Abstract
The role of hematopoietic growth factors in lineage commitment and differentiation is unclear. We present evidence that heterologous expression of an erythroid specific receptor allows granulocytic differentiation of a myeloid cell line. We have previously characterized a truncation mutant of the erythropoietin receptor (EpoR), which is associated with familial erythrocytosis (Blood89:4628, 1997). This truncated EpoR lacks the distal 70 amino acids of the cytoplasmic domain. To study the functional role of this distal receptor domain, 32D cells, a murine interleukin-3 (IL-3)–dependent myeloid line, were transfected with the wild-type EpoR (32D/EpoR WT) or the truncated EpoR (32D/EpoR FE). 32D cells expressing either the full-length or truncated EpoR display equivalent proliferative rates in saturating concentrations of Epo. There is a dramatic difference in maturational phenotype between the two cell lines, however. The 32D/EpoR FE cells and mock transfected 32D cells have an immature, monoblastic morphology and do not express the primary granule protein myeloperoxidase. The 32D/EpoR WT cells, on the other hand, demonstrate granulocytic differentiation with profuse granulation, mature, clumped chromatin, and myeloperoxidase expression. There is no evidence of erythroid differentiation in 32D cells transfected with either the full-length or truncated EpoR. Treatment of the cells with the specific Jak2 inhibitor tyrphostin AG 490 inhibits myeloid differentiation driven by the distal EpoR. We conclude that: (1) the distal cytoplasmic domain of the EpoR is able to induce a specific myeloid differentiation signal distinct from mitogenic signaling, and (2) these data extend to myelopoiesis the growing body of evidence that the cellular milieu, not the specific cytokine receptor, determines the specificity of differentiation after cytokine receptor activation.
© 1998 by The American Society of Hematology.
HEMATOPOIETIC GROWTH factors such as interleukin-3 (IL-3), granulocyte colony-stimulating factor (G-CSF), and erythropoietin (Epo) are potent mitogens for their target cells. The mitogenic effects of these growth factors are mediated through homo or hetero-dimerization of their cognate receptors1 and subsequent activation of several signal transduction pathways including the JAK/STAT pathway,2 and the mitogen-activated protein (MAP) kinase pathway.3 In addition, these factors are able to suppress apoptosis. For instance, Epo is able to prevent apoptosis of erythroid precursors.4
Several lines of evidence suggest that hematopoietic growth factors are also capable of inducing differentiation of appropriate immature precursor cells. For instance, hematopoietic cells that normally do not express β globin can be induced to express this erythroid specific protein in an Epo-dependent manner after overexpression of the erythropoietin receptor.5 6
Other data are consistent with a more passive role for hematopoietic growth factors, ie, the growth factor merely allows a progenitor cell that is already “predetermined” to proceed with its differentiation program. Suppression of apoptosis by overexpression of Bcl-2 allowed spontaneous, cytokine independent differentiation of a multipotent progenitor cell line into several hematopoietic lineages.7 In another example, the permissive effect of Epo in erythropoiesis can be mimicked by prolactin in transfected erythroid cells that overexpress the prolactin receptor.8
We have recently described a family with a novel truncation mutation of the human EpoR associated with the disease familial erythrocytosis (FE).9 FE is a rare disease of red blood cell overproduction caused by EpoR mutations resulting in truncation of the cytoplasmic domain of the EpoR. This truncation deletes a negative regulatory domain of the EpoR and results in hyperactivity of the receptor.10 This mutation (EpoR FE) was characterized in the erythroid precursors of affected individuals and in murine myeloid 32D cells transfected with the mutant EpoR. EpoR FE was shown to result in hypersensitivity to Epo9 and to increased activation of the JAK/STAT pathway (Arcasoy M, Harris K, Forget B, manuscript submitted). These effects are thought to be due to deletion of the binding site on the EpoR for the negative regulatory tyrosine phosphatase SHP-1.10 It has been shown that the proximal cytoplasmic region of the EpoR containing the Jak binding domains are sufficient to support mitogenesis of transfected hematopoietic cells.6 8
During these studies, we noticed that 32D cells transfected with the native, full-length EpoR had several obvious morphologic differences from mock transfected cells and cells transfected with EpoR FE, despite identical growth rates and mitogenic signaling. We demonstrate here that the full-length human EpoR is capable of inducing granulocytic differentiation in this myeloid cell line. This differentiative signal requires the distal 70 amino acids of the receptor, as well as intact Jak2 kinase activity.
MATERIALS AND METHODS
Cell lines.
Murine myeloid IL-3–dependent 32D cells (clone 3, a gift of Dr Arati Khanna-Gupta, Yale University) have been previously described.11,12 Cells were grown in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO Life Technologies, Gaithersburg, MD) with 10% fetal calf serum (FCS) and 2 ng/mL murine IL-3 (StemCell Technologies, Vancouver, Canada) at 37°C in 5% CO2. Cloning and expression of the mutant human EpoR cDNA and generation of 32D cells stably transfected with pRc/CMV expression vector (Invitrogen, San Diego, CA) containing the wild-type (32D/EpoR WT cells) or mutant (32D/EpoR FE cells) EpoR cDNAs have been described.9 As a control, 32D cells were similarly transfected with empty pRc/CMV vector (32D/neo cells). The stably transfected pools of 32D/EpoR WT and 32D/EpoR FE cells 32D were maintained in 2 U/mL Epo (a gift of Ortho Biotech, Raritan, NJ) and 0.2 mg/mL G418 (Sigma, St Louis, MO) or 2 ng/mL IL-3 and 0.4 mg/mL G418 (32D/neo cells). For some experiments, the transfected cells were grown in 100% defined serum-free media (Aim-V; GIBCO BRL, Grand Island, NY) containing the above growth factors. The experiments reported here were all performed with pools of transfected clones. Identical results were obtained with several single cell clones of each transfectant obtained by limiting dilution (see Fig 4 and data not shown).
Cell growth assays.
32D EpoR transfectants (5,000 cells per well) were cultured in 96-well plates in IMDM/10% FCS containing 2 U/mL Epo. After 3 days, the viable cells were assayed in triplicate with MTT (dimethylthiazol-2-yl-2,5-diphenyltetrazolium) as previously described.13 Aliquots were removed from similarly treated wells and assayed for viable cells by the trypan blue exclusion technique. For thymidine incorporation assays, 20,000 cells were cultured in 1.5 mL of IMDM/10% FCS containing 2 U/mL Epo. 0.2 μCi of3H-thymidine was added and, after an additional 4 hours of culture, trichloroacetic acid precipitable radioactivity was measured.
AG 490 inhibition of cells.
32D transfectants (10,000 cells per mL) were cultured in 24-well plates in 2 mL of IMDM/10% FCS containing 2 ng/mL IL-3 (32D/neo) or 2 U/mL Epo (32D/EpoR WT and 32D/EpoR FE). The Jak2 inhibitor AG 490 (Calbiochem, San Diego, CA) was added at varying concentrations (0 to 10 μmol/L) at 0 hours and again at 24 and 48 hours. At 72 hours, myeloperoxidase (Mpo) cytochemical stains were performed on cytospin preparations.
Immunoblots.
The 32D parent line and the 32D EpoR transfectants were grown for 3 days in IMDM/10% FCS containing 10% WEHI conditioned media (as a source of IL-3) with varying concentrations of Epo. 1 × 105 cells were then electrophoresed on a 10% sodium dodecyl sulfate (SDS) gel, transferred to nitrocellulose, and immunoblotted with polyclonal rabbit antibody against mouse hemoglobin (Cappel, Durham, NC). Blots were developed with chemiluminescent reagent (Sigma). A total of 1 × 104 mouse reticulocytes were used as a positive control. Reticulocytes were prepared from phenylhydrasine-treated mice and represented about 30% of the total number of red blood cells in the specimen used.
Cytochemical staining of cells.
Mpo cytochemical assay was performed on cytospin preparations with p-phenylenediamine/catechol reagent (Sigma) as described by the manufacturer. For assay of percent Mpo positive cells, 10,000 cells were stained for Mpo as described above. At least 200 cells per slide were counted and only unequivocally stained cells were considered positive. Each point was performed in triplicate using cytospins from three independent wells. Wright-Giemsa staining was performed by standard methods.14
RESULTS
32D cells transfected with the full-length (32D/EpoR WT) and truncated (32D/EpoR FE) human EpoR constructs (Fig 1) were analyzed for growth in the presence of Epo. 32D cells were chosen for these experiments because they do not express endogenous murine EpoR, as judged by ligand binding studies using125I-Epo.9 At 2 U/mL of Epo, both cell lines grow at an equivalent rate as indicated by 3H-thymidine incorporation (Fig 2). Similar results were obtained using MTT cell proliferation assay or viable cell counts using trypan blue (data not shown). This differs from the Epo-stimulated growth at lower Epo concentrations (0.01 to 0.1 U/mL) at which the 32D/EpoR FE cells proliferate much faster than the 32D/EpoR WT cells.9 When analyzed with Hoescht staining, fewer than 1% apoptotic cells were noted during Epo-induced log phase growth in both 32D/EpoR WT and 32D/EpoR FE cells (data not shown). In the absence of Epo, both 32D/EpoR WT and 32D/EpoR FE cells undergo apoptosis, although 32D/EpoR FE cells initiate apoptosis at a slower rate than EpoR WT cells after growth factor withdrawal (M. Arcasoy, K. Harris, and B. Forget, manuscript submitted). Thus, at 2 U/mL Epo, both 32D/EpoR WT and 32D/EpoR FE cells have similar mitogenic and antiapoptotic signaling. Neither parental 32D nor 32D/neo cells will survive in Epo alone, presumably as a result of the absence of endogenous EpoR.9
Structures of normal EpoR and EpoR truncation mutant. The deletion found in the familial erythrocytosis mutation studied here is shown (EpoR FE). There is a frameshift at the coding sequence of amino acid 433 resulting in 17 novel amino acids (black box) followed by a premature stop codon. The 70 terminal amino acids of the normal EpoR (EpoR WT) are deleted in this mutation. The deletion includes 6 of the 9 cytoplasmic tyrosine residues (short vertical lines). The region required for mitogenesis (which includes the box 1 and box 2 domains) is shown in the open box.
Structures of normal EpoR and EpoR truncation mutant. The deletion found in the familial erythrocytosis mutation studied here is shown (EpoR FE). There is a frameshift at the coding sequence of amino acid 433 resulting in 17 novel amino acids (black box) followed by a premature stop codon. The 70 terminal amino acids of the normal EpoR (EpoR WT) are deleted in this mutation. The deletion includes 6 of the 9 cytoplasmic tyrosine residues (short vertical lines). The region required for mitogenesis (which includes the box 1 and box 2 domains) is shown in the open box.
32D cells transfected with either the full-length or truncated EpoR demonstrate equivalent growth at high Epo concentrations. Cells were grown in 2 U/mL Epo for 3 days and then assayed in triplicate for 3H-thymidine incorporation. 32D/neo cells do not proliferate in Epo because they lack Epo receptors. Standard error bars are indicated.
32D cells transfected with either the full-length or truncated EpoR demonstrate equivalent growth at high Epo concentrations. Cells were grown in 2 U/mL Epo for 3 days and then assayed in triplicate for 3H-thymidine incorporation. 32D/neo cells do not proliferate in Epo because they lack Epo receptors. Standard error bars are indicated.
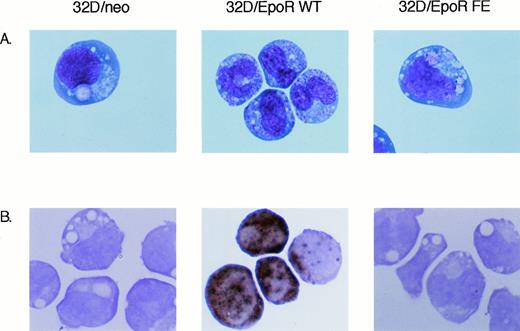
Despite similar proliferation under these conditions, the two EpoR-transfected cell lines have a markedly different morphology. Wright-Giemsa–stained preparations (Fig3A) demonstrate that the mock transfected 32D/neo cell line, as well as the 32D/EpoR FE cells, have a monocytoid appearance with large vacuoles and little evidence of granules. The nuclei have frequent nucleoli and an open chromatin pattern. This morphology for 32D cells is very similar to that described in previous reports.12,15 16
Morphology of 32D transfectants. Cells were grown for 3 days in either 2 ng/mL IL-3 (32D/neo), or 2 U/mL Epo (32D/EpoR WT and FE cells). Cytospin preparations were prepared for Wright-Giemsa stain or Mpo stain. Original magnification is 500X.
Morphology of 32D transfectants. Cells were grown for 3 days in either 2 ng/mL IL-3 (32D/neo), or 2 U/mL Epo (32D/EpoR WT and FE cells). Cytospin preparations were prepared for Wright-Giemsa stain or Mpo stain. Original magnification is 500X.
The 32D/EpoR WT cells, on the other hand, have a dimorphic morphology on passage in Epo. About 50% of the cells are similar to the 32D parent line and the 32D/EpoR FE cells. The other 50% have a granulocytic morphology with a decreased number of vacuoles and many granules. The nucleus has a more mature morphology with condensed chromatin and infrequent nucleoli. The morphology of these cells indicates differentiation to the stage of metamyelocytes and bands. This morphology for 32D/EpoR WT is consistent with previously described G-CSF–induced granulocytic maturation of 32D cells.12 In our hands, G-CSF induces similar granulocytic differentiation in 32D, 32D/neo, 32D/EpoR WT, and 32D/EpoR FE cells (data not shown). The increased granulation of the 32D/EpoR WT cells in the presence of Epo was also demonstrated by an increase in side scattering on flow cytometric analysis (data not shown).
To verify that the 32D/EpoR WT cells were undergoing granulocytic differentiation, the cell lines were stained for the granulocytic enzyme myeloperoxidase. As shown in Fig 3B, there are numerous Mpo positive cells among the 32D/EpoR WT cells. 32D/EpoR FE and 32D/neo routinely stains negative (Fig 3B), as do parental 32D cells (data not shown). Analogous results were obtained using northern blots, ie, strong expression of Mpo mRNA in 32D/EpoR WT cells and no detectable expression in 32D and 32D/EpoR FE cells (data not shown).
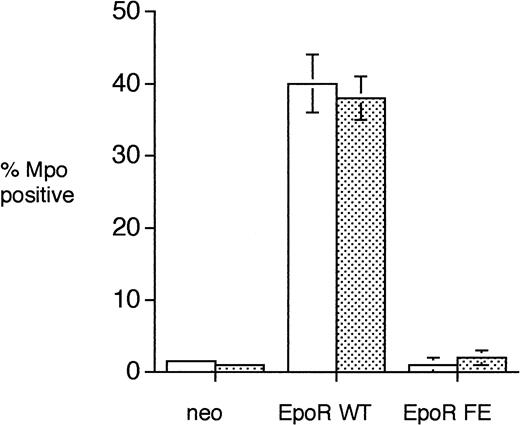
To ensure that the results described above were not due to a spurious integration event in a subpopulation of cells in the pools tested, single cell clones of the 32D transfectants were tested for Mpo expression. Figure 4 demonstrates that in multiple single cell clones approximately 40% 32D/EpoR WT cells express Mpo. Both 32D/neo and 32D/EpoR FE express Mpo in 1% to 3% of cells. Similar results were obtained when cells were grown in either 10% fetal calf serum (Fig 4, open bars) or in 100% defined serum-free media (Fig 4, dotted bars).
Expression of Mpo in single cell clones of 32D transfectants. Single cell clones of the 32D transfectants (obtained by limiting dilution) were grown in either 10% FCS (open bars) or 100% serum-free media (dotted bars) in the presence of 2 ng/mL IL-3 (32D/neo) or 2 U/mL Epo (32D/EpoR WT and 32D/EpoR FE) for 3 days and then cytochemical stains for Mpo were performed. A total of 200 cells were counted for each individual clone tested. The number of clones tested was 2 for neo, 5 for EpoR WT, and 5 for EpoR FE. The bars indicate standard error.
Expression of Mpo in single cell clones of 32D transfectants. Single cell clones of the 32D transfectants (obtained by limiting dilution) were grown in either 10% FCS (open bars) or 100% serum-free media (dotted bars) in the presence of 2 ng/mL IL-3 (32D/neo) or 2 U/mL Epo (32D/EpoR WT and 32D/EpoR FE) for 3 days and then cytochemical stains for Mpo were performed. A total of 200 cells were counted for each individual clone tested. The number of clones tested was 2 for neo, 5 for EpoR WT, and 5 for EpoR FE. The bars indicate standard error.
Neither the full-length nor truncated EpoR was capable of inducing erythroid differentiation of 32D cells. Figure 5 demonstrates a Western blot for murine hemoglobin of lysates from 32D, 32D/EpoR WT, and 32D/EpoR FE cells grown at various concentrations of Epo. There is no expression of globin proteins in any of the three cell lines.
32D transfectants do not express globin. Cells were grown for 3 days in 10% WEHI conditioned media (as a source of IL-3) containing 0 (lane 1), 0.1 (lane 2), 0.5 (lane 3), 1 (lane 4), or 5 U/mL Epo (lane 5). The lysate from 1 × 105 cells was electrophoresed in a 10% SDS gel and then immunoblotted with antimouse hemoglobin. Lane labeled “Ret” indicates mouse reticulocytes (1 × 104 cells) as a positive control.
32D transfectants do not express globin. Cells were grown for 3 days in 10% WEHI conditioned media (as a source of IL-3) containing 0 (lane 1), 0.1 (lane 2), 0.5 (lane 3), 1 (lane 4), or 5 U/mL Epo (lane 5). The lysate from 1 × 105 cells was electrophoresed in a 10% SDS gel and then immunoblotted with antimouse hemoglobin. Lane labeled “Ret” indicates mouse reticulocytes (1 × 104 cells) as a positive control.
Regulation of the Jak/Stat pathway after Epo stimulation differs in 32D/EpoR FE compared with 32D/EpoR WT cells (M. Arcasoy, K. Harris, and B. Forget, manuscript submitted), probably as a result of deletion of the C terminal negative regulatory domain of the EpoR.10 17 In 32D/EpoR FE cells, Jak2 and Stat5 remain phosphorylated and active for a longer time after Epo withdrawal than in 32D/EpoR WT cells. Because these two cell lines also differ in their ability to differentiate after Epo exposure, we wondered whether the granulocytic differentiation driven by the distal EpoR cytoplasmic domain is mediated by the level of activation of the Jak/Stat pathway.
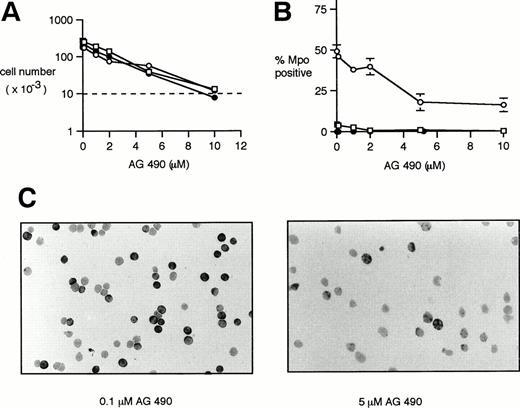
The specific Jak2 inhibitor tyrphostin AG 49018 was used to inhibit Jak2 signaling in the 32D/neo cell line and the cells transfected with the full-length and truncated EpoR. As expected, there was a dose-dependent effect of the inhibitor on cellular proliferation of all three cell lines (Fig 6A), although there was some cell proliferation at all concentrations of inhibitor used except the 10 μmol/L concentration (note the dotted line indicating the initial cell number). At 10 μmol/L AG 490, an increased number of cells demonstrated nuclear fragmentation (data not shown) suggesting that at this concentration of inhibitor, cellular proliferation was approximately balanced by cell death.
Effect of the Jak2 inhibitor AG 490 on the growth and differentiation of 32D transfectants. Cells were cultured in IMDM/10% FCS containing 2 ng/mL IL-3 (32D/neo cells) or 2 U/mL Epo (32D/EpoR WT and 32D/EpoR FE cells). The indicated concentration of the Jak2 inhibitor AG 490 was added at 0 hours and again at 24 and 48 hours. The cells were studied at 72 hours. AG 490 concentrations were 0, 0.1, 1, 2, 5, and 10 μmol/L. 32D/neo (□), 32D/EpoR WT (○), 32D/EpoR FE (•). (A) The viable cells were counted and expressed as a function of increasing AG 490. The initial cell number (10,000 cells) is indicated by the dotted line. Each point was done in triplicate. Standard error at each point was 10% or less of indicated value and has been omitted for clarity. (B) Cytospins were stained for Mpo. Mpo positive cells were counted and expressed as a function of increasing AG 490. Each point was done in triplicate with standard error bars shown. (C) Representative fields of Mpo-stained cytospins of 32D/EpoR WT cells at the indicated AG 490 concentrations.
Effect of the Jak2 inhibitor AG 490 on the growth and differentiation of 32D transfectants. Cells were cultured in IMDM/10% FCS containing 2 ng/mL IL-3 (32D/neo cells) or 2 U/mL Epo (32D/EpoR WT and 32D/EpoR FE cells). The indicated concentration of the Jak2 inhibitor AG 490 was added at 0 hours and again at 24 and 48 hours. The cells were studied at 72 hours. AG 490 concentrations were 0, 0.1, 1, 2, 5, and 10 μmol/L. 32D/neo (□), 32D/EpoR WT (○), 32D/EpoR FE (•). (A) The viable cells were counted and expressed as a function of increasing AG 490. The initial cell number (10,000 cells) is indicated by the dotted line. Each point was done in triplicate. Standard error at each point was 10% or less of indicated value and has been omitted for clarity. (B) Cytospins were stained for Mpo. Mpo positive cells were counted and expressed as a function of increasing AG 490. Each point was done in triplicate with standard error bars shown. (C) Representative fields of Mpo-stained cytospins of 32D/EpoR WT cells at the indicated AG 490 concentrations.
In addition to these effects on cell growth, there was a dose-dependent inhibition of granulocytic differentiation, as determined by Mpo cytochemical staining (Fig 6B). In this experiment, there was a dose-dependent decrease in Epo-induced Mpo positivity in the 32D/EpoR WT cells from 49% ± 4% to 16% ± 2% over the range of 0 to 10 μmol/L AG 490. The inhibitor had little effect on Mpo positivity of the 32/EpoR FE or 32D/neo cells (0% to 3% positivity at all concentrations of inhibitor). Figure 6C shows representative Mpo stains of 32D/EpoR WT cells at two concentrations of inhibitor. Similar results were obtained in two other experiments. Wright-Giemsa–stained preparations of this experiment confirmed the inhibition of granulocytic differentiation in 32D/EpoR WT, ie, there was a dose-dependent decrease in the number of morphologically recognizable granulocytic cells with increasing AG 490 (data not shown).
DISCUSSION
The results we present here demonstrate that myeloid differentiation in 32D cells does not require stimulation by G-CSF. Signal transduction via an hematopoietic growth factor receptor from another cell lineage is able to effectively substitute for G-CSF. When stimulated with Epo, transfected EpoR was able to induce myeloid differentiation of this cell line, as judged by Mpo expression, granule formation, and characteristic nuclear condensation and clumping (Figs 3 and 4). The Epo/EpoR interaction was sufficient to induce granulocytic differentiation of 32D cells, as equivalent levels of differentiation were obtained in either FCS or serum-free media (Fig 4). This indicates that other maturation factors are not required for this effect. The Epo/EpoR interaction was not able to induce erythroid differentiation in this cell line, as judged by morphology (Fig 3) or globin expression (Fig 5).
These results are similar to previous reports with other hematopoietic lineages. The prolactin receptor is able to substitute for the EpoR in mediating erythroid differentiation,8 and the G-CSFR cytoplasmic domain is able to substitute for the thrombopoietin receptor cytoplasmic domain in megakaryocyte differentiation.19 We have now extended these observations to the myeloid lineage. It seems clear from these data that growth factors are not “instructive” in hematopoietic differentiation.
Nor do our results support a simple permissive model of the role of growth factors in myeloid differentiation. Both the 32D/EpoR WT and 32D/EpoR FE cell lines grow at equivalent rates in the presence of 2 U/mL Epo (Fig 2). Very few apoptotic cells were noted in either cell line in the presence of Epo. Upon Epo withdrawal, 32D/EpoR FE cells actually initiate apoptosis at a slower rate than 32D/EpoR WT cells. Clearly, the full-length EpoR is providing signals that do more than support the cell long enough to allow expression of a predetermined endogenous program. This conclusion is consistent with similar work in 32D cells demonstrating that suppression of apoptosis by overexpression of Bcl-2 is not sufficient to support myeloid differentiation.15 Likewise, Pless et al20 have shown that erythroid differentiation of BaF3 cells requires dimerization of two full-length hematopoietic receptor subunits. In their study, combinations of receptor subunits that did not include two distal receptor domains were not able to induce differentiation, despite intact mitogenic signaling.
32D cells were chosen for our experiments because they represent the most commonly studied cell line for in vitro myeloid differentiation11,12,15,16 (and the references contained therein). 32D cells have been extensively used because they are growth factor-dependent, nontumorigenic, and have a normal karyotype.11 12 They may thus more accurately reflect the molecular events leading to granulocytic differentiation than other nongrowth factor-dependent tumor cell lines such as HL-60 or M1.
Our results indicate that the distal 70 amino acids of the EpoR are required for myeloid differentiation in this system. This is similar to results described for the G-CSFR. For instance Fukunaga et al21 demonstrated that the distal half of the G-CSFR is required for myeloid differentiation in the FDCP cell line. This differentiation domain, like the domain we have described here, is dispensable for mitogenesis. Interestingly, similar results have not been forthcoming with the EpoR. In TSA8 cells6 and primary erythroid precursor cells,8 the distal EpoR is not required for erythroid differentiation. It is possible that myeloid differentiation is intrinsically different than erythroid differentiation in the requirement for distal receptor signaling. A suggestion that this is the case comes from the human mutations that result in the truncation of hematopoietic growth factor receptors. In these conditions, the EpoR truncation results in erythrocytosis,22 while the G-CSFR truncation results in neutropenia.16 The role of the Jak/Stat pathway in hematopoietic differentiation is controversial. Some investigators have seen a direct correlation between increased Stat activation by the EpoR and erythroid differentiation,23 while others have seen an inverse relationship.20 Our results suggest that intact Jak signaling is required, but not sufficient for Epo-induced myeloid differentiation in 32D/EpoR WT cells. It is important to keep in mind that Jak2 may have other physiological substrates in addition to the Stat proteins.
The EpoR binds and activates Jak2, while the G-CSFR binds and activates numerous members of this family including Jak1, Jak2, and Tyk2.24 Jak1 activation, however, may be critical in G-CSFR signaling.24 If Jak signaling is indeed required for myeloid differentiation, our results would suggest that Jak2 can substitute for Jak1 in this process.
The findings presented here, in conjunction with those of others mentioned above, suggest that it is likely that under most situations an hematopoietic growth factor-induced signal is required for differentiation of hematopoietic precursor cells. This signal is distinct from mitogenic and antiapoptosis signaling and, at least in the case of myeloid differentiation, requires the distal domain of an hematopoietic receptor. The specificity of the signal delivered, however, is determined by the cell, presumably through some unknown molecular mechanism associated with commitment. The availability of numerous well characterized EpoR mutants, as well as the recent rapid development of other tools for the study of signal transduction pathways, should allow identification of the mechanism by which the distal 70 amino acids of the EpoR are able to induce myeloid differentiation in this system.
ACKNOWLEDGMENT
We thank Qing Shen for technical help, David Haile for the reticulocyte lysate, Yair Gazitt for help with flow cytometry, Katri Selander for performing the Hoescht stain analysis of apoptosis, and Ortho Biotech for a gift of human Epo.
Supported by a Veterans Administration Merit Review grant, San Antonio Cancer Institute grant, and a University of Texas Health Science Center at San Antonio Howard Hughes Medical Institute Institutional Resources grant (to K.W.H.), and Grant No. DK 44058 from the National Institutes of Health, Bethesda, MD (to B.G.F.).
Address reprint requests to Kevin W. Harris, MD, PhD, Department of Medicine, Division of Hematology, University of Texas Health Science Center, 7703 Floyd Curl Dr, San Antonio, TX 78284-7880.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal