 1011. In children, the medium, high, and very high doses corresponded to transfusion of respectively 2 to 4, 4 to 6, and > 6 × 1011 platelets. The doses were defined relative to the number of platelets contained in the APC rather than the patient’s body weight, as we usually transfuse the highest available dose, without adapting it precisely to the patient’s weight; however, the doses received per kg of body weight were retrospectively calculated for each transfusion. The sequence of administration of the different doses depended solely on APC availability, as the other contraints of the transfusion protocol did not permit randomization and as each patient serves as their own control.
Patients were classified as having or not having clinical factors known to impair platelet recovery; in patients with such factors, clinical status had to remain unchanged during the transfusion protocol.
Patients previously alloimmunized or who developed anti-HLA and/or antiplatelet antibodies during the study, patients submitted to particular platelet transfusion policies (eg, patients who received heparin or antithrombotic drugs), patients with a change in clinical status during the transfusion protocol, and patients who did not receive the three doses as planned were excluded from the analysis.
In Vivo Evaluation
The following parameters were evaluated: (1) the platelet increment, ie, the difference between the pretransfusion platelet count and that measured 12 hours after transfusion (both measured in the morning); (2) platelet recovery, calculated according to the following formula: Posttransfusion − Pretransfusion Platelet Count (× 109/L) × Patient Total Blood Volume (L) × 100 Number of Platelets Transfused (× 1011); (3) the transfusion interval, defined as the time between the transfusion analyzed and the next required transfusion, ie, when the platelet count fell to 15 to 25 × 109/L. The transfusion interval was measured in whole days; and (4) the daily platelet requirement, calculated as the fall in the number of platelets between 2 consecutive days.
Furthermore, in view of these results, we attempted to determine the optimal platelet dose per kg of body weight in adult patients. This dose was arbitrarily defined as the dose leading to a platelet increment of at least 50 × 109/L and to a transfusion interval of more than 2 days in patients without factors favoring platelet consumption. In patients with such factors, the required platelet increment and transfusion interval were at least 20 × 109/L and 2 days, respectively.
Statistical Analysis
Results are expressed as means ± standard deviation (SD). As each patient received all three doses of platelets, we compared the platelet increment, platelet recovery, and the transfusion interval by using a paired t-test (the results of the transfusion with the medium dose was compared with those of the high dose, and results of the high dose with those of the very high dose). The difference was considered significant if P < .05.
The relationship between the doses received per kg of body weight and the platelet increment and the transfusion interval were studied separately in patients with and without clinical factors favoring platelet consumption. For cut-off of platelet doses ranging from 0.06 to 0.14/kg, we calculated the proportion of transfusions responsible for the platelet increment and the transfusion interval as defined above. Statistical analysis was performed using BMDP statistical software.
RESULTS
Evaluation of the Dose-Effect of Platelet Transfusions
Adults.
A total of 196 adults were enrolled in the study, but only 69 could be evaluated: 41 patients with anti-HLA and/or antiplatelet antibodies and 21 patients who required special transfusion policies were excluded, as were 32 patients who failed to receive the correct dose of platelets at the time planned, and 33 patients whose clinical status changed during the protocol. The 69 assessable patients had a mean age of 38 (range, 17 to 58) and comprised 37 men and 32 women; 27 were treated for AML and 42 had undergone allogeneic BMT. Their mean weight was 64 kg (range, 49 to 110 kg). In the medium, high, and very high dose groups, the mean numbers of transfused platelets were 4.6, 6.5, and 8.9 × 1011, respectively, corresponding to a mean number of platelets per kg body weight of 0.08, 0.10, and 0.14 × 1011. As shown in Table 1, mean platelet recoveries were similar in the three doses groups at between 28% and 30%. The posttransfusion platelet increment increased significantly with the number of platelets transfused (P < .01): the mean pretransfusion platelet counts were 19, 22, and 21 × 109/L in the three dose groups, and the mean posttransfusion counts were 52, 73, and 83 × 109/L, respectively. In parallel, we observed a significantly longer interval between transfusions after the high dose (3.3 days) and very high dose (4.1 days) compared with the medium dose (2.6 days) (P < .01).
Prospective Comparison of the Platelet Increment, Platelet Recovery, and Transfusion Interval After Medium, High, and Very High Doses of Platelets in 69 Adults
| . | Medium Dose 4 to 6 × 1011 . | High Dose 6 to 8 × 1011 . | (p) . | Very High Dose >8 × 1011 . | (P) . |
|---|---|---|---|---|---|
| Platelet transfused (1011) | 4.6 ± 0.6 | 6.5 ± 0.5 | 8.9 ± 0.7 | ||
| Platelet increment (109/L) | 33 ± 22 | 51 ± 29 | (p < .01) | 62 ± 34 | (P < .01) |
| Recovery (%) | 28 ± 13 | 30 ± 15 | NS | 29 ± 13 | NS |
| Time to the next transfusion (days) | 2.6 ± 0.7 | 3.3 ± 1.2 | (p < .01) | 4.1 ± 1.4 | (P < .01) |
| . | Medium Dose 4 to 6 × 1011 . | High Dose 6 to 8 × 1011 . | (p) . | Very High Dose >8 × 1011 . | (P) . |
|---|---|---|---|---|---|
| Platelet transfused (1011) | 4.6 ± 0.6 | 6.5 ± 0.5 | 8.9 ± 0.7 | ||
| Platelet increment (109/L) | 33 ± 22 | 51 ± 29 | (p < .01) | 62 ± 34 | (P < .01) |
| Recovery (%) | 28 ± 13 | 30 ± 15 | NS | 29 ± 13 | NS |
| Time to the next transfusion (days) | 2.6 ± 0.7 | 3.3 ± 1.2 | (p < .01) | 4.1 ± 1.4 | (P < .01) |
Results are expressed as means ± SD.
Abbreviations: NS, not significant; p, statistical difference between medium and high dose; P, statistical difference between high and very high dose. Comparisons were made using a pairedt-test.
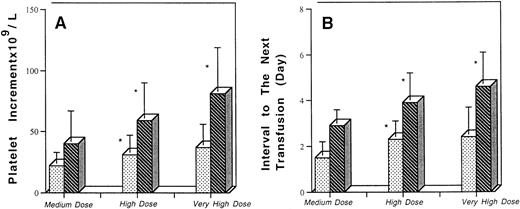
To determine if factors known to affect platelet recovery could mask the dose-response, patients were distinguished according to their clinical status. As shown in Fig 1, the dose-response effect was more evident in the 29 patients without clinical factors likely to affect platelet recovery: the interval between transfusions reached an average of 2.9 and 3.9 days after transfusion of the medium and high doses (P < .01) and increased to 4.5 days after transfusion of the very high dose (P < .05). In the remaining 40 patients with clinical factors potentially influencing platelet consumption, the difference remained significant between the medium and high doses, with a mean platelet increment of 22 and 31 109/L and a mean interval between transfusion of 1.4 and 2.1 days, respectively (P < .05), but there was no further significant improvement with the very high dose.
Comparison of the platelet increment and transfusion interval after medium, high, and very high doses of platelets according to pretransfusion clinical status. * Significant difference between medium and high dose and high dose and very high dose. Comparisons were made using a paired t-test. (▧) Clinical factors of platelet consumption. (▧) No clinical factors of platelet consumption.
Comparison of the platelet increment and transfusion interval after medium, high, and very high doses of platelets according to pretransfusion clinical status. * Significant difference between medium and high dose and high dose and very high dose. Comparisons were made using a paired t-test. (▧) Clinical factors of platelet consumption. (▧) No clinical factors of platelet consumption.
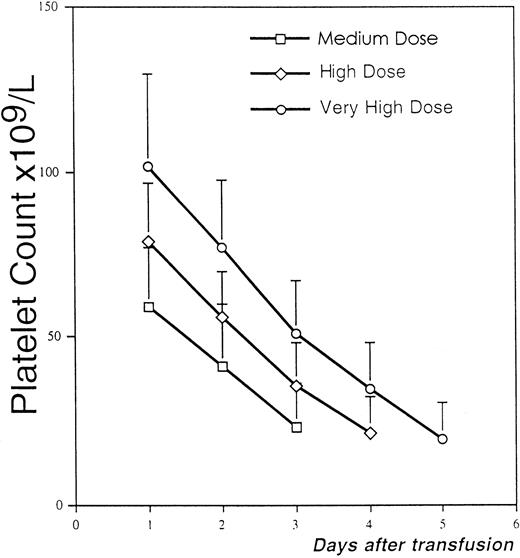
In the group of patients without clinical factors likely to affect platelet recovery, we calculated the fall in the platelet count from day to day after the three doses of platelets (Fig 2). We observed that the rate of fall was similar after transfusion of the medium, high, and very high doses, with a mean daily requirement of respectively 18, 19, and 20 x 109/L (range, 11 to 44 × 109/L); it was not significantly different when platelet counts were from 50 to 100 × 109/L (mean, 23 × 109/L) in comparison with counts <50 × 109/L (mean, 17 × 109/L).
Evaluation of the mean daily fall of the platelet count after medium, high, and very high doses of platelets in patients without clinical factors favoring platelet consumption. * In seven patients, the platelet counts on days 3, 4, and 5 after medium, high, and very high doses were not relevant because the patients were retransfused on the day before; so in these patients, the counts on these days were estimated and extrapolated from the fall in the platelet count in the previous days.
Evaluation of the mean daily fall of the platelet count after medium, high, and very high doses of platelets in patients without clinical factors favoring platelet consumption. * In seven patients, the platelet counts on days 3, 4, and 5 after medium, high, and very high doses were not relevant because the patients were retransfused on the day before; so in these patients, the counts on these days were estimated and extrapolated from the fall in the platelet count in the previous days.
Children.
Thirty-three children undergoing allogeneic BMT were enrolled. Eleven were excluded because they failed to receive the correct dose at the planned time, and nine because of a change in clinical status during the protocol. Thirteen children were thus assessable. Their mean age was 11 years (range, 7 to 14) and their mean weight 34 kg (range, 24 to 44).
The mean absolute numbers of platelets transfused were 3.3, 4.9, and 7 × 1011, respectively, in the medium, high, and very high dose groups, corresponding to a mean number of platelets per kg of body weight of 0.10, 0.15, and 0.22 × 1011, respectively. Mean recovery was similar to that obtained in the adults (28% to 32%), but some low-weight, clinically stable children showed recovery of the majority of platelets transfused (up to 75%). As shown in Table 2, the dose effect was very pronounced in the children. The posttransfusion platelet increment increased with the dose of platelets transfused, from 37 × 109/L with the medium dose to 64 × 109/L with the high dose (P < .05) and 98 × 109/L with the very high dose (P < .01), and the interval before the next transfusion also increased significantly from 2.5 days to 3.4 and 4.4 days.
Prospective Comparison of the Platelet Increment, Platelet Recovery and Transfusion Interval After Medium, High, and Very High Doses of Platelets in 13 Children
| . | Medium Dose 2 to 4 × 1011 . | High Dose 4 to 6 × 1011 . | (p) . | Very High Dose >6 × 1011 . | (P) . |
|---|---|---|---|---|---|
| Platelet transfused (1011) | 3.3 ± 0.3 | 4.9 ± 0.6 | 7 ± 0.7 | ||
| Platelet increment (109/L) | 37 ± 28 | 64 ± 33 | (p < .05) | 98 ± 34 | (P < .01) |
| Recovery (%) | 28 ± 19 | 29 ± 17 | NS | 32 ± 15 | NS |
| Time to the next transfusion (days) | 2.5 ± 1 | 3.4 ± 1.5 | (p < .05) | 4.4 ± 1.5 | (P < .05) |
| . | Medium Dose 2 to 4 × 1011 . | High Dose 4 to 6 × 1011 . | (p) . | Very High Dose >6 × 1011 . | (P) . |
|---|---|---|---|---|---|
| Platelet transfused (1011) | 3.3 ± 0.3 | 4.9 ± 0.6 | 7 ± 0.7 | ||
| Platelet increment (109/L) | 37 ± 28 | 64 ± 33 | (p < .05) | 98 ± 34 | (P < .01) |
| Recovery (%) | 28 ± 19 | 29 ± 17 | NS | 32 ± 15 | NS |
| Time to the next transfusion (days) | 2.5 ± 1 | 3.4 ± 1.5 | (p < .05) | 4.4 ± 1.5 | (P < .05) |
Results are expressed as means ± SD.
Abbreviations: NS, not significant; p, statistical difference between medium and high dose; P, statistical difference between high and very high dose. Comparisons were made using a pairedt-test.
Definition of the Optimal Platelet Dose per kg of Body Weight in Adult Patients
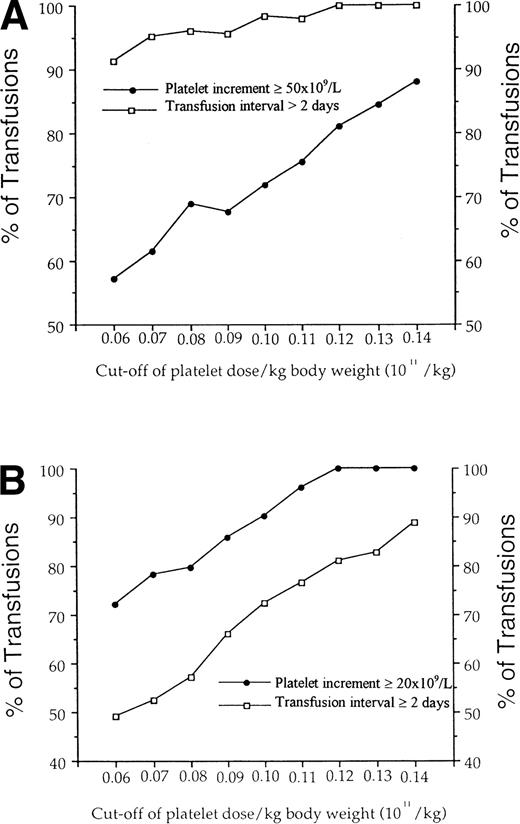
For each transfusion, the dose transfused per kg of body weight was calculated. We determine for doses ranging from 0.06 to 0.14 × 1011 per kg, the percent of transfusions leading to the required platelet increment and transfusion interval (Fig 3). In patients without clinical factors favoring platelet consumption, a threshold seemed to be achieved with a dose of 0.07 × 1011 per kg of body weight, for which more than 95% of transfusions yielded a transfusion interval of more than 2 days. At this dose, 62% of the patients achieved the required increment of at least 50 × 109/L platelets. In contrast, in patients with clinical factors known to affect platelet recovery, the more platelets we transfused, the higher the proportion of transfusions leading to a platelet increment ≥ 20 × 109/L (from 72% to 100% for doses from 0.06 to 0.14/kg body weight). In parallel, the percent of transfusions leading to the required transfusion interval increased from 49% to 89%. With a platelet dose of 0.10 × 1011/kg, the required interval was obtained in 70% of cases.
Definition of an optimal dose of platelets per kg body weight according to pretransfusion clinical status. For cut-off of doses ranging from 0.06 to 0.14 × 1011 per kg body weight in adult patients, the percent of transfusions leading to a required platelet increment and transfusion interval was determined. (A) Required platelet increment and transfusion interval were arbitrarily defined as increment ≥ 50 × 109/L and interval > 2 days in patients without clinical factors known to affect platelet consumption. (B) In patients with factors favoring platelet consumption, required platelet increment and interval were at ≥ 20 × 109/L and 2 days, respectively.
Definition of an optimal dose of platelets per kg body weight according to pretransfusion clinical status. For cut-off of doses ranging from 0.06 to 0.14 × 1011 per kg body weight in adult patients, the percent of transfusions leading to a required platelet increment and transfusion interval was determined. (A) Required platelet increment and transfusion interval were arbitrarily defined as increment ≥ 50 × 109/L and interval > 2 days in patients without clinical factors known to affect platelet consumption. (B) In patients with factors favoring platelet consumption, required platelet increment and interval were at ≥ 20 × 109/L and 2 days, respectively.
DISCUSSION
Platelet recovery is usually about 60% of the number of autologous platelets transfused, but may be as low as 20% to 40% after homologous transfusion in patients with factors affecting platelet recovery.17 Given the mean number of platelets in 1 U of blood (around 0.5 × 1011), the calculated platelet increment per platelet unit transfused is of the order of 12 to 25 × 109. Morrison12 suggested that 5 U of platelets were needed for an adult. This empiric figure has rarely been challenged. The NIH consensus conference suggested 1 U/10 kg body weight or an APC per transfusion3 and the British Committee for Standards in Hematology7 proposed a formula taking into account the desired platelet increment, the patient’s blood volume, and a recovery factor, resulting in recommended platelet doses of approximately 3 × 1011 for adults.
Very few teams have investigated the platelet dose-response. In 1983, Roy et al18 compared two platelet doses in children who received a mean of 2.6 and 3.4 U per transfusion. The average 1-hour increments were very small with both doses (18 and 25 × 109/L) and the incidence of bleeding was similar; the lower dose was thus recommended. More recently, Andreu19 reviewed results obtained with four doses of platelets in patients with AML and reported a significantly better response to the higher doses.
We report the first prospective comparison of three different doses of platelets. We attempted to eliminate all factors other than the number of platelets transfused that could affect efficiency: platelets were fresh, ABO-compatible, and administered in similar clinical conditions to the same patient. APC were routinely leukocyte-depleted before storage, as the quality of stored platelets can be improved by reducing the number of contaminating leukocytes. Platelets were irradiated, if needed, just before transfusion. In these conditions, we observed beneficial effects of high platelet doses not only in terms of higher posttransfusion platelet counts, but also longer intervals between transfusions. The larger platelet increments obtained with higher platelet doses increased the transfusion interval from 2 to 4 days.
The longer platelet life span after high doses could be explained by previously reported platelet kinetics in patients with bone marrow hypoplasia. Hanson and Slichter20 forwarded the concept of a given platelet requirement to support vascular integrity, random platelet utilization averaging 18% of overall platelet turnover in normal individuals; however, this proportion increased rapidly as the platelet count fell below 100 × 109/L.Using51Cr-labeled platelets, they found that autologous and homologous platelet survival correlated directly with the platelet count, with a reduction in the platelet life span to 3 to 4 days when the count fell below 50 × 109/L. This agrees with our observation, in patients without clinical factors favoring platelet consumption, of a mean regular daily platelet requirement of 19 × 109/L (corresponding to a mean of 31% of circulating platelets). Our study thus supports the concept that after the number of platelets needed for maintenance has been reached, the remaining platelets will continue to circulate, and that the higher the count, the longer the platelets will circulate. This could also account for the weaker dose-effect relationship in patients with clinical factors potentially inducing platelet destruction, independantly of platelet numbers and random utilization.
The inclusion criteria were very restrictive as regards the characteristics of the APC transfused and allowed for no change in clinical status; this led to the exclusion of a large number of patients. We therefore confirmed our results by a retrospective analysis of all the transfusions received by the cohort of adult patients included in this prospective study; we found a posttransfusion platelet increment of 31, 52, and 64 × 109/L, respectively after transfusion of the medium, high, and very high platelets doses; in addition, transfusion intervals increased with the dose of platelets transfused, from 2.4 to 3.1 and 4 days, respectively, showing that the beneficial effects of high doses are also observed in routine use whatever the characteristics of APC trasfused (data not shown). Interestingly, our results are very close to those obtained by Andreu19 in a retrospective study of AML patients.
The dose-effect relationship was much more marked in children, for whom lower doses are usually recommended.21 If we compare the same dose of 4 to 6 × 1011 in adults and children, there was a 1.5-fold to twofold higher platelet increment and transfusion interval in the latter. As in adults, the results of the prospective study were confirmed by the retrospective analysis of our transfusion practice. These excellent results explain why, in our experience, some children underwent BMT with platelets from only five donors.
Having demonstrated the dose-effect of platelet transfusions, it may be helpful to determine an “optimal dose” of platelets. Our study suggests that the optimal dose may be 0.07 × 1011platelets/kg for patients without clinical factors favoring platelet consumption (1.5 U/10 kg body weight in patients receiving random platelet concentrates). In patients with clinical factors known to affect platelet comsumption, the more platelets we give, the larger the proportion of patients achieving an optimal platelet increment and transfusion interval.
We conclude that high doses of APC can significantly reduce the need for transfusion support in thrombocytopenic patients, and thereby reduce donor exposure. This transfusion policy is practical, as concentrates containing more than 5 to 6 × 1011platelets can routinely be obtained with new apheresis devices. In our experience and that of others,22 such doses can be obtained in more than 60% of apheresis procedures. Furthermore, initial studies suggest that very high platelet yields (>10 × 1011) can be obtained after administration of thrombopoietin to platelet donors.23 We therefore recommend that APC should not be split, but transfused entirely to increase the transfusion interval and reduce donor exposure.
ACKNOWLEDGMENT
We are grateful to G. Van Waeg for editing of the manuscript and B. Raisonnier for data collection.
Address reprint requests to Françoise Norol, MD, Etablissement de Transfusion Sanguine, Hôpital Henri Mondor, 51, Av du Maréchal de Lattre de Tassigny, 94000 Creteil, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal