Abstract
Deficiency of the granulocyte-macrophage colony-stimulating factor (GM-CSF)/interleukin-3 (IL-3)/IL-5 receptors common β chain (βc) is a cause of fatal respiratory failure. βc deficiency manifests as pulmonary alveolar proteinosis (PAP). PAP has heterogenous etiologies that may be genetic or aquired. Some cases of PAP have been reported to be associated with hematologic malignancies such as acute myeloid leukemia (AML). In mice, the PAP phenotype was generated by targeted deletion of the gene for βc and can be treated by transplantation of wild-type bone marrow into βc −/− mice. Thus, our findings in βc −/− mice provide evidence for a causal relationship between the lung disease and the hematopoietic system. We describe here expression defects of βc or βc plus GM-CSF receptor chain (GM-CSFR ) in 3 pediatric patients with AML and PAP symptoms. All of the patients’ leukemic cells failed to express normal levels of βc. The leukemic cells of patients no. 2 and 3 additionally lacked the expression of GM-CSFR , as shown by flow cytometry. Strikingly reduced or absent function of βc was demonstrated in clonogenic progenitor assays with absent colony-forming unit (CFU) growth after GM-CSF or IL-3 stimulation. The response to growth factors acting via a growth factor receptor distinct from the GM-CSF/IL-3/IL-5 system (recombinant human granulocyte colony-stimulating factor [rhG-CSF]) was normal. After antileukemic treatment, the pulmonary symptoms resolved and βc or βc plus GM-CSFR expression was normal. Our findings provide evidence that a defect in the expression of βc or βc plus GM-CSFR on AML blasts can be associated with respiratory failure in patients with AML.
© 1998 by The American Society of Hematology.
PATIENTS WITH MYELOID leukemias can present with severe pulmonary symptoms. Those pulmonary affections may be interpreted as interstitial pneumonia, infiltration of the lung with leukemic cells, or as a disease of an unknown cause. Single cases have been reported to be associated with pulmonary alveolar proteinosis (PAP).1-3 PAP is a rare disease histopathologically diagnosed by an accumulation of proteinaceous, periodic acid Schiff (PAS) stain-positive material in the alveolar space. Additionally, the diagnostic value of broncheo alveolar lavage fluid (BAL) has been shown for adults4,5 and pediatric patients.6 The BAL fluid contains large quantities of serum proteins and glyceroproteins.7 Furthermore, elevated amounts of surfactant proteins (SP) have been found in adults and some pediatric patients.8 9
In children, the disease has been described less frequently than in adults and imposes as a heterogeneous disorder that can be associated with different diseases. Whereas most pediatric cases remain idiopathic, a well-defined group of some congenital PAP patients has been found to be associated with the hereditary SP-B deficiency.10,11 Other cases have been reported in patients with lysinuric protein intolerance,12immunodeficiencies,13 or hematological disorders such as acute myeloid leukemia (AML) or chronic myeloid leukemia (CML).1-3 However, with the exception of the SP-B–deficient patients, the etiology and pathophysiological mechanisms in pediatric PAP are unknown. Recently, we described an expression defect of common β chain (βc) in pediatric patients with PAP and in 1 patient with severe lung disease suspected to be PAP.14 15
In mice, PAP has been shown to be associated with a deletion of the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene16,17 or with a deficiency of the GM-CSF/interleukin-3 (IL-3)/IL-5 receptor βc chain.18 The lung patholgy in both types of mutant mice shows significant similarities to human PAP.16-18
The receptors for human GM-CSF, IL-3, and IL-5 are composed of two subunits.19,20 The respective private α chain mediates the specific binding, whereas βc is required to confer high-affinity binding21 and is essential for signal transduction.22
We now describe our findings on expression deficiencies and functional absence of the βc chain and/or the GM-CSFR α chain in bone marrow (BM), peripheral blood (PB), and/or broncheo alveolar lavage fluid myeloblasts of 3 pediatric AML patients associated with signs of compromised respiratory function. Two of them have been diagnosed for PAP. One patient has had noninfectious severe respiratory failure of unknown causes suspected to be PAP.
MATERIALS AND METHODS
Flow cytometry analysis.
Surface receptor expression on cells from patients, control AML patients, and healthy controls was assessed in whole blood samples. For indirect flow cytometry, the following unconjugated monoclonal antibodies (MoAbs) were used: the βc-specific MoAb (CDw131) S-16, the GM-CSF receptor α chain MoAbs (CD116) S-16 (and S-50; not shown), and the IL-3 receptor α chain MoAb (CDw123) S-12 (all Santa Cruz Biotechnology, Santa Cruz, CA). The receptor antibodies recognize extracellular domains of βc and GM-CSFR α, respectively. Receptor antibodies were developed with a fluorochrome-conjugated secondary goat-antimouse antibody (Coulter, Krefeld, Germany). For cell type distribution and diagnosis of AML (data are included in the case reports), the following fluorochrome-conjugated antibodies were used: CD3 (Leu 4), CD4 (Leu 7), CD14 (Leu M3), CD15 (Leu M1), CD20 (Leu 16), CD33 (Leu M9), CD34 (HPCA2), CD45 (HLe-1), HLA-DR (L243), CD56 and (Leu 19) (Becton Dickinson, Heidelberg, Germany); CD7 (IOT7), CD11b (IOM1b), CD14 (IOM2), and CD117 (17F11) (Immunotech, Krefeld, Germany); and fluorochrome-conjugated isotype-matched control antibodies (Dianova, Hamburg, Germany). Cell staining and analysis by FACScan (Becton Dickinson) was performed according to standing laboratory procedure as we have previously described.15 Briefly, for indirect staining, cells were incubated with the first antibody for 15 to 30 minutes at room temperature (RT) according to manufacturer’s guidelines, washed twice with phosphate-buffered saline (PBS), and were stained for 30 minutes with the fluorochrome-conjugated secondary antibody. For direct staining, fluorochrome-conjugated antibodies were incubated for 10 minutes at RT. After washing with PBS, both indirectly and directly stained samples were incubated with the FACS lysing solution according to the manufacturer’s procedure (Becton Dickinson). In BM and PB, the receptor expression was analyzed in the myeloid gate (CD34+ and CD33+). Fluorescence intensity is expressed as the percentage of positive cells.
Progenitor clonogenic assays.
Patients’, control AML patients’, and controls’ BM mononuclear cells (BMMNCs) or PB mononuclear cells (PBMNCs) were separated by Ficoll-Hypaque density gradient, washed twice with PBS, and cultured at a concentration of 1.5 × 105 cells/mL culture medium in 24-well culture clusters (Costar, Cambridge, MA). The culture medium consisted of 30% fetal calf serum (FCS; Sigma, Deisenhofen, Germany), 1% 2-mercaptoethanol (Sigma), 1% penicilline-streptomycin solution (GIBCO, Eggenstein, Germany), 1% L-glutamin, 10% bovine serum albumine (Behring, Marburg, Germany) with sodium bicarbonate, 0.5 U recombinant human erythropoietin (rhEPO), 40% methylcellulose, and 500 to 5,000 U of rhIL-3 (Behring), rhGM-CSF (Leucomax; Sandoz, Nürnberg, Germany), and granulocyte colony-stimulating factor (G-CSF; Neupogen; Amgen/Roche, München, Germany). After 14 days of culture at 37°C, 5% CO2, 100% humidified atmosphere, the colony-forming units (>50 cells/colony) of granulocytes and macrophages were determined by inverse microscopy.
Cytopathological analysis.
BAL samples were collected from patients no. 1 and 2. They were examined by light microscopy after cytocentrifugation and staining by May-Grünwald-Giemsa, PAS, and Ziehl.
Electron microscopy analysis.
Electron microscopy was performed in glutaraldhyde-fixed BAL samples according to standard procedures.
CASE REPORTS
Patient no. 1, a boy, was admitted to the hospital at the age of 1 month because of severe respiratory distress and generalized swelling of lymph nodes (Table 1). Despite pulmonary symptoms, the first x-ray showed no severe pulmonary changes. His high leukocyte count was suspicious for a hematological malignancy. A BM harvest was performed, leading to the diagnosis of AML French-American-British (FAB) classification M4. Immunophenotyping of the PB cells showed 91% myeloblasts expressing CD33, CD34, CD11b, CD15, and they were negative for CD14 and HLA-DR. Because of the high leukocyte count (129,000 cells/μL), the patient underwent an exchange transfusion before the start of chemotherapy. The antileukemic treatment was combined with a high-dose antibiotic and antimycotic therapy. During the first course of chemotherapy, the respiratory symptoms got worse and the patient required exogenous oxygenation. The x-ray showed perihilar infiltrates and increased interstitial markings. After a short-lasting improvement, his respiratory condition got worse, necessitating intubation and mechanical ventilation. Under a combined therapy with antibiotics, antimycotics, exchange transfusion, and lavages through the tubes, with 5 mL NaCl in each lung three times a day, the symptoms got better. Within 3 weeks of therapy, he became oxygen-independent. He was scheduled for myeloablative therapy and unrelated cord blood transplantation. Before transplantation, the patient received high-dose chemotherapy, which was well tolerated. At day +42, autologous reconstitution was diagnosed by analysis of chimerism. Three months after the graft failure, he relapsed under maintenance therapy initially with 20% M4 myeloblasts in the BM aspirate. The blast count increased up to 70% within 10 days. He again developed tachypnoea. Two weeks after relapse, the patient underwent a myeloablative therapy and an allogeneic BM transplantation (BMT). After BMT, he suffered from recurrent severe infections. At day +75, he became septicemic with progress to death at day +90. Within the septicemia, he developed renal, liver, and respiratory failure. At day +87, the breathing became periodic and required exogenous oxygenation. The septicemia condition deteriorated with progression to death.
Clinical Presentation of the Patients and Investigations
| Patient No. . | Age at Diagnosis . | AML . | PAP Diagnosis . | Therapy . | Outcome . |
|---|---|---|---|---|---|
| 1 | 2 mo | M4 | BAL, x-Ray | ChTx, ventil, ther. BAL, SCT | Relapse, no PAP symptoms |
| ChTx, BMT | No PAP symptoms, died of septicemia | ||||
| 2 | 132 mo | Unclass. | BAL, x-Ray | ChTx, ventil, ther. BAL | Planned for SCT No PAP symptoms, died of septicemia |
| 3 | 9 mo | M5 | Suspected, x-Ray | ChTx, BMT | 5 yr post-BMT remission, no PAP symptoms |
| Patient No. . | Age at Diagnosis . | AML . | PAP Diagnosis . | Therapy . | Outcome . |
|---|---|---|---|---|---|
| 1 | 2 mo | M4 | BAL, x-Ray | ChTx, ventil, ther. BAL, SCT | Relapse, no PAP symptoms |
| ChTx, BMT | No PAP symptoms, died of septicemia | ||||
| 2 | 132 mo | Unclass. | BAL, x-Ray | ChTx, ventil, ther. BAL | Planned for SCT No PAP symptoms, died of septicemia |
| 3 | 9 mo | M5 | Suspected, x-Ray | ChTx, BMT | 5 yr post-BMT remission, no PAP symptoms |
Abbreviations: BAL, broncheo alveolar lavage fluid; BMT, bone marrow transplantation; Chemotx, chemotherapy; PAP, alveolar proteinosis; SCT, stem cell transplantation; ther., therapeutic; ventil., ventilation; Unclass, unclassified.
Patient no. 2, a girl, was diagnosed for AML (FAB, unclassified) at the age of 132 months with 95% myelocytic blasts expressing CD34, CD33, CD11b, CD56, CD13 (21%), and c-kit (23%); they were negative for CD7, HLA-DR. The absolute leukocyte count at diagnosis was 143,000/μL.
At the time of diagnosis, she presented with dyspnea and a nonproductive cough. The x-ray showed a bilateral pleural effusion but no pulmonary changes. Because of the instable pulmonary condition, she was treated with a modified BFM AML 93 protocol with split chemotherapy course including longer intervals. Within a few days, the pulmonary symptoms got worse and she presented with increasing shortness of breath. This time the chest x-ray showed marked infiltrates of the right middle and lower lobe. Despite high-dose antibiotic and antimycotic therapy, her general condition deteriorated with increasing dyspnea and oxygen dependency. The x-ray now showed bilateral infiltrates. A diagnostic BAL was performed and she required mechanical ventilation.
Alternating therapeutic lavage of each lung gave a short-lasting improvement in oxygenation, but no long-lasting demonstrable changes in pulmonary mechanics. Because her blasts were increasing up to 65% in the BM, the antileukemic treatment was intensified. Under this regimen, the pulmonary symptoms got better. However, her blast count persisted at approximately 25%. She developed septicemia and a capillary leakage syndrom with progression to death 4 months after diagnosis. At that time, the pulmonary symptoms were minor and she had no clinical evidence for PAP. A BAL was not performed and the parents refused to have an autopsy performed.
Patient no. 3, a girl, has been diagnosed for AML M5 at the age of 9 months. At time of diagnosis, she presented with severe pulmonary symptoms. She presented with myeloid skin infiltrates and 50% myeloid blasts in her BM aspirate expressing CD33, CD34, CD56, CD11b, CD11c, CD4, and CD15; they were negative for HLA-DR. Her leukocyte count was 3,900/μL. Right after the beginning of the first course of chemotherapy, her pulmonary symptoms got worse and she developed dyspnea and orthopnea. This time the x-ray showed bilateral perihilar infiltrates and increased interstitial markings. Under a combined therapy of antibiotics and chemotherapy, her pulmonary situation slowly improved. Because she showed a persistence of her myeloblasts (5%) after 6 months of therapy, she underwent a myeloablative chemotherapy and unrelated BMT. At present, she is alive 5 years after BMT. She is in good clinical condition and had no clinical or radiological PAP symptoms ever since.
We examined 17 control patients with myeloid leukemias, 3 pediatric patients (1 M0, 2 M2, and 1 refractory anemia with blast excess [RAEB]), and 14 adults (3 M1, 2 M2, 6 M4, and 3 M5). Furthermore, we examined 3 age-matched healthy controls. None of them has had clinical or radiological PAP or PAP-like symptoms (see Table 3B).
RESULTS
Diagnosis of alveolar proteinosis in pediatric AML patients.
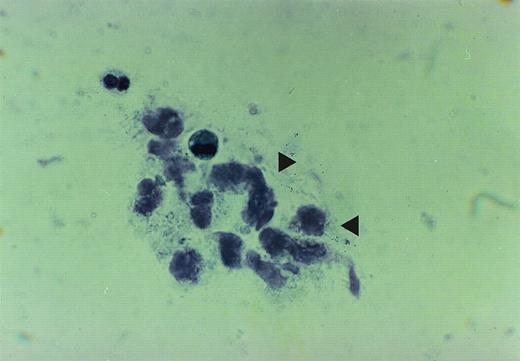
The cytopathologic analysis of the lavage of patients no. 1 and 2 showed a PAS-positive amorphous, granular material. The material contained some enlarged alveolar macrophages and showed vacuolization of their cytoplasma. The amount of extracellular proteinaceous material was strikingly augmented. A count of BAL cells was not performed, because it has been shown to be of no prognostic value23(Fig 1A).
The figure is taken from cytospin material of broncheo alveolar lavage fluid from patient no. 2. The accumulation of the proteinaceous material is depicted by arrows.
The figure is taken from cytospin material of broncheo alveolar lavage fluid from patient no. 2. The accumulation of the proteinaceous material is depicted by arrows.
Electron microscopy was performed in BAL samples of patients no. 1 and 2 demonstrating enlarged alveolar macrophages containing lamellar bodies and multilamellar bodies with electron dense central regions. Furthermore, large amounts of surfactant were found in the surrounding of the macrophages. These findings indicate either an maldigestion or an overproduction of the surfactant material and have been described in PAP4,6 (data not shown).
Quantification of surfactant proteins.
The testing for surfactant apoproteins A (SP-A) and SP-B has recently been established as being of diagnostic value. Quantification of surfactant proteins was performed as described previously.24,25 SP-B was readily detected in both patients; thus, the hereditary SP-B deficiency as a possible underlying cause of PAP in children was excluded.11 However, both patients had elevated levels of total protein and SP-A and detectable levels of SP-B, proving that the proteinaceous PAS-positive material seen after cytocentrifugation were proteins and surfactant (Table 2).
Quantification of Surfactant Proteins B and A in BAL
| . | BAL . |
|---|---|
| Patient no. 1 | |
| SP-B (ng/mL) | 003.3 μg/mL |
| SP-A (μg/mL) | 008.0 μg/mL |
| Total protein | 1230 μg/mL |
| Phospholipids | 0200 μg/mL |
| Patient no. 2 | |
| SP-B (ng/mL) | 002.4 μg/mL |
| SP-A (μg/mL) | 006.7 μg/mL |
| Total protein | 982.0 μg/mL |
| Phospholipids | 089.0 μg/mL |
| Normal range of proteins in BAL fluid | |
| SP-B (ng/mL) | 00.7 ± 0.1 μg/mL |
| SP-A (μg/mL) | 01.5 ± 0.2 μg/mL |
| Total protein | 73.0 ± 6.0 μg/mL |
| Phospholipids | 30.0 ± 14.0 μg/mL |
| . | BAL . |
|---|---|
| Patient no. 1 | |
| SP-B (ng/mL) | 003.3 μg/mL |
| SP-A (μg/mL) | 008.0 μg/mL |
| Total protein | 1230 μg/mL |
| Phospholipids | 0200 μg/mL |
| Patient no. 2 | |
| SP-B (ng/mL) | 002.4 μg/mL |
| SP-A (μg/mL) | 006.7 μg/mL |
| Total protein | 982.0 μg/mL |
| Phospholipids | 089.0 μg/mL |
| Normal range of proteins in BAL fluid | |
| SP-B (ng/mL) | 00.7 ± 0.1 μg/mL |
| SP-A (μg/mL) | 01.5 ± 0.2 μg/mL |
| Total protein | 73.0 ± 6.0 μg/mL |
| Phospholipids | 30.0 ± 14.0 μg/mL |
Quantitative analysis of patients’ surfactant protein content in broncheo alveolar lavage fluid (BAL) and tracheal aspirate (TA). Patients produced high amounts of surfactant protein B and A. Normal range data are from Krämer et al.35
Abbreviation: ND, not determined.
Before the first course of chemotherapy, the expression of βc and the private α chain of the GM-CSF receptor and the private α chain of the human IL-3 receptor was analyzed by flow cytometry. The data are obtained by analysis of the respective receptor expression in the gate representing the myeloid cells. Analysis was performed in PB and pooled BAL fluid from patient no. 1 and in BM and pooled BAL fluid cells from patients no. 2 and 3. All patients’ cells showed a strikingly reduced expression of βc on their myeloid blasts in all samples (<1%). GM-CSFR α was readily detected in cells from patient no. 1 (90%), but was not detectable in cells from patients no. 2 (<1%) and 3 (<1%), whereas IL-3R-α was detectable in each patients’ sample (25% in patient no. 1, 39% in patient no. 2, and 27% in patient no. 3). All data were obtained in comparison to healthy controls and to 16 AML controls and 1 RAEB control without PAP symptoms (Table 3B).
Flow Cytometry Analyses
| (A) Flow Cytometry Analysis in Children With PAP Before Therapy . | ||||||
|---|---|---|---|---|---|---|
| Patient No. . | Age at Diagnosis . | AML . | βc . | GM-CSFR− α . | IL-3R− α . | Material . |
| 1 | 0.5 mo | M4 | 5% | 90% | 25% | PB |
| <1% | 18% | 10% | BAL | |||
| 2 | 11 yr | M0 | <1% | <1% | 39% | BM |
| <1% | <1% | 8% | BAL | |||
| 3 | 9 mo | M5 | <1% | <1% | 27% | BM(th) |
| ND | ND | ND | BAL |
| (A) Flow Cytometry Analysis in Children With PAP Before Therapy . | ||||||
|---|---|---|---|---|---|---|
| Patient No. . | Age at Diagnosis . | AML . | βc . | GM-CSFR− α . | IL-3R− α . | Material . |
| 1 | 0.5 mo | M4 | 5% | 90% | 25% | PB |
| <1% | 18% | 10% | BAL | |||
| 2 | 11 yr | M0 | <1% | <1% | 39% | BM |
| <1% | <1% | 8% | BAL | |||
| 3 | 9 mo | M5 | <1% | <1% | 27% | BM(th) |
| ND | ND | ND | BAL |
| (B) Flow Cytometry Analysis in Controls . | |||||
|---|---|---|---|---|---|
| Healthy Controls . | Age . | βc . | GM-CSFR α . | IL-3R− α . | Material . |
| 1 | 1 mo | 85% | 88% | 34% | PB |
| 2 | 4 yr | 86% | 90% | 9% | BM(th) |
| 3 | 12 yr | 83% | 88% | 22% | BM |
| (B) Flow Cytometry Analysis in Controls . | |||||
|---|---|---|---|---|---|
| Healthy Controls . | Age . | βc . | GM-CSFR α . | IL-3R− α . | Material . |
| 1 | 1 mo | 85% | 88% | 34% | PB |
| 2 | 4 yr | 86% | 90% | 9% | BM(th) |
| 3 | 12 yr | 83% | 88% | 22% | BM |
| Control AML Patients . | Age at Diagnosis . | AML . | βc . | GM-CSFR α . | IL-3R− α . | Material . |
|---|---|---|---|---|---|---|
| 1 | 1 mo | M0 | 85% | 75% | 11% | BM |
| 2 | 42 yr | M1 | 86% | 67% | 20% | BM |
| 3 | 40 yr | M1 | 75% | 85% | 54% | BM |
| 4 | 27 yr | M1 | 95% | 92% | 19% | BM |
| 5 | 66 yr | M2 | 61% | 77% | 12% | BM |
| 6 | 11 yr | M2 | 61% | 80% | 35% | BM |
| 7 | 53 yr | M2 | 92% | 78% | 18% | BM |
| 8 | 54 yr | M4 | 67% | 65% | 23% | BM |
| 9 | 63 yr | M4 | 53% | 54% | 55% | BM |
| 10 | 69 yr | M4 | 62% | 87% | 42% | BM |
| 11 | 54 yr | M4 | 65% | 64% | 16% | BM |
| 12 | 28 yr | M4 | 94% | 92% | 65% | BM |
| 13 | 63 yr | M4 | 96% | 67% | 44% | BM |
| 14 | 25 yr | M5 | 95% | 95% | 10% | BM |
| 15 | 35 yr | M5 | 48% | 95% | 32% | BM |
| 16 | 52 yr | M5 | 60% | 70% | 35% | BM |
| 17 | 4 yr | RAEB | 92% | 63% | 12% | BM |
| Control AML Patients . | Age at Diagnosis . | AML . | βc . | GM-CSFR α . | IL-3R− α . | Material . |
|---|---|---|---|---|---|---|
| 1 | 1 mo | M0 | 85% | 75% | 11% | BM |
| 2 | 42 yr | M1 | 86% | 67% | 20% | BM |
| 3 | 40 yr | M1 | 75% | 85% | 54% | BM |
| 4 | 27 yr | M1 | 95% | 92% | 19% | BM |
| 5 | 66 yr | M2 | 61% | 77% | 12% | BM |
| 6 | 11 yr | M2 | 61% | 80% | 35% | BM |
| 7 | 53 yr | M2 | 92% | 78% | 18% | BM |
| 8 | 54 yr | M4 | 67% | 65% | 23% | BM |
| 9 | 63 yr | M4 | 53% | 54% | 55% | BM |
| 10 | 69 yr | M4 | 62% | 87% | 42% | BM |
| 11 | 54 yr | M4 | 65% | 64% | 16% | BM |
| 12 | 28 yr | M4 | 94% | 92% | 65% | BM |
| 13 | 63 yr | M4 | 96% | 67% | 44% | BM |
| 14 | 25 yr | M5 | 95% | 95% | 10% | BM |
| 15 | 35 yr | M5 | 48% | 95% | 32% | BM |
| 16 | 52 yr | M5 | 60% | 70% | 35% | BM |
| 17 | 4 yr | RAEB | 92% | 63% | 12% | BM |
(A) Flow cytometry analysis of the GM-CSF/IL-3/IL-5 receptors’ common β chain (βc), GM-CSF receptor α chain (GM-CSFR α), and IL-3 receptor α chain (IL-3Rα) in peripheral blood (PB), bone marrow aspirate (BM), or broncheo alveolar lavage fluid (BAL) from 2 patients with AML and PAP and 1 child with AML and severe lung disease. Data are expressed as the percentage of cells in the myeloid gate. The AML classification was performed according to FAB.
(B) Flow cytometry analysis of the GM-CSF/IL-3/IL-5 receptors common β chain (βc), GM-CSF receptor α chain (GM-CSFR α), and IL-3 receptor α chain (IL-3Rα) in peripheral blood (PB), bone marrow aspirate (BM), or broncheo alveolar lavage fluid (BAL) from 16 patients with AML without PAP, 1 refractory anemia with blast excess (RAEB), 3 age-matched controls, and 1 control BAL from a child after peanut aspiration. Data are expressed as the percentage of cells in the myeloid gate. The AML classification was performed according to FAB.
Abbreviations: ND, not determined; th, thawed.
Thus, pulmonary symptoms in the AML patients were found to be associated with βc or βc plus GM-CSFR α deficiency on patients’ myeloid cells (Table 3A). Remarkably, in BAL fluid from patients no. 1 and 2, the alveolar macrophages did not express mature markers (CD14+ and CD15+) but expressed early myeloid markers (CD34+, CD33+), indicating that the alveolar macrophages in these patients might be descendating from the leukemic clone.
Expression of the GM-CSF/IL-3/IL-5 receptor common β chain, the GM-CSF receptor α chain (GM-CSFR α), and the IL-3R α chain (IL-3R α) after therapy.
After elimination of the leukemic clone by high-dose chemotherapy (ChTx), βc was readily detected on cells from patient no. 1. At the time of the relapse, the leukemic cells failed to express βc, whereas βc was detectable on the remaining mature myeloid cells. After myeloablative therapy (ChTx) and total body irradiation (TBI), βc was found to be expressed on the myeloid cells.
Patient no. 2 did not get into remission after the first split course of ChTx with 60% remaining blasts. After intensified ChTx, the cell count of the leukemic blasts remained stable at around 20%, and roughly 80% nonleukemic βc+/GM-CSFR α+cells were detectable in the myeloid population.
After myeloablative ChTx, TBI, and allogeneic BMT, cells from patient no. 3 expressed βc and GM-CSFR α (Table4).
Flow Cytometry Analysis in Children With PAP After Therapy
| Patient No. . | AML Therapy . | βc . | GM-CSFR α . | IL-3R α . | Material . |
|---|---|---|---|---|---|
| 1 | ChTx + CBT | 95% | 93% | 25% | BM |
| 98% | 92% | ND | BAL | ||
| 13-150 | ChTx; TBI + BMT | 99% | 87% | 10% | BM |
| 2 | Chtx | 82% | 80% | 13% | BM |
| 3 | ChTx; TBI + BMT | 92% | 92% | 25% | BM (th) |
| Patient No. . | AML Therapy . | βc . | GM-CSFR α . | IL-3R α . | Material . |
|---|---|---|---|---|---|
| 1 | ChTx + CBT | 95% | 93% | 25% | BM |
| 98% | 92% | ND | BAL | ||
| 13-150 | ChTx; TBI + BMT | 99% | 87% | 10% | BM |
| 2 | Chtx | 82% | 80% | 13% | BM |
| 3 | ChTx; TBI + BMT | 92% | 92% | 25% | BM (th) |
Flow cytometry analysis of the GM-CSF/IL-3/IL-5 receptors common β chain (βc), GM-CSF receptor α chain (GM-CSFR α), and IL-3 receptor α chain (IL-3Rα) in bone marrow aspirate (BM) or broncheo alveolar lavage fluid (BAL) from 3 children with AML and PAP symptoms. Data are expressed as the percentage of cells in the myeloid gate.
Abbreviations: BMT, allogeneic bone marrow transplantation; CBT, allogeneic cord blood transplantation; Chtx, chemotherapy; TBI, total body irradiation; th, thawed.
Patient no. 1 relapsed with his AML 3 months after allogeneic cord blood transplantation, graft failure, and autologous reconstitution (see case report).
Clonogenic progenitor growth in the presence of βc-dependent and βc-independent stimuli.
Hematopoietic progenitor cloning assays were performed to examine the response of the βc and βc plus GM-CSFR α chain-deficient MNCs to growth factors dependent on the GM-CSF/IL-3/IL-5 receptor system, such as GM-CSF and IL-3 to G-CSF as a growth factor that acts independently of the GM-CSF/IL-3/IL-5 receptors system. PB or BM cells from the βc-deficient AML patients did not respond to GM-CSF and IL-3 when compared with controls. However, normal multilineage colonies were formed in response to a stimulus that acts independently of the βc receptor, such as G-CSF. The data obtained by progenitor cloning assays prove the functional absence of βc. There was no significant difference between the patient lacking βc only (βc−) and the patients lacking both βc and GM-CSFR α (βc− plus GM-CSFR α−; Table 5).
Colony-Forming Unit Assays
| Stimulation . | EPO/GM-CSF . | EPO/IL-3 . | EPO/G-CSF . |
|---|---|---|---|
| PB | |||
| Patient no. 1 | 0 ± 0 | 0 ± 0 | 143 ± 16 |
| Controls | 55 ± 20 | 45 ± 15 | 84 ± 46 |
| BM | |||
| Patient no. 2 | 4 ± 0 | 5 ± 0 | 372 ± 15 |
| Controls | 325 ± 55 | 456 ± 79 | 439 ± 66 |
| Thawed BM | |||
| Patient no. 3 | 0 ± 0 | 0 ± 0 | 72 ± 15 |
| Controls | 98 ± 65 | 135 ± 76 | 182 ± 78 |
| Stimulation . | EPO/GM-CSF . | EPO/IL-3 . | EPO/G-CSF . |
|---|---|---|---|
| PB | |||
| Patient no. 1 | 0 ± 0 | 0 ± 0 | 143 ± 16 |
| Controls | 55 ± 20 | 45 ± 15 | 84 ± 46 |
| BM | |||
| Patient no. 2 | 4 ± 0 | 5 ± 0 | 372 ± 15 |
| Controls | 325 ± 55 | 456 ± 79 | 439 ± 66 |
| Thawed BM | |||
| Patient no. 3 | 0 ± 0 | 0 ± 0 | 72 ± 15 |
| Controls | 98 ± 65 | 135 ± 76 | 182 ± 78 |
Granulocyte-macrophage colony-forming unit assay from cells of βc-deficient AML patients with PAP and from healthy controls. Each value represents the mean of triplicate determinations.
DISCUSSION
Respiratory failure is a severe complication in patients with myeloid leukemias. The pulmonary affections can be interpreted as pneumonia, as infiltration of the lung with myeloid cells, or as a rare lung disease such as alveolar proteinosis (PAP). The diagnosis of PAP is based on typical histology or cytopathology showing the accumulation of proteinaceous material. Several theories have been advanced to explain the association between myeloid leukemias and PAP. Some investigators focused on PAP as a sequel of chemotherapy-induced lung injury,2,26 altered cell immunity,3 or impaired function of the alveolar macrophages.27 28 However, the underlying molecular mechanism leading to this respiratory failure remains to be determined.
In this report, we describe our investigations in 2 pediatric AML patients with accompanying alveolar proteinosis and 1 AML patient with severe respiratory failure suspected to be PAP. In mice, a disease similar to human PAP has been shown to be associated with a deletion of the GM-CSF gene16,17 or with a deficiency of βc.18 Thus, we analyzed the βc expression and function in our AML patients. All patients’ myeloid blasts in BAL fluid, BM, or PB were analyzed for the expression of βc and GM-CSFR α, the second subunit of the GM-CSF receptor. In all of them we found a striking reduction of βc (βc−). Blasts from patients no. 2 and 3 additionally lacked the expression of GM-CSFR α (GM-CSFR α−) but not the IL-3 receptor α chain (IL-3Rα). Remarkably, in the flow cytometry analysis of BAL fluid, βc− myeloblasts were detected, but no mature alveolar macrophages. This finding indicates that the leukemic clone may have displaced the mature alveolar macrophages.
All data were obtained in comparison to healthy controls and to AML control patients without PAP.
For characterization of the functional consequences of the absence of βc or βc plus GM-CSFR α, we examined clonogenic growth of the patients’ hematopoietic progenitor cells in response to growth factors. We used rhGM-CSF and rhIL-3 functioning through binding to members of the GM-CSF/IL-3/IL-5 receptors family and the independently effecting rhG-CSF. GM-CSF and IL-3 had almost no effect on clonogenic growth of the patients’ progenitor cells, whereas growth in response to G-CSF was normal. The clonogenic growth in patients with a combined deficiency of βc and GM-CSFR α was not different from the patient lacking βc only, indicating the specific α subunit of the receptor alone is not sufficient for the receptor function. Furthermore, the combined deficiency of both βc and GM-CSFR α expression in AML patients did not exercerbate the pulmonary disease in these patients when compared with the patient with an isolated βc expression defect. These findings indicate that βc deficiency eliminates the entire GM-CSF/IL-3/IL-5 receptors system. This corresponds to results showing βc to be essential for signal transduction.22
Following hematopoietic reconstitution after myeloablative therapy, BM and BAL cells from patient no. 1 beared the βc receptor, and the PAP symptoms completely resolved. When he relapsed, we again found his leukemic clone to be βc−. This time he also developed tachypnoea but did not progress to severe respiratory failure. Because the kinetic of pulmonary invasion with malignant myeloid cells is unknown, the question of why the patient did not develop all PAP symptoms at this time remains speculative. It has been demonstrated that replacement of alveolar macrophages from the BM is completed within 35 to 100 days.29 Although it is not known whether this is true for malignant myeloid cells, we suggest that the short time span of only a few days between relapse and myeloablative therapy may have prevented from invasion of the lung with βc− macrophages and from the development of full PAP symptoms.
After one course of chemotherapy, patient no. 2 still had 60% leukemic cells and the pulmonary symptoms got worse; she required mechanical ventilation. However, no focus for an infection was found, and analysis of the BAL fluid gave strong evidence for the diagnosis of PAP. She recieved an intensified chemotherapy protocol that was not able to completely erradicate the blasts. However, the blast count remained stable at around 20%, and the normal hematopoiesis recovered with 10% mature myeloid cells (CD14+/CD15+). Whithin the next 4 weeks, her pulmonary situation slowly improved and she was breathing spontaneously. She died of septicemia with clinically nonrelevant pulmonary symptoms.
After allogeneic BMT, cells from patient no. 3 beared the βc and GM-CSF receptor α chain. She is in remission of her leukemia for 5 years and has had no PAP symptoms ever since.
Our data suggest that some cases of human PAP in patients with AML may be caused by a defect of βc expression on the alveolar macrophages. The involvement of alveolar macrophages in the pathophysiology of PAP has been suggested.27,30,31 In particular, the frequent association of PAP with hematological diseases1,3,32immunodeficiencies13 implicates that the alteration of macrophage function may play an important role in the pathogenesis of PAP.23 33
Our data implicate the following model for the pathogenesis of PAP in AML patients. Alveolar macrophages in AML may be derived from the leukemic clone. It has been established that alveolar macrophages are of BM origin.29 Because myeloid blasts have a growth advantage, the blast clone may be able to replace the normal alveolar macrophages. This has been demonstrated by our FACS analysis showing solely the βc− or βc− plus GM-CSFR α− leukemic clone in the respective patients’ BAL fluid before the antileukemic treatment. The βc− or βc− plus GM-CSFR α− alveolar macrophages may have an impaired surfactant catabolism and clearance of the alveolar space from surfactant, leading to PAP symptoms. Investigations on the kinetics of the alveolar macrophages showed a mean turnover time of 5.6 days, with 70% influx of monocytes and a local division of 30%.34 Elimination or marked reduction of the leukemic βc− or βc− plus GM-CSFR α− clone by high-dose chemotherapy and repopulation of the lung with an allogeneic or autologous βc+ or βc plus GM-CSFR-α+ clone may reverse PAP symptoms.
This study indicates that infantile PAP in AML patients can be associated with a βc− or βc− plus GM-CSFR α− expression, at least in some cases. This conclusion has been corroborated by the following findings. PAP symptoms in the described AML patients occurred at the time of diagnosis of the AML. In the BAL fluid, solely βc− or βc− plus GM-CSFR α− leukemic blasts were found by flow cytometry, indicating an absence of normal alveolar macrophages. Elimination of the leukemic clone and expansion of βc+ and GM-CSFR α+ cells reversed PAP symptoms. These data provide evidence that PAP in these patients may be related to a dysfunction of alveolar macrophages due to a deficiency in the GM-CSF/IL-3/IL-5 receptor system.
ACKNOWLEDGMENT
The authors thank Paul Stevens and Matthias Griese for performing the SP-B and SP-A ELISA, Dr Orthmann for helpful advice, and Petra Genutt and Ulla Wieczoreck for technical assistance.
Supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 503; by the Bundesministerium für Bildung, Wissenschaft und Technologie (BMBF) Germany BEO BioRegio 311661; by the Dr. Mildred Scheel Stiftung der Deutschen Krebshilfe; and by the Elterninitiative Kinderkrebsklinik Düsseldorf e.V. U.D. was the recipient of the Resident Physician Merit Award of the American Society of Hematology 1996.
Presented in part at the 38th Annual Meeting of the American Society of Hematology, Orlando, FL. Some data presented here are part of the thesis of U.H.
Address reprint requests to Uta Dirksen, MD, Department of Pediatric Hematology/Oncology, Children’s Hospital Medical Center, 14.82, Moorenstr. 5, D-40225 Duesseldorf, Germany; e-mail:dirksen@uni-duesseldorf.de; or Stefan Burdach, MD, Children’s Hospital Medical Center, Ernst-Gruberstr. 40, 06097 Halle, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal