Abstract
Erythrocyte production in mammals is known to depend on the exposure of committed progenitor cells to the glycoprotein hormone erythropoietin (Epo). In chimeric mice, gene disruption experiments have demonstrated a critical role for Epo signaling in development beyond the erythroid colony-forming unit (CFU-e) stage. However, whether this might include the possible Epo-specific induction of red blood cell differentiation events is largely unresolved. To address this issue, mechanisms of induced globin expression in Epo-responsive SKT6 cells have been investigated. Chimeric receptors containing an epidermal growth factor (EGF) receptor extracellular domain and varied Epo receptor cytoplasmic domains first were expressed stably at physiological levels in SKT6 cells, and their activities in mediating induced hemoglobinization were assayed. While activity was exerted by a full-length chimera (EE483), truncation to remove 7 of 8 carboxyl-terminal tyrosine sites (EE372) markedly enhanced differentiation signaling. Moreover, mutation of a STAT5 binding site in this construct (EE372-Y343F) inhibited induced globin expression and SKT6 cell hemoglobinization, as did the ectopic expression of dominant-negative forms of STAT5 in parental SKT6 cells. As in normal CFU-e, SKT6 cells also were shown to express functional receptors for stem cell factor (SCF). To further define possible specific requirements for differentiation signaling, effects of SCF on SKT6 cell hemoglobinization were tested. Interestingly, SCF not only failed to promote globin expression but inhibited this Epo-induced event in a dose-dependent, STAT5-independent fashion. Thus, effects of Epo on globin expression may depend specifically on STAT5-dependent events, and SCF normally may function to attenuate terminal differentiation while promoting CFU-e expansion.
© 1998 by The American Society of Hematology.
DURING RED BLOOD CELL development, stringent control is exerted over the proliferation, survival, and maturation of erythroid progenitor cells. An early step in this process is the commitment of pluripotent hematopoietic progenitor cells to the erythroid lineage, and this is thought to be dictated by the coexpression of select lineage-restricted trans-factors, including GATA1,1Friend of GATA1 (FOG),2 and erythroidKrüppel-like factor (EKLF).3 Subsequently, the development of these committed erythroid progenitor cells as erythrocytes has been shown to depend on the lineage- and stage-restricted expression of the single transmembrane receptor for erythropoietin (Epo).4,5 Epo has long been recognized to be required for the efficient formation of red blood cells from erythroid colony-forming units (CFU-e) in vitro6 and, recently, this role for Epo has been confirmed in chimeric mice via disruption of the Epo7 and Epo receptor genes.7-9 Specifically, in the presence of stem cell factor and pokeweed mitogen-stimulated spleen cell conditioned medium, the development of CFU-e from Epo receptor−/− fetal hepatocytes proceeds efficiently.7,8 However, development beyond this stage is either undetectable9 or markedly inhibited in that, in studies by Lin et al,8 limited frequencies of benzidine-positive fetal liver cells were detected. These findings and observations that Epo blocks DNA fragmentation in normal erythroid progenitor cells10 provide clear evidence that Epo critically supports progenitor cell survival and expansion beyond the CFU-e stage.
By comparison, whether Epo might also selectively promote differentiation is an unresolved issue. Using Epo receptor−/− fetal hepatocytes as a model, two types of experiments recently have been performed in which heterologous receptors have been demonstrated to promote CFU-e differentiation in the absence of Epo. First, based on the observed expression of thrombopoietin (Tpo) receptor transcripts in these cells, the ability of Tpo to support erythroid differentiation has been tested.9 In the presence of either stem cell factor (SCF) or interleukin-3 (IL-3) plus IL-11, limited levels of Tpo-dependent red blood cell formation could be demonstrated. Second, Epo receptor−/− fetal hepatocyte differentiation also has been shown to be supported after the ectopic overexpression and activation of a full-length rabbit prolactin receptor form.11 These studies provoke two alternate interpretations. As a strict interpretation, Epo might be considered to be nonessential for differentiation signaling and to be important only as a general survival factor for preprogrammed erythroid progenitor cells. However, high conservation exists in signaling events that are activated by Epo,12 Tpo,13 and prolactin14 via their structurally related receptors,15 and Tpo and prolactin receptors therefore may specifically compensate for the Epo receptor upon their expression and activation in late erythroid progenitor cells.
Consistent with the notion that Epo can specifically promote at least certain late erythroid differentiation events, in several murine,16-18 human,19 and avian20cell lines, Epo has been shown to induce globin gene expression. Murine erythroleukemic SKT6 cells comprise one such example,17 and in recent studies we have isolated stable SKT6 cell sublines that readily hemoglobinize in response to Epo at physiological concentrations.21 In addition, we have demonstrated that chimeric receptors composed of the human epidermal growth factor (EGF) receptor extracellular domain and defined Epo receptor cytoplasmic domains support ligand-induced hemoglobinization, and in preliminary studies we21 as well as Wakao et al22 and Iwatsuki et al18 have provided evidence that STAT5, a signal transducer andactivator of transcription,23 may regulate this Epo response pathway. In the present study, we now show that chimeric receptors containing highly truncated Epo or prolactin Nb2 receptor cytoplasmic domains in fact mediate ligand-induced SKT6 cell hemoglobinization significantly more efficiently than do endogenous Epo receptors. In addition, the point mutation of tyrosine sites for STAT5 binding in these minimal chimeras as well as the ectopic expression of dominant-negative forms of STAT5 are demonstrated to effectively inhibit induced SKT6 cell differentiation. Thus, extended evidence is provided that STAT5 may function as a regulator of Epo-induced globin expression and hemoglobinization. STAT5 is 1 of 8 related transcription factors that have been shown to be activated by most hematopoietic cytokines.23 As defined first in the interferon-α receptor system, cytokine activation of STATs proceeds from the SH2 domain-mediated binding of STATs to phosphotyrosine sites within activated receptor complexes.24 Receptor-associated Jak kinases then phosphorylate STATs at a conserved C-terminal tyrosine residue, and phosphorylated STATs then self-associate. Subsequently dimeric or multimeric STATs undergo nuclear translocation, bind to conserved cis-elements via central DNA binding domains, and modulate transcription at targeted promoters and enhancers via C-terminal transactivation domains. Epo has been shown to selectively activate Jak2 and to recruit primarily STAT5 A and B (2 highly related isoforms in mice)25 to a receptor Y343site.26 However, Epo activation of STATs 1 and/or 3 also has been observed in normal rat fetal liver CFU-e,27Friend virus-infected murine erythroid splenocytes,28 HCD57 cells,29 and SKT6 cells.21
In addition, Epo effects on red blood cell production are augmented by several alternate hematopoietic growth factors. Specifically, IL-3,30 granulocyte-macrophage colony-stimulating factor (GM-CSF),31 IL-9,32 and SCF33promote the expansion of early erythroid progenitor cells (burst-forming units-erythroid), whereas Tpo,34IL-6,35 and again SCF36 have been shown to exert effects at later developmental stages. SCF has perhaps been best-defined as a coregulator. In mice with mutations in the genes for SCF37 or c-Kit,38 anemias are prevalent, and SCF and Epo synergistically promote red blood cell production ex vivo.39 Furthermore, in HCD57 cells, signaling via c-Kit has been suggested to depend on the trans-phosphorylation of the Epo receptor40; in at least certain cell lines c-Kit may occur in constitutive association with Jak2,41,42 and in marrow-derived mast cells SCF may activate STAT5.43 Thus, in the context of red blood cell development, important questions are raised regarding possible effects of SCF on erythropoietic proliferation versus differentiation events. Like normal CFU-e, SKT6 cells presently are shown to express functional receptors for SCF, and the effects of SCF on induced globin expression and SKT6 cell hemoglobinization also have been investigated. Interestingly, SCF proved to inhibit Epo-induced SKT6 cell differentiation in a concentration-dependent, STAT5-independent fashion. Overall, investigations provide novel evidence that specific signaling events may underlie the ability of Epo to advance the development of CFU-e and indicate that SCF may attenuate this pathway to terminal differentiation.
MATERIALS AND METHODS
Chimeric receptor and STAT cDNA constructs.
Receptor constructs studied include the wt Epo receptor and the chimeric constructs EE483, EE372, and EENb2. In each chimera, the extracellular domain is derived from the EGF receptor (Leu1-Cys620) and the transmembrane domain from the Epo receptor (Pro225-Leu247).44EE483 contains the full-length cytoplasmic domain of the Epo receptor, EE372 contains Epo receptor residues Ser248-Met372,45 and EENb2 contains 8 membrane cytoplasmic residues of the Epo receptor (Ser248-Lys256) fused to cytoplasmic residues Ile243-His393 of the rat prolactin Nb2 receptor.46 cDNAs encoding these receptor forms were constructed and cloned into pCIneo (Promega, Madison, WI) as follows. For pCIneo-EE483, an EGF receptor/Epo receptor chimeric cDNA47 was cloned into pGEM5Zf+ (to acquire a 5′ Spe I site) and then into a pCIneo vector (pCIneoΔBII)21 at Nhe I and Sal I sites. pCIneo-EE372 was prepared by restricting pCIneo-EE483 with BglII and Sal I (to excise codons encoding Ile257-Ser483 of the Epo receptor) and by replacing this fragment with an Epo receptor cDNA encoding residues Ile257-Met372.47 pCIneo-EENb2 was constructed by first using polymerase chain reaction (PCR) to prepare a cDNA fragment from a wild-type Nb2 construct.21,46 This modified 3′ Nb2 cDNA then was cloned into pCIneoEE483 atBgl II and Sal I sites. pCINeo-EE372Y343F was constructed by cloning the Bgl II-Xho I fragment of pSL1180-ER396 into pSP72 and by mutating Y343 to phenylalanine by overlap extension.48 In overlap extension, the primers 5′-CCAGGACACCTTCTTGGTATTGGAT-3′ and 5′-ATCCAATACCAAGAAGGTGTCCTGG-3′ were used together with M13 reverse and forward sequencing primers, respectively. The derived PCR product was then cloned into pSP72, excised as a BglII-Xho I fragment, and cloned stepwise into pCINeo-EE483 atBgl II and Sal I sites. pMK1059-EE375Y343F was constructed using pXM190 as a template together with the following PCR primers: 5′-GATCGGGCCCTTACTGGAGCCGGTGGGCAGTGAGCATGCCCAGGACACCTTCTTGGTATTGGATAAGTGG-3′ and 5′-GCTCTAGACTAAGCTTCATCCATAGTCACAGGGTCCAC-3′. This PCR product was cloned into pSP72 at Apa I and Xba I sites, was excised as a 360-bp Bgl II-Xba I fragment, and was used to construct the expression vector pMK1059-EE375Y343F. In the constructs S5ΔD and S3ΔD, the predicted DNA binding domains of murine STAT5A (Thr409-Arg523) and STAT3 (Asn400-Leu508) were deleted using a Pfu polymerase, PCR-based method (Exsite System; Stratagene, La Jolla, CA). The PCR primers used were 5′-GGGCTGGTGGTACTCCATGACGCAA-3′, 5′-GGGTTGACCAAGGAGAACCTCGTGTTC-3′ (S5ΔD), and 5′-AGCCTCCTCCATGTTCATCACTTTTGTGTTCG-3′, 5′-AGCTGGCAGTTCTCGTCCACCACC-3′ (S3ΔD). For S5ΔC, a stop codon was inserted after Ala713 and a 3′ XbaI site was introduced using 5′-CAGCAACCACCTCGAGGACTACAACA-3′ and 5′-CGTCAATGCATCCACAGATGCCTGATCTAGAGC-3′ as PCR primers. All PCR products were sequenced to confirm deletions, recovery of reading frames, and insertions. cDNAs encoding S5ΔD, S5ΔC, and S3ΔD then were cloned to pCINeo. In addition, S5ΔD and S5ΔC were cloned into the dicistronic expression vector pMK1059.49
SKT6 cell culture, transfections, and derived cell lines.
The ability of the above-described chimeric receptor forms to mediate mitogenic and differentiation signaling was studied via their stable expression in SKT6 cells. SKT6 cells were maintained and electrotransfected as described previously.21 Stably transfected lines were selected in G418 (1.2 mg/mL), and low complexity subclones were isolated by dilution. Expression of chimeric receptor forms in derived cell lines was assayed by Northern blotting. Expression of STAT5 mutants from pCINeo and pMK1059 vectors was assayed by Western blotting of cell lysates with antibodies to STAT5A (α-S5#1; Santa Cruz Biotechnology, Santa Cruz, CA) or STAT5A/B (α-S5#2; Transduction Laboratories, Lexington, KY).
Assays of ligand-induced SKT6 cell proliferation.
In assays of ligand-induced proliferation, exponentially growing SKT6 cells were cultured for 10 hours in OptiMem I supplemented with 1% fetal bovine serum (FBS), penicillin (1 U/mL), streptomycin (1 μg/mL), amphotericin B (2.5 ng/mL), and 10−5 mol/L β-mercaptoethanol. Cells then were adjusted to 3 × 105 cells/mL in 96-well plates (50 μL/well). Epo or EGF was added (50 μL) at 48 hours of culture. [methyl-3H] thymidine (1 μCi) was added. At 2 hours of incubation, rates of incorporation were assayed by scintillation counting (Beckman 1205 Betaplate reader; Beckman Instruments, Palo Alto CA).
Assays of ligand-induced SKT6 cell hemoglobinization and globin expression.
In assays of ligand-induced SKT6 cell hemoglobinization, cultures were initiated at 1.5 × 105 cells/mL (1.5 mL/well, 6-well plates) and were exposed to Epo (±10 U/mL) or EGF (±15 ng/mL). At 36 hours, an equal volume of media was added, and at 48 and 72 hours, hemoglobin-positive cells were assayed by staining with 2, 7 diaminofluorene (DAF).21 In assays of globin expression, ligand-exposed cells were collected (1,500g for 8 minutes), washed in 1.5 mL phosphate-buffered saline (PBS; 137 mmol/L NaCl, 2.7 mmol/L KCl, 4.3 mmol/L NaHPO4-7H2O, 1.4 mmol/L KH2PO4, pH 7.3), and lysed by gentle vortexing and incubation (10 minutes at 4°C) in RIPA buffer (1% nonidet-P40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, and 2.2 μg/mL aprotinin). Cleared lysates were assayed for protein (BCA assay; Pierce, Rockford, IL) and were denatured for SDS-polyacrylamide gel electrophoresis (1.7% SDS, 0.1 mol/L dithiothreitol, 0.001 mmol/L bromophenyl blue, 5% glycerol, and 0.06 mol/L Tris-HCl, pH 6.8). Electrophoresis (15% gels) and Western blotting was performed as described previously21 using 0.1-μm nitrocellulose membranes (Protran; Schleicher & Schuell, Keene, NH).
Assays of STAT5 binding activity and tyrosine phosphorylation.
STAT5 activation was based on binding to a biotinylated prolactin response element (PRE; 5′-TTAGATTTCTAGGAATTCAAATC-3′, and 5′-biotin-GATTTGAATTCCTAGAAATCT-3′).21SKT6-EE372 and SKT6-EENb2 cells were exposed to Epo (20 U/mL) or EGF (33 ng/mL), chilled immediately to 0°C, washed in PBS, and lysed in 10 mmol/L CHAPS, 2 mmol/L Na2EDTA, 0.1 mmol/L Na2VO4, 5 mmol/L NaF, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.5 μg/mL leupeptin, 0.7 μg/mL pepstatin A, 2.2 μg/mL aprotinin, 50 mmol/L Tris, pH 8.0, at 0°C with sonication (30 seconds, 50% duty; Branson Sonifier 250; Branson Ultrasonics, Danbury, CT). Cleared lysates (175 μL of 600 μL total per 1.5 × 107 cells) were then combined with 10 μg of poly dIdC (Pharmacia, Piscataway, NJ), biotinylated PRE cassette (250 μmol/L final concentration), and binding buffer to yield 300 μL of sample in 2% glycerol, 60 mmol/L KCl, 0.5 mmol/L Na2EDTA, 1 mmol/L dithiothreitol, 4 mmol/L Tris, 12 mmol/L HEPES, pH 7.9. Samples were incubated stepwise for 20 minutes at 4°C and at 25°C and then were adsorbed to streptavidin Agarose CL4B (40 μL of gel per sample). Gels were washed four times in binding buffer, and bound STAT5 was eluted in sample buffer for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and assayed by ECL Western blotting (STAT5A antibodies; Santa Cruz Biotech). For assay of STAT5 tyrosine phosphorylation, cleared lysates from above were incubated stepwise with antibodies to STAT5A (α-S5#1) at 4°C for 3 hours and with 25 μL of Protein-A Agarose CL4B (Boehringer Mannheim Biochemicals, Indianapolis, IN) at 4°C for 1 hour. STAT5/gel complexes were then washed four times in 5 mmol/L Na2EDTA, 5 mmol/L NaF, 0.05% SDS, 0.05% Na-deoxycholate, 0.1 mmol/L Na2VO4, and 40 mmol/L Tris, pH 7.4, and samples were eluted and electrophoresed (7.5% polyacrylamide, SDS gels). Western blotting was performed using antibodies to phosphotyrosine (UBI, Lake Placid, NY) and ECL (Amersham, Arlington Heights, IL).
Northern blot analyses.
Total RNA was isolated from SKT6 cells using the method of Chomczynski and Sacchi50 and TRIzol reagent (1 × 107 cells/mL; Life Technologies, Gaithersburg, MD). Total RNA was electrophoresed in 1.5% agarose gels containing 5.8% formadehyde, was transferred to Nytran membranes (Schleicher & Schuell), and was UV and heat-fixed. Hybridizations were performed in Quick-Hyb solution, as described previously.5132P-labeled probes were prepared by random priming using the following cDNA fragments: EGF receptor, EcoRI-BglII fragment of pCINeo-EE483; Epo receptor, Bgl II-Xba I of pUC19-EpoR429; Cis, EcoRI-Not I of pCRV-Cis; Myb,Bgl II-Not I of pBluescript-h-cMyb; c-Myc, XhoI fragment of pCINeo-c-myc; c-Kit, Xba I fragment of pCDM8-m-cKit; GAPDH, Kpn I-Xho I fragment of pSP-GAPDH. The 7S rRNA probe was generated by PCR of pSP-7S using 5′-TGTAGTTCCAGCTACTCGGGAGGCT-3′ and 5′-TCCCGCCTGGTCGTTCACCCCT-3′ primers.
RESULTS
Chimeric receptor signaling of SKT6 cell differentiation: Elevated activities of carboxyl-terminal truncated receptor forms.
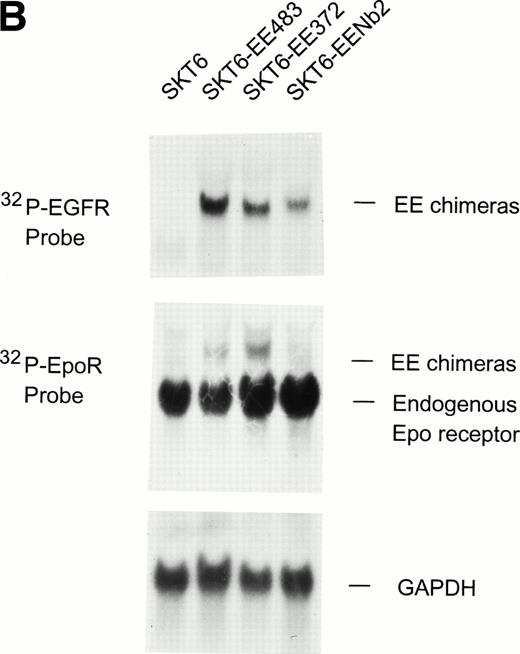
In studies aimed at defining Epo receptor subdomains and associated effectors that regulate induced globin expression in SKT6 cells, chimeric receptor cDNAs were constructed that encode the extracellular domain of the human EGF receptor and cytoplasmic domains of either the Epo receptor (EE483), an Epo receptor form truncated to remove 7 of 8 tyrosine sites for effector binding (EE372), or a naturally occurring truncated form of the rat prolactin Nb2 receptor (EENb2) (Fig 1A). These chimeric constructs were cloned into pCINeo vectors and were expressed stably in SKT6 cells. In derived cell lines, expression of EE483, EE372, and EENb2 receptor transcripts first was assayed by Northern blotting using a human EGF receptor 32P-cDNA probe, and sublines expressing transcripts at comparable levels were isolated (Fig 1B, upper panel). In addition, to compare chimera expression levels with levels of endogenous Epo receptor transcripts, blots were stripped and probed with a 32P-Epo receptor 3′ cDNA fragment (Fig 1B, lower panel). As assayed by phosphor-imaging, levels of chimeric receptor transcript expression were shown to be approximately fivefold lower than endogenous Epo receptor transcript expression levels. In addition, levels of chimeric receptor expression at the cell surface were assayed by fluorescence-activated cell sorting (FACS) using nonactivating phycoerythrin (PE)-labeled antibody to the human EGF receptor extracellular domain. Via this approach, receptor densities were shown to be in the range of 200 to 600 receptors per cell (N. Jiang, data not shown). These initial experiments served to confirm the expression of receptor chimeras at uniform and physiological levels in selected SKT6-EE483, -EE372, and -EENb2 sublines. Previously, overexpression of mutated Epo receptor forms or chimeras has been indicated to activate effectors and response pathways that otherwise would be uncoupled,52 53 and expression levels therefore are a nontrivial consideration.
Chimeric receptor constructs and expression in erythroleukemic SKT6 cells. (A) Diagrammed are wild-type Epo, prolactin Nb2, and EGF receptors together with derived chimeric constructs composed of the human EGF receptor extracellular domain, and cytoplasmic domains of the Epo receptor (full-length EE483, C-truncation EE372) or the Nb2 receptor (EENb2). (B) SKT6 cells were transfected with pCIneo vectors encoding EE483, EE372, or EENb2, and expression of chimeric receptor transcripts in derived cell lines was assayed by Northern blotting. Hybridization was to either a human EGF receptor probe (32P-EGFR) or to a murine Epo receptor probe (32P-EpoR). Equivalence in RNA loading was assessed by hybridization to a GAPDH cDNA.
Chimeric receptor constructs and expression in erythroleukemic SKT6 cells. (A) Diagrammed are wild-type Epo, prolactin Nb2, and EGF receptors together with derived chimeric constructs composed of the human EGF receptor extracellular domain, and cytoplasmic domains of the Epo receptor (full-length EE483, C-truncation EE372) or the Nb2 receptor (EENb2). (B) SKT6 cells were transfected with pCIneo vectors encoding EE483, EE372, or EENb2, and expression of chimeric receptor transcripts in derived cell lines was assayed by Northern blotting. Hybridization was to either a human EGF receptor probe (32P-EGFR) or to a murine Epo receptor probe (32P-EpoR). Equivalence in RNA loading was assessed by hybridization to a GAPDH cDNA.
In initial experiments, the abilities of the above chimeric receptor forms to modulate SKT6 cell growth were assessed. Parental SKT6 cells or derived SKT6-EE483, -EE372, and -EENb2 cell lines were cultured for 10 hours in the presence of 1% serum to limit proliferation and were exposed to either Epo or EGF at increasing concentrations. At 48 hours of cytokine exposure, stimulated rates of [3H] thymidine incorporation were assayed. Exposure of each derived SKT6 cell line to Epo led to an apparent inhibition of growth (Fig 2A). In SKT6-EE372 and SKT6-EENb2 cells, exposure to EGF likewise resulted in a significant inhibition of [3H] thymidine incorporation (Fig 2B). In contrast, EGF-activation of the full-length chimeric receptor form EE483 in SKT6-EE483 cells affected only a modest inhibition of proliferation. Thus, Epo-induced differentiation of parental SKT6 cells is associated with a growth-inhibitory effect, and this response was mediated efficiently in SKT6-EE372 and -EENb2 cells expressing carboxyl-truncated chimeric receptor forms.
Inhibition of SKT6 cell proliferation via C-terminal truncated chimeric receptor forms. To test possible effects of EGF (or Epo as an internal control) on SKT6-EE483, -EE372, and -EENb2 cell proliferation, cells were cultured for 48 hours in the presence of Epo (upper panel, solid symbols) or EGF (lower panel, open symbols) at increasing concentrations. Rates of [methyl-3H] thymidine ([3H]dT) incorporation then were assayed. Graphed are the normalized mean rates of [3H]dT incorporation (n = 3).
Inhibition of SKT6 cell proliferation via C-terminal truncated chimeric receptor forms. To test possible effects of EGF (or Epo as an internal control) on SKT6-EE483, -EE372, and -EENb2 cell proliferation, cells were cultured for 48 hours in the presence of Epo (upper panel, solid symbols) or EGF (lower panel, open symbols) at increasing concentrations. Rates of [methyl-3H] thymidine ([3H]dT) incorporation then were assayed. Graphed are the normalized mean rates of [3H]dT incorporation (n = 3).
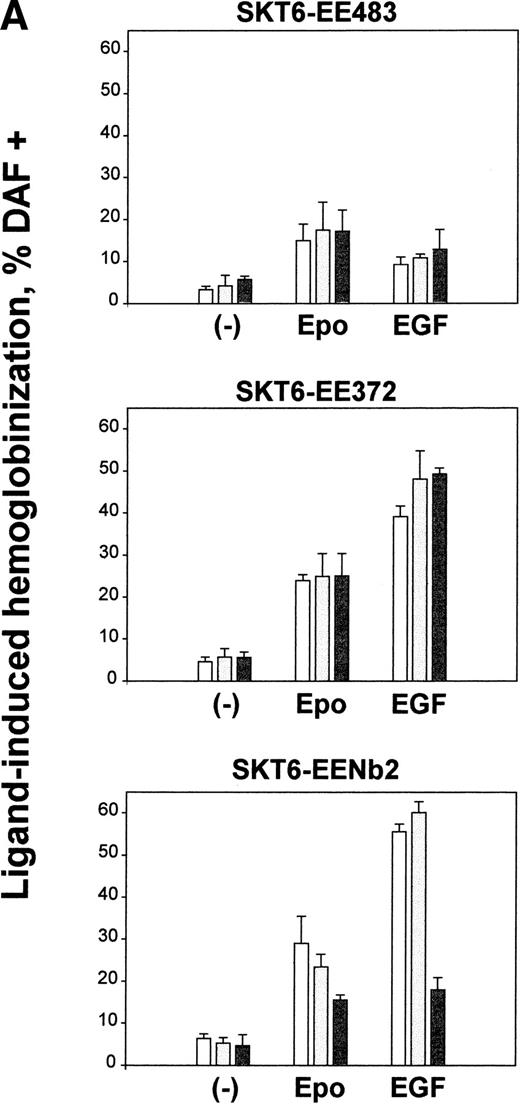
In SKT6-EE483, -EE372, and -EENb2 cells, the ability of EGF to induce differentiation next was assayed. Initially, this was based on EGF-dependent induction of hemoglobinization (Fig 3A). Here, three independent sublines of SKT6-EE483, -EE372, and -EENb2 cells were analyzed, and cells also were exposed in parallel to Epo as an internal positive control. In SKT6-EE483 cells, the chimeric receptor EE483 was shown to mediate EGF-induced hemoglobinization at frequencies (11% on average) approaching those supported by Epo (17% on average; Fig 3A, upper panel). By comparison, the truncated chimeric receptor forms EE372 and EENb2 each proved to be significantly more active in mediating EGF-induced hemoglobinization, with mean frequencies of 46% and 45% hemoglobin-positive cells observed, respectively, upon exposure to EGF (Fig 3A, lower panels). Among SKT6-EENb2 sublines, somewhat lower frequencies of induced hemoglobinization were observed for one subline. However, in this subline, Northern blot analyses showed an apparent inefficiency in chimeric receptor transcript processing (R.C.G., data not shown). Also, and as shown in previous studies,21 exposure of parental SKT6 cells to h-EGF did not affect differentiation (or any other assayed signaling events).
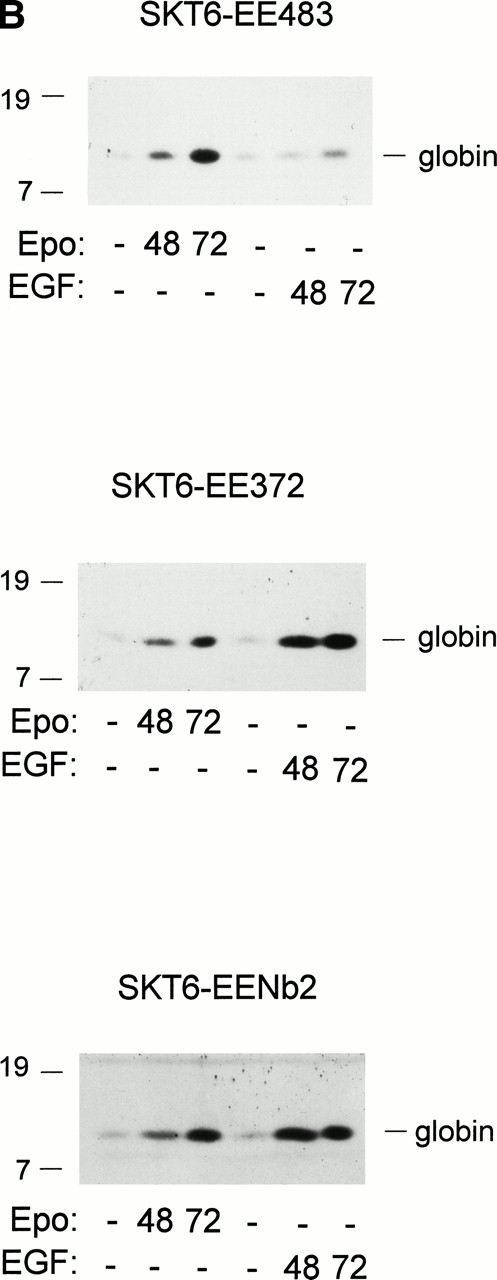
Activities of EE483, EE372, and EENb2 chimeras in mediating induced SKT6 cell hemoglobinization and globin expression. (A) In SKT6 cells stably expressing the above chimeric receptor forms (see Fig 1), hemoglobinization as induced by EGF first was analyzed. SKT6-EE483, -EE372, and -EENb2 cell lines (3 sublines for each designated by open, closed, and shaded histograms, respectively) were exposed to either EGF (15 ng/mL) or Epo (10 U/mL) and, at 72 hours of cytokine exposure, hemoglobin-positive cells were stained with 2, 7 diaminofluorene (DAF) and scored (>200 cells per sample). Graphed are mean frequencies of DAF-positive cells ± standard deviations (n = 3). (B) EGF-induced globin expression in SKT6-EE483, -EE372, and -EENb2 cell lines. Cells were exposed to EGF (15 ng/mL) or Epo (10 U/mL) for the indicated intervals (0, 48, and 72 hours), and levels of globin expression were assayed by direct Western blotting of cell lysates using purified antibodies to murine hemoglobin.
Activities of EE483, EE372, and EENb2 chimeras in mediating induced SKT6 cell hemoglobinization and globin expression. (A) In SKT6 cells stably expressing the above chimeric receptor forms (see Fig 1), hemoglobinization as induced by EGF first was analyzed. SKT6-EE483, -EE372, and -EENb2 cell lines (3 sublines for each designated by open, closed, and shaded histograms, respectively) were exposed to either EGF (15 ng/mL) or Epo (10 U/mL) and, at 72 hours of cytokine exposure, hemoglobin-positive cells were stained with 2, 7 diaminofluorene (DAF) and scored (>200 cells per sample). Graphed are mean frequencies of DAF-positive cells ± standard deviations (n = 3). (B) EGF-induced globin expression in SKT6-EE483, -EE372, and -EENb2 cell lines. Cells were exposed to EGF (15 ng/mL) or Epo (10 U/mL) for the indicated intervals (0, 48, and 72 hours), and levels of globin expression were assayed by direct Western blotting of cell lysates using purified antibodies to murine hemoglobin.
Activities of the above-mentioned chimeric receptor forms in supporting ligand-induced SKT6-EE483, -EE372, and -EENb2 cell differentiation also were assayed by Western blotting of globins. Results are shown in Fig3B and are presented quantitatively in Table 1 (together with the overall results of the above assays of induced hemoglobinization and proliferation). For each cell line and assay, data are normalized versus mean Epo-response values. These data show that, despite their expression at levels below those of endogenous wt Epo receptors in SKT6 cells, the truncated chimeric receptor forms EE372 and EENb2 nonetheless promote these differentiation events at enhanced efficiencies as compared with endogenous Epo receptors. Therefore, the membrane proximal cytoplasmic region of the Epo receptor (Ser248-Met372) mediates induced hemoglobinization, and effectors whose activation normally depends on C-terminal receptor domains may attenuate this differentiation response.
EGF-Induced Globin Expression in SKT6-EE483, -EE372, and -EENb2 Cells
| EGF Exposure (h) . | SKT6-EE483 . | SKT6-EE372 . | SKT6-EENb2 . | |||
|---|---|---|---|---|---|---|
| W-Blots . | DAF(+) . | W-Blots . | DAF(+) . | W-Blots . | DAF(+) . | |
| 48 | 72% | ND | 154% | ND | 184% | ND |
| 72 | 62% | 66.5% | 152% | 225.5% | 128% | 196.4% |
| EGF Exposure (h) . | SKT6-EE483 . | SKT6-EE372 . | SKT6-EENb2 . | |||
|---|---|---|---|---|---|---|
| W-Blots . | DAF(+) . | W-Blots . | DAF(+) . | W-Blots . | DAF(+) . | |
| 48 | 72% | ND | 154% | ND | 184% | ND |
| 72 | 62% | 66.5% | 152% | 225.5% | 128% | 196.4% |
Values shown are normalized averages versus levels of globin expression induced by Epo in each distinct cell line. Levels of globin expression were assayed quantitatively by high-resolution scanning denstometry of Western blots. Mean values represent percentages of pixel densities for EGF/Epo at each time point (n = 3). Means represent frequencies of DAF (+) cells, and likewise are presented as ratios (EGF-induced/Epo-induced). For Western blots and DAF analyses, observed values ranged 17% and 7% from mean values, respectively.
Abbreviation: ND, not determined.
Roles for STAT5 in Epo-induced globin expression and SKT6 cell hemoglobinization.
Based on the elevated activity of the truncated chimeric Epo receptor form EE372 in mediating ligand-induced SKT6 cell differentiation and on the retention of a Y343 site for STAT5 recruitment in this construct,26 54 possible roles for STAT5 in regulating this response were next investigated. First, in analyses of EGF- versus Epo-induced STAT5 DNA-binding activity, SKT6-EE372 cells were exposed to cytokines and, at defined intervals, cells were rapidly chilled to 0°C and lysed. Lysates then were incubated with a biotinylated PRE element to quantitatively retrieve activated STAT5 complexes and bound STAT5 was assayed by adsorption to streptavidin agarose, elution from washed gels, and Western blotting. As shown in Fig 4, the duration of STAT5 activation in response to EGF (via EE372) was sustained and, unlike Epo, persisted beyond 6 minutes to nominally 18 minutes. In independent experiments (and in independent SKT6-EE372 and SKT6-EENb2 sublines), this was observed reproducibly and is at least consistent with the notion that sustained STAT5 activation might contribute to the enhanced activity of C-terminal truncated receptor forms in promoting SKT6 cell differentiation.
Sustained activation of STAT5 via the truncated chimeric receptor form EE372. In SKT6-EE372 cells, the time course of STAT5 activation as induced by EGF versus Epo was assayed as follows. Cells were exposed to either EGF or Epo at concentrations shown to promote mitogenesis of myeloid FDCW2-wtER and FDCW2-EE483 cells at 50% maximal rates (35 ng/mL or 90 nmol/L and 20 U/mL or 120 nmol/L, respectively; R.C.G., unpublished data). At the indicated intervals of cytokine exposure, SKT6 cell lysates were prepared by sonication in the presence of CHAPS. Activated STAT5 then was bound to a biotinylated PRE cassette, adsorbed to streptavidin agarose, eluted from washed gels, and assayed by Western blotting. Data shown are representative of two independent experiments.
Sustained activation of STAT5 via the truncated chimeric receptor form EE372. In SKT6-EE372 cells, the time course of STAT5 activation as induced by EGF versus Epo was assayed as follows. Cells were exposed to either EGF or Epo at concentrations shown to promote mitogenesis of myeloid FDCW2-wtER and FDCW2-EE483 cells at 50% maximal rates (35 ng/mL or 90 nmol/L and 20 U/mL or 120 nmol/L, respectively; R.C.G., unpublished data). At the indicated intervals of cytokine exposure, SKT6 cell lysates were prepared by sonication in the presence of CHAPS. Activated STAT5 then was bound to a biotinylated PRE cassette, adsorbed to streptavidin agarose, eluted from washed gels, and assayed by Western blotting. Data shown are representative of two independent experiments.
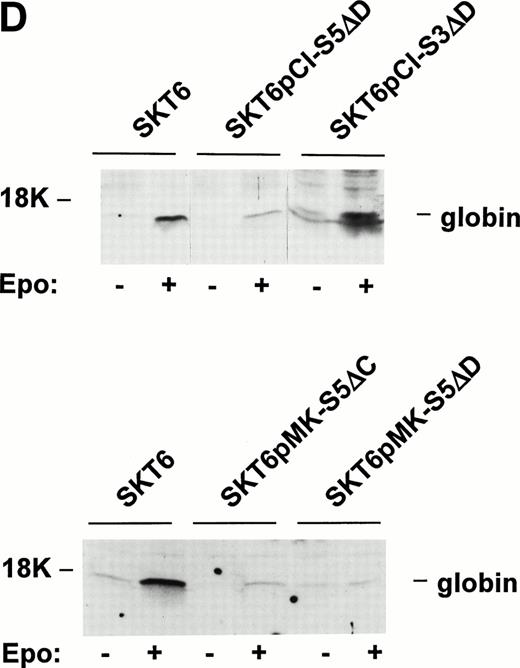
Next, to test the extent to which STAT5 might regulate Epo-induced SKT6 cell hemoglobinization, two approaches were used. First, effects on Epo-induced SKT6 cell differentiation of expressing dominant-negative forms of STAT5 were assessed. For these experiments, two distinct dominant-negative forms of STAT5A were prepared: S5ΔD, in which the predicted DNA-binding domain was deleted, and S5ΔC, in which the carboxyl-terminal transactivation domain was removed (Fig 5A). Towards assessing specificity, a DNA-binding domain deletion mutant of STAT3 (S3ΔD) also was prepared. These STAT constructs each were expressed in SKT6 cells, and in stably transfected and derived sublines (SKT6-pCI-S5ΔD, -pMK-S5ΔD, and -pMK-S5ΔC cells) expression was assessed (Fig 5B). For STAT5ΔD, expression first was accomplished using pCINeo as a vector. Based on Western blotting with C-terminal directed antibodies (α-S5#1), expression levels were appreciable, yet were somewhat below levels of endogenous wt STAT5A. Therefore, STAT5ΔD also was expressed stably in SKT6 cells using pMK as a vector. This increased expression to levels approximating those of endogenous STAT5A (Fig 5B, upper panel). Using pMK as a vector, STAT5ΔC independently was expressed in SKT6 cells at comparable levels (Fig 5B, lower panel). For STAT5ΔC, Western blotting was accomplished using antibodies directed against a more central epitope (α-S5#2). Cells stably expressing these deletion constructs next were tested for their ability to support Epo-induced hemoglobinization. SKT6-pCI-S5ΔD, -pMK-S5ΔD, -pMK-S5ΔC, and -pCI-S3ΔD cells were exposed to Epo for 48 or 72 hours, and frequencies of induced hemoglobinization were assayed. As shown in Fig5C, ectopic expression of either STAT5ΔD or STAT5ΔC inhibited Epo-induced hemoglobinization by approximately 10-fold, and in cells expressing these constructs only low frequencies of hemoglobin-positive cells were detected (ie, <2 %). In addition, lysates from the above-mentioned cell lines were prepared and levels of globin expression at 72 hours of Epo exposure (±Epo) were assessed by Western blotting (Fig 5D). Here, STAT5ΔD- and STAT5ΔC-dependent inhibition of Epo-induced differentiation likewise was observed.
Dominant-negative forms of STAT5A inhibit Epo-induced SKT6 cell hemoglobinization and globin expression. SKT6 cells were transfected stably with expression vectors encoding forms of STAT5A in which either the predicted DNA binding domains (pCIneo-S5▵D, pMK-S5▵D) or the C-terminal transactivation domains were deleted (pMK-S5▵C) (Wang-Ihle). As a control, a STAT3 DNA binding domain deletion mutant (S3▵D) also was constructed and was expressed stably (SKT6-pCIneo-S3▵D cells). Effects of the expression of these STAT mutants on Epo-induced SKT6 cell differentiation were then assessed. (A) Shown diagrammatically are wt STAT5A, wt STAT3, and the derived deletion constructs S5▵D, S5▵C, and S3▵D. (B) Levels of expression of STAT5A deletion constructs in SKT6-pCIneo-S5▵D, -pMK-S5▵D, and -pMK-S5▵C cells as assayed by Western blotting of total cell lysates. The designated antibody S5#1 recognizes a C-terminal epitope of murine STAT5A (Asp774-Ser793), whereas S5#2 recognizes an epitope within a central domain (Phe451-Tyr649) of STAT5-A and -B. As controls, lysates from parental SKT6 cells as well as STAT5A immunoprecipitated from SKT6 cells were coanalyzed. (C) In assays of induced hemoglobinization, SKT6 cells expressing either STAT5▵D, STAT5▵C, or STAT3▵D (as a control) were exposed to Epo (2 U/mL) and, at the indicated intervals (48 and 72 hours), hemoglobinized cells were stained with DAF and scored (>200 cells per sample). Values are the mean frequencies of DAF-positive cells ± standard deviations (n = 3). Frequencies of DAF staining-positive cells scored in the absence of Epo were subtracted as background from these numbers. (D) In assays of induced globin expression, SKT6-pCIneo-S5▵D and SKT6-pCIneo-S3▵D cells (top panel) or SKT6-pMK-S5▵C and SKT6-pMK-S5▵D cells (lower panel) were exposed to Epo (2 U/mL) and, at 72 hours, cell lysates were prepared. Globin levels then were assayed by Western blotting. As a positive control, lysates from parental SKT6 cells (±Epo exposure) were coanalyzed.
Dominant-negative forms of STAT5A inhibit Epo-induced SKT6 cell hemoglobinization and globin expression. SKT6 cells were transfected stably with expression vectors encoding forms of STAT5A in which either the predicted DNA binding domains (pCIneo-S5▵D, pMK-S5▵D) or the C-terminal transactivation domains were deleted (pMK-S5▵C) (Wang-Ihle). As a control, a STAT3 DNA binding domain deletion mutant (S3▵D) also was constructed and was expressed stably (SKT6-pCIneo-S3▵D cells). Effects of the expression of these STAT mutants on Epo-induced SKT6 cell differentiation were then assessed. (A) Shown diagrammatically are wt STAT5A, wt STAT3, and the derived deletion constructs S5▵D, S5▵C, and S3▵D. (B) Levels of expression of STAT5A deletion constructs in SKT6-pCIneo-S5▵D, -pMK-S5▵D, and -pMK-S5▵C cells as assayed by Western blotting of total cell lysates. The designated antibody S5#1 recognizes a C-terminal epitope of murine STAT5A (Asp774-Ser793), whereas S5#2 recognizes an epitope within a central domain (Phe451-Tyr649) of STAT5-A and -B. As controls, lysates from parental SKT6 cells as well as STAT5A immunoprecipitated from SKT6 cells were coanalyzed. (C) In assays of induced hemoglobinization, SKT6 cells expressing either STAT5▵D, STAT5▵C, or STAT3▵D (as a control) were exposed to Epo (2 U/mL) and, at the indicated intervals (48 and 72 hours), hemoglobinized cells were stained with DAF and scored (>200 cells per sample). Values are the mean frequencies of DAF-positive cells ± standard deviations (n = 3). Frequencies of DAF staining-positive cells scored in the absence of Epo were subtracted as background from these numbers. (D) In assays of induced globin expression, SKT6-pCIneo-S5▵D and SKT6-pCIneo-S3▵D cells (top panel) or SKT6-pMK-S5▵C and SKT6-pMK-S5▵D cells (lower panel) were exposed to Epo (2 U/mL) and, at 72 hours, cell lysates were prepared. Globin levels then were assayed by Western blotting. As a positive control, lysates from parental SKT6 cells (±Epo exposure) were coanalyzed.
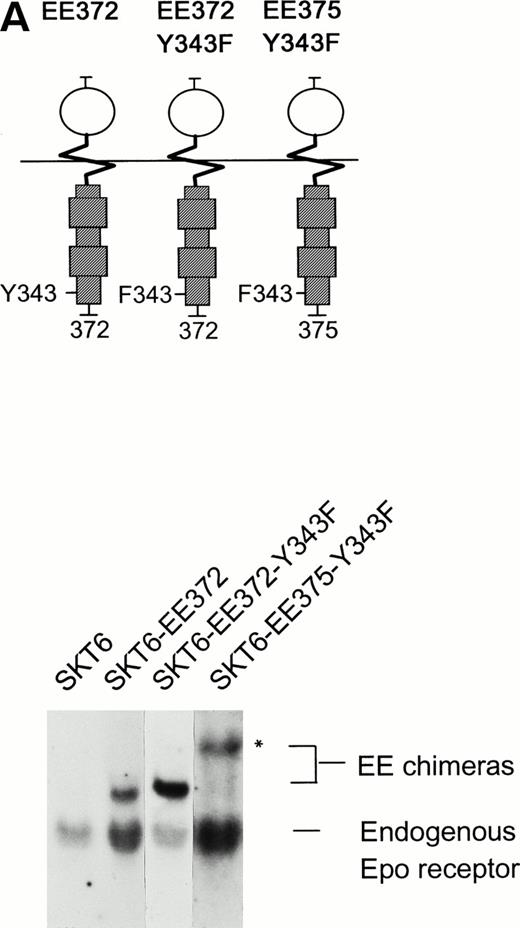
To test more directly roles for STAT5 in Epo receptor-induced SKT6 cell hemoglobinization, the STAT5 binding site Y343 within the chimeric receptor form EE372 was mutated to phenylalanine, and effects of this mutation on receptor activity in mediating SKT6 cell differentiation were assayed. In addition, based on its previous use in studies of mitogenic signaling in DA-3 cells,54 a related receptor form EE375-Y343F was prepared (as an EGF receptor chimera) and also was expressed in SKT6 cells. Northern blot analyses of derived SKT6-EE372-Y343F and -EE375-Y343F cells first were performed, and sublines were selected that expressed chimera transcripts at levels approximating those of the endogenous Epo receptor (Fig 6A). Next, the ability of these receptor forms to mediate the ligand-induced transcription of an established STAT5 target gene, cis,55 was assessed. As shown in Fig 6B, cis gene transcription was induced by Epo in each of these SKT6 cell lines. However, among the above chimeric constructs, only EE372 (but neither EE372-Y343F nor EE375-Y343F) mediated this STAT5-dependent response. EGF-induction of SKT6 cell hemoglobinization likewise was supported only in SKT6-EE372 cells, and not in SKT6-EE372-Y343F or -EE375-Y343F cells (R.C.G., data not shown).
Mutation of the STAT5 binding site Y343 in chimeric EE372 and EE375 receptor forms inhibits EGF-induced SKT6 cell hemoglobinization. (A) To further test roles for STAT5 during ligand-induced SKT6 cell differentiation, the Y343 STAT5 binding site within two distinct truncated chimeric receptor forms, EE372-Y343F and EE375-Y343F, was mutated to phenylalanine. These point-mutated chimeras are illustrated, and levels of transcript expression for these chimeras and endogenous Epo receptors in SKT6-EE372, EE372-Y343F, and -EE375-Y343F cell lines are presented. For EE375-Y343F, increased transcript size (*) is due to expression from the dicistronic vector. (B) To functionally confirm loss of STAT5 signaling via the receptor forms EE372-Y343F and EE375-Y343F, derived SKT6 cell lines were exposed to either EGF (±35 ng/mL) or Epo (±20 U/mL) for 180 minutes, and induced transcription of the STAT5-regulated gene, cis, was assayed by Northern blotting. Equivalence in loading was confirmed by hybridization to a 32P-GAPDH cDNA.
Mutation of the STAT5 binding site Y343 in chimeric EE372 and EE375 receptor forms inhibits EGF-induced SKT6 cell hemoglobinization. (A) To further test roles for STAT5 during ligand-induced SKT6 cell differentiation, the Y343 STAT5 binding site within two distinct truncated chimeric receptor forms, EE372-Y343F and EE375-Y343F, was mutated to phenylalanine. These point-mutated chimeras are illustrated, and levels of transcript expression for these chimeras and endogenous Epo receptors in SKT6-EE372, EE372-Y343F, and -EE375-Y343F cell lines are presented. For EE375-Y343F, increased transcript size (*) is due to expression from the dicistronic vector. (B) To functionally confirm loss of STAT5 signaling via the receptor forms EE372-Y343F and EE375-Y343F, derived SKT6 cell lines were exposed to either EGF (±35 ng/mL) or Epo (±20 U/mL) for 180 minutes, and induced transcription of the STAT5-regulated gene, cis, was assayed by Northern blotting. Equivalence in loading was confirmed by hybridization to a 32P-GAPDH cDNA.
SCF-inhibition of Epo-induced SKT6 cell differentiation.
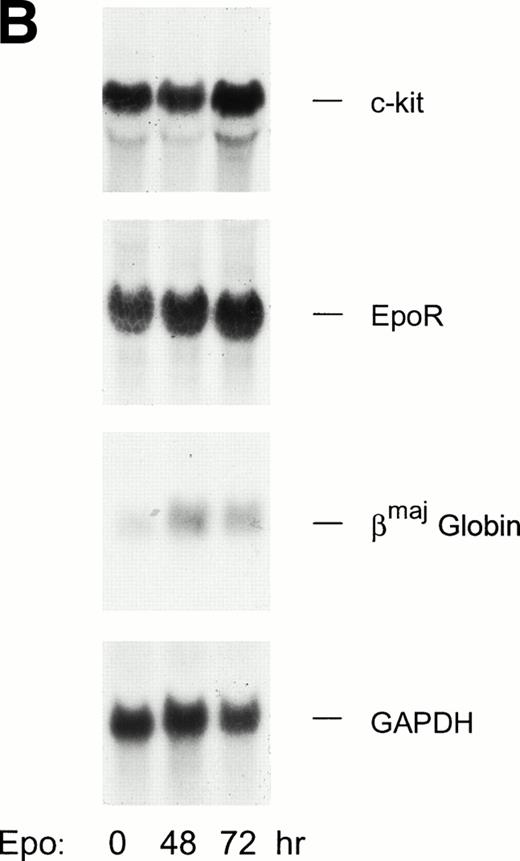
SCF is known to act synergistically with Epo in promoting red blood cell production,36 and c-Kit function recently has been suggested to depend on Epo receptor expression and possibly on receptor trans-activation events.40 Also, because SCF and Epo activate at least an overlapping set of signaling events, possible effects of SCF on SKT6 cell differentiation were investigated. Initial experiments served to establish that, like CFU-e, SKT6 cells express functional SCF receptors. In these experiments, SKT6-EE372 cells were cultured in 0.2% FBS for 10 hours and were then exposed to either Epo, EGF, or SCF. Levels of c-myb, c-myc, and cis transcripts then were assayed by Northern blotting (Fig 7A). Each cytokine detectably induced the expression of c-myb transcripts with the highest levels of induction by c-Kit. c-myc transcription also was induced by SCF, Epo, and EGF. In contrast, cis gene transcription was activated strongly by EGF and Epo, but not by SCF. In addition, levels of c-Kit transcript expression in Epo-exposed SKT6 cells also were assayed by Northern blotting to assess any possible effects on c-Kit expression (Fig 7B). Predicted increases in levels of globin transcripts were observed, but levels of c-Kit (or Epo receptor) transcripts were not modulated significantly during this period of induction.
SCF activation of c-kit response pathways in SKT6-EE372 cells. (A) SCF/c-kit induced transcription of c-myc and c-myb genes in SKT6-EE372 cells. SKT6-EE372 cells were cultured for 10 hours in 0.2% FBS and were exposed to Epo (20 U/mL), EGF (25 ng/mL), or SCF (100 ng/mL) for 0, 90, or 180 minutes. At the indicated intervals, cells were lysed, total RNA was isolated, and levels of c-myc, c-myb, and cis transcript expression were analyzed by Northern blotting. Equivalence in loading was assessed by hybridization to a 7S rRNA cDNA probe. (B) c-kit transcript expression levels are not downmodulated during Epo-induced SKT6 cell hemoglobinization. SKT6 cells were exposed to Epo at 10 U/mL and, at the indicated intervals (0, 48, and 72 hours), levels of c-kit, Epo receptor, and βmaj-globin transcripts were assayed by Northern blotting. Equivalence in loading was assessed by hybridization to a 32P-GAPDH cDNA.
SCF activation of c-kit response pathways in SKT6-EE372 cells. (A) SCF/c-kit induced transcription of c-myc and c-myb genes in SKT6-EE372 cells. SKT6-EE372 cells were cultured for 10 hours in 0.2% FBS and were exposed to Epo (20 U/mL), EGF (25 ng/mL), or SCF (100 ng/mL) for 0, 90, or 180 minutes. At the indicated intervals, cells were lysed, total RNA was isolated, and levels of c-myc, c-myb, and cis transcript expression were analyzed by Northern blotting. Equivalence in loading was assessed by hybridization to a 7S rRNA cDNA probe. (B) c-kit transcript expression levels are not downmodulated during Epo-induced SKT6 cell hemoglobinization. SKT6 cells were exposed to Epo at 10 U/mL and, at the indicated intervals (0, 48, and 72 hours), levels of c-kit, Epo receptor, and βmaj-globin transcripts were assayed by Northern blotting. Equivalence in loading was assessed by hybridization to a 32P-GAPDH cDNA.
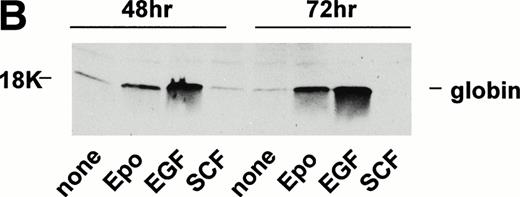
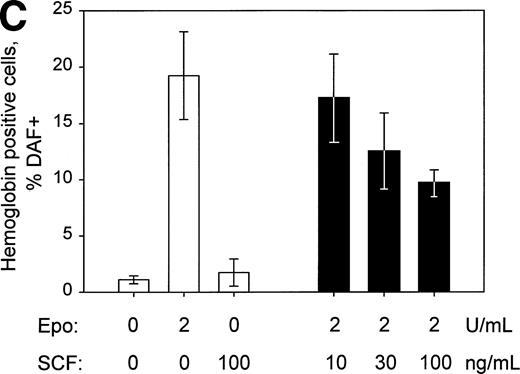
Given the above-indicated occurrence of functional c-Kit receptors in SKT6-EE372 and SKT6 cells, possible effects of SCF on induced hemoglobinization next were tested. SKT6-EE372 cells first were cultured for 72 hours in the presence of either SCF, EGF, or Epo and hemoglobinization was assayed by staining with DAF and by Western blotting of globins (Fig 8A and B). Whereas Epo and EGF each efficiently induced these differentiation responses, no such effects were induced by SCF. Based on these results, whether SCF might act to modulate Epo-induced SKT6 cell differentiation next was tested. SKT6 cells were exposed to SCF at 10, 30, or 100 ng/mL for 8 hours and subsequently to Epo for 72 hours. Interestingly, DAF (Fig 8C) and Western blot analyses (R.C.G., data not shown) showed that SCF exerted a dose-dependent inhibition of Epo-induced globin expression and SKT6 cell hemoglobinization.
SCF-dependent inhibition of Epo-induced SKT6 cell hemoglobinization. (A and B) SKT6-EE372 cell hemoglobinization is induced by Epo and EGF, but not by SCF. SKT6-EE372 cells were exposed to Epo (20 U/mL), EGF (25 ng/mL), or SCF (100 ng/mL) and, at 72 hours of culture, hemoglobin-positive cells were stained with DAF and scored (>200 cells per sample). Graphed (A) are mean frequencies of hemoglobin-positive cells ± standard deviation (n = 3). Also analyzed by Western blotting were levels of globin expression in Epo-, EGF-, and SCF-exposed SKT6 EE372 cells (B). (C) Concentration-dependent SCF-inhibition of Epo-induced SKT6 cell hemoglobinization. SKT6 cells were exposed for 8 hours to SCF at the concentrations indicated and subsequently were stimulated with Epo (2 U/mL) for 72 hours. As controls, SKT6 cells also were exposed independently to either SCF (100 ng/mL) or Epo (2 U/mL). Hemoglobin-positive cells were stained with DAF, and positive cells were scored (> 200 cells per sample). Mean frequencies of DAF-positive cells ± standard deviations (n = 3) are illustrated.
SCF-dependent inhibition of Epo-induced SKT6 cell hemoglobinization. (A and B) SKT6-EE372 cell hemoglobinization is induced by Epo and EGF, but not by SCF. SKT6-EE372 cells were exposed to Epo (20 U/mL), EGF (25 ng/mL), or SCF (100 ng/mL) and, at 72 hours of culture, hemoglobin-positive cells were stained with DAF and scored (>200 cells per sample). Graphed (A) are mean frequencies of hemoglobin-positive cells ± standard deviation (n = 3). Also analyzed by Western blotting were levels of globin expression in Epo-, EGF-, and SCF-exposed SKT6 EE372 cells (B). (C) Concentration-dependent SCF-inhibition of Epo-induced SKT6 cell hemoglobinization. SKT6 cells were exposed for 8 hours to SCF at the concentrations indicated and subsequently were stimulated with Epo (2 U/mL) for 72 hours. As controls, SKT6 cells also were exposed independently to either SCF (100 ng/mL) or Epo (2 U/mL). Hemoglobin-positive cells were stained with DAF, and positive cells were scored (> 200 cells per sample). Mean frequencies of DAF-positive cells ± standard deviations (n = 3) are illustrated.
Finally, experiments were performed to initially investigate possible mechanisms of SCF-inhibition of SKT6 cell differentiation. Specifically, based on the apparent roles for STAT5 during Epo-induced globin expression and hemoglobinization, whether SCF might modulate STAT5 tyrosine phosphorylation or DNA-binding activity was tested. SKT6 cells were exposed first to SCF for 0, 7.5, and 15 minutes and subsequently to Epo (±) for 7.5 minutes. Cells then were rapidly chilled to 0°C and lysed. Phosphotyrosine Western blotting of immunoprecipitated STAT5 from these lysates first showed that SCF did not detectably induce STAT5 tyrosine phosphorylation and did not detectably modulate this response as induced by Epo (Fig 9A). Furthermore, in STAT5 DNA-binding assays, no effects of SCF on Epo-induced PRE cassette binding were observed (Fig 9B). Therefore, SCF does not stimulate but rather inhibits SKT6 cell differentiation, and mechanisms underlying this inhibition apparently do not involve direct effects of SCF and c-Kit on Jak2-STAT5 activation.
Epo-induced activation of STAT5 in SKT6 cells is not modulated by SCF. To test whether SCF-inhibition of Epo-induced SKT6 cell differentiation might involve effects on STAT5 activation, SKT6 cells were pre-exposed to SCF at 100 ng/mL for increasing intervals and subsequently were exposed to Epo at 20 U/mL for 7.5 minutes. Lysates were then prepared by sonication in the presence of CHAPS. (A) Levels of cytokine-induced tyrosine phosphorylation of STAT5 were analyzed by immunoprecipitation and ECL-Western blotting using antibodies to phosphotyrosine. (B) Activated STAT5 was assayed by binding to a biotinylated PRE-element, adsorption to streptavidin-agarose, elution from washed gels, and Western blotting using antibodies to STAT5A.
Epo-induced activation of STAT5 in SKT6 cells is not modulated by SCF. To test whether SCF-inhibition of Epo-induced SKT6 cell differentiation might involve effects on STAT5 activation, SKT6 cells were pre-exposed to SCF at 100 ng/mL for increasing intervals and subsequently were exposed to Epo at 20 U/mL for 7.5 minutes. Lysates were then prepared by sonication in the presence of CHAPS. (A) Levels of cytokine-induced tyrosine phosphorylation of STAT5 were analyzed by immunoprecipitation and ECL-Western blotting using antibodies to phosphotyrosine. (B) Activated STAT5 was assayed by binding to a biotinylated PRE-element, adsorption to streptavidin-agarose, elution from washed gels, and Western blotting using antibodies to STAT5A.
DISCUSSION
One central issue in hematopoietic growth factor (HGF) signaling concerns the extent to which HGFs may affect lineage-specific blood cell differentiation, and an important yet unresolved example is provided by Epo. As introduced above, the ability of at least certain heterologous receptors of the type 1 superfamily to substitute for the Epo receptor in supporting terminal differentiation events9,11,21,22 suggests that Epo receptor-derived signals may be generic ones that serve only to support survival during a preprogrammed course of terminal differentiation. However, in several in vitro systems, Epo readily activates at least select late erythroid differentiation events,16-19 and in transgenic mice the enforced expression of Bcl-2 in erythroid progenitor cells recently has been shown to support BFU-e and CFU-e survival but not red blood cell formation.56 Thus, these latter observations suggest that at least some degree of specificity for differentiation signaling is provided by Epo. To investigate mechanisms associated with such Epo-induced differentiation responses, responsive SKT6 cells presently have been used together with a chimeric receptor approach to define Epo receptor domains and effectors that regulate induced globin expression and hemoglobinization. In these investigations in this model system, three findings merit discussion: the enhanced activity of highly truncated receptor forms, apparent roles for STAT5 in this differentiation response pathway, and an observed repression of Epo-induced SKT6 cell differentiation that occurs upon SCF activation of c-Kit.
With regards first to the observed abilities of truncated chimeric receptor forms to mediate SKT6 cell differentiation at enhanced levels, negative roles for Epo receptor C-terminal domains previously have been defined first in the context of proliferation. A murine Epo receptor construct lacking 40 C-terminal amino acids (including tyrosine residues 443, 460, 464, and 479) originally was shown in BaF/3 cells to support mitogenesis at increased efficiencies.44Mitogenesis since has been shown to also be attenuated by more proximal Y429 and Y431 receptor sites for the recruitment of hematopoietic cell phosphatase (HCP).57 In the truncated chimeric receptor form EE372, each of the above-noted negative regulatory domains is lacking and one possible explanation for the enhanced activity of this chimera in SKT6 cells is that effectors that negatively regulate mitogenesis may act similarly to dampen differentiation signaling (Fig10). For HCP, this appears to be the case, because its forced expression in SKT6 cells recently has been shown to inhibit Epo-induced hemoglobinization, possibly by attenuating Jak2-STAT5 signaling.58 However, evidence exists to suggest that certain other effectors may act to differentially regulate growth versus differentiation events. For example, the expression of an Epo receptor form retaining only Y464 has been shown to efficiently support BaF/3 cell proliferation, but not CFU-e differentiation in Epo receptor−/− fetal hepatocytes.59 Therefore, the enhanced activity of EE372 and EENb2 chimeras in mediating hemoglobinization in SKT6 cells may depend additionally on an uncoupling of effectors that normally favor a mitogenic response. Consistent with these findings in SKT6 cells, in ELM-I-1 cells (a murine erythroleukemic cell line that likewise hemoglobinizes in response to Epo),18 a chimeric receptor form truncated at K348 recently has been reported to mediate ligand-induced hemoglobinization at frequencies somewhat above those supported by a full-length control chimera. However, in studies of the activity of retrovirally expressed Epo receptor mutants in fetal hepatocytes, conflicting results have been observed. Specifically, Wu et al59 observed that an Epo receptor form mutated at all cytoplasmic tyrosine sites except Y343 was attenuated in its ability to support CFU-e maturation, whereas Socolovsky et al11 observed enhanced activity for an Epo receptor form truncated at E374. Within this system, these discrepancies may derive from varied retroviral overexpression of heterologous receptor forms or possibly from partial misfolding of the former Epo receptor mutant.
Candidate effectors of Epo- and SCF-regulated SKT6 cell hemoglobinization. In this model, STAT5 may act to promote globin gene expression via one (or more) primary target genes. By comparison, inhibitory effects of SCF may dependent on the downmodulation of Jak2 by HCP.
Candidate effectors of Epo- and SCF-regulated SKT6 cell hemoglobinization. In this model, STAT5 may act to promote globin gene expression via one (or more) primary target genes. By comparison, inhibitory effects of SCF may dependent on the downmodulation of Jak2 by HCP.
With regards to possible roles for STAT5 during Epo-induced erythroid differentiation, mutation of a defined Y343 site for STAT5 binding (Y343F) in the C-terminal truncated chimera EE372 (and the related chimera EE375) resulted in a loss in signaling of SKT6 cell hemoglobinization. In addition, induced differentiation via endogenous Epo receptors likewise was inhibited efficiently upon the expression of either of two dominant negative forms of STAT5A, S5ΔD, or S5ΔC. While these investigations were ongoing, Wakao et al22reported an observed attenuated differentiation signaling in SKT6 cells for a receptor form point-mutated at Y343 and an inhibition of Epo-induced globin expression upon the expression of a point mutated form of ovine STAT5A (Y694F). Also, the expression of a C-terminally truncated form of STAT5A recently has been shown to diminish frequencies of hemoglobinization in ELM-I-1 cells.18 Thus, the present investigation and these related latter studies each demonstrate at least a partial failure in Epo-induced globin expression and hemoglobinization upon the disruption of STAT5 activation. Also, in the case of exogenously expressed STAT5A mutants, this is presumed in each study to include dominant-negative inhibition of any contributions by STAT5B based on the ability of A and B isoforms to heterodimerize.25 Therefore, these observations consistently suggest that STAT5 normally may mediate Epo effects on globin gene expression and possibly to additional events involved in the terminal differentiation of red blood cells. Beyond this, it is tempting to at least speculate that these responses might involve the STAT5-dependent activation of select targeted genes within late erythroid progenitor cells. However, these possibilities are tempered by recent investigations of STAT5 function in three alternate systems. First, in human erythroleukemic TF-1 cells, a mutated form of the Epo receptor has been shown to be expressed and to limit levels of STAT5 activation.19 Expression of the wt murine Epo receptor in these cells increased levels of STAT5 activation, provided for prolonged proliferation in the presence of Epo, and detectably reduced frequencies of benzidine-positive cells. Based on these observations, STAT5 was suggested to promote mitogenesis and to attenuate differentiation signaling by Epo in this model. Second, in studies in Epo receptor−/− fetal hepatocytes Epo receptor forms retaining any 1 of 8 cytoplasmic tyrosine residues each has been demonstrated upon retroviral overexpression to support CFU-e maturation.59 Although differences in the apparent activities of these Epo receptor mutants were limited, relatively high activities were exerted by receptor forms retaining either Y479 (a site for PI3-kinase binding) or Y343(STAT5 binding site). Therefore, findings in this system are at least consistent with the notion that STAT5 may contribute in an important fashion to Epo-induced differentiation. Finally, effects of disrupting the expression of either STAT5A or STAT5B genes in chimeric mice recently have been reported. In STAT5A−/− mice, mammary gland development is inhibited, and this is consistent with a previously defined role for STAT5 in mediating prolactin-induced whey acidic protein gene expression as a direct differentiation response.60 Also, in STAT5A−/−mice, macrophage proliferative responsiveness to GM-CSF is attenuated detectably.61 By comparison, STAT5B−/− mice display growth hormone-related defects in liver gene expression.62 However, no overt defects in hematopoiesis or erythropoiesis have been reported to date in STAT5-deficient mice. In the context of erythropoiesis, this can be interpreted to mean that either no important roles are exerted by STAT5 or that these roles are compensated for either by alternate Epo receptor-activated effectors and possibly other STATs. Interestingly, in STAT5B−/− mice, an increased expression of STAT1 and possibly STAT3 is apparent in at least certain tissues.62 Although this has not yet been analyzed in erythroid progenitor cells, it is noteworthy that Epo-activation of STAT1 and 3 has been reported in normal erythroid rat fetal hepatocytes27 and in SKT6 cells.21
Finally, with regards to c-Kit signaling, the present studies of effects of SCF on SKT6 cell differentiation were promoted by the consideration that c-Kit function recently has been suggested to involve trans-signaling through Epo receptor complexes40and by the predication that if signals for differentiation in fact are generic ones, then coactivation of c-Kit and Epo receptor complexes might augment differentiation. However, contrary to this prediction, SCF was shown to effectively inhibit Epo-induced SKT6 cell hemoglobinization. This finding first raises the question as to nature of SCF effects on erythroid progenitor cell development. Although SCF has been demonstrated to act as a mitogen and survival factor for erythroid progenitor cells,33,36 SCF interestingly also has been shown to retard Epo-dependent hemoglobinization in normal erythroid colony-forming cells.63 These observations indicate that, although SCF supports the expansion of late erythroid progenitor cells, c-Kit signaling also may attenuate terminal differentiation events. Second, given the demonstrated ability of SCF to act as both a mitogen and viability factor,33,36 51 this observed SCF-dependent inhibition of late erythroid differentiation also indirectly supports the notion that Epo may provide signals beyond those required simply for survival. Indicated specific effects of Epo on late differentiation might depend on the activation of discrete effectors (such as STAT5) or possibly on factors that differentially regulate cell growth and/or cycle status. Further investigations using SKT6 cells expressing minimal chimeric receptor forms should serve to advance mechanistic resolutions of this problem in red blood cell development.
ACKNOWLEDGMENT
The authors thank Amgen, Inc (Thousand Oaks, CA) for the generous provision of recombinant human Epo and Drs Akihiko Yoshimura, Kenneth Lord, and Peter Besmer for the generous provision of cis, c-myb, and c-Kit cDNAs, respectively.
First authorship is merited for N.J. and R.C.G.
Supported by National Institutes of Health Grants No. HL44491 and RCDA HL 03042 to D.M.W. and Sigma Xi Grants-in-Aid of Research to R.C.G. and N.J.
Address reprint requests to Don M. Wojchowski, PhD, 115 William L. Henning Bldg, The Pennsylvania State University, University Park, PA 16802; e-mail: dmw1@email.psu.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Inhibition of SKT6 cell proliferation via C-terminal truncated chimeric receptor forms. To test possible effects of EGF (or Epo as an internal control) on SKT6-EE483, -EE372, and -EENb2 cell proliferation, cells were cultured for 48 hours in the presence of Epo (upper panel, solid symbols) or EGF (lower panel, open symbols) at increasing concentrations. Rates of [methyl-3H] thymidine ([3H]dT) incorporation then were assayed. Graphed are the normalized mean rates of [3H]dT incorporation (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/4/10.1182_blood.v92.4.1104/5/m_blod41638002x.jpeg?Expires=1769180638&Signature=SX3YW7xNTb-EkxiQQ1ATvajiDP9zwR~NqUOWUvYakPIhrKWdb2wzkwlZ8wNHBQJtmsgiRpZhdUk99d3Y3rLjlL4V8csEvcHQ-jlkPkcmEgMRA-WdNJepK3B5Ojek6DB~gWZcUdzqLGeELZ48tHX2fBfsSajD-4wwnxwncSKt9wgRcFpt8d3-2rF8k0oQ2DWHDxv2EcIL9WA4s4E5H1RQXQJtHp6Z6u9WBIKiEteJ~C3~yWGxkBgdaERdgJUVFvBXJ5XoOV0LJQxtnRRt9C4nQ8SUdtn9TWAfC8PyceCuvkB4D5tW7KP~3IqLh1Qq860VQeCw8TCT5fi2Pvw7JxgsLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal